Introduction
Prostate cancer is a very common type of cancer,
accounting for 1/4 of all cancer cases in men, and is the second
most common cause of cancer-related mortality among men (1).
At least 90% of prostate cancers are initially
diagnosed as acinar adenocarcinomas (2), which are almost always
androgen-dependent (3). This reliance
of the development of prostatic adenocarcinoma on androgens working
through the androgen receptor (AR), is the basis for the use of
chemical or surgical castration and AR inhibition as standard
therapies (3). However, after an
initial period of disease control through targeting the androgen
axis, the disease almost inevitably progresses to
castration-resistant prostate cancer (CRPC) (4).
A neuroendocrine pattern is frequently observed in
the cellular composition of CRPC, which was not present in the
initial diagnosis (5). The emergence
of this neuroendocrine pattern in CRPC has been attributed to the
effect of androgen deprivation therapy (3,4,6) and two main mechanisms have been
hypothesized. The first hypothesis suggests that, under prolonged
hormonal manipulation, the resistant neuroendocrine-like tumor cell
populations are selected from an initially heterogeneous tumor
(3). The second hypothesis suggests
that prolonged androgen deprivation may activate a process referred
to as neuroendocrine transdifferentiation, which enables prostatic
adenocarcinoma cells to acquire neuroendocrine characteristics
(4).
There remains the question of whether the assessment
of the neuroendocrine pattern in CRPC may be useful in the
selection of potential responders to target therapies, such as
somatostatin analogues (5), as in the
present case.
Case report
A 69-year-old man was initially diagnosed with
Gleason 4+4 prostate adenocarcinoma in 2007, and was treated by
radiotherapy along with the antiandrogen bicalutamide and the
gonadotrophin-releasing hormone agonist triptorelin. In 2009, the
patient underwent nephrectomy due to severe trauma of the left
kidney during an accident. Between 2009 and 2013, the patient was
intermittently treated with bicalutamide, degarelix and other
luteinizing hormone-releasing hormone (LHRH) agonists, due to
responsive biochemical recurrence with mild elevation of the
prostate-specific antigen (PSA) levels, the highest being 2.2
ng/ml. In the 3 months from October, 2013 to January, 2014, there
was an elevation of PSA from 3.49 to 22.14 ng/ml, despite ongoing
treatment with LHRH agonists. The patient also complained of
dyspnea and chest computed tomography (CT) revealed a 7.5-cm tumor
in the upper lobe of the left lung, which caused stenosis of the
respective bronchus. There were also enlarged mediastinal lymph
nodes, with a maximum diameter of 17 mm. Skeletal radionuclide
imaging with 99mTc-methylene diphosphonate also revealed
numerous osseous metastases in the thoracic and lumbar spine, the
anterior 6th right rib, the right scapula and the left femur.
Bronchial biopsy of the lung metastasis revealed infiltration by
non-small-cell adenocarcinoma of prostatic origin, with a small
proportion of CD56- and synaptophysin-positive cells, indicating
neuroendocrine differentiation. The Ki-67 index was 40%. In
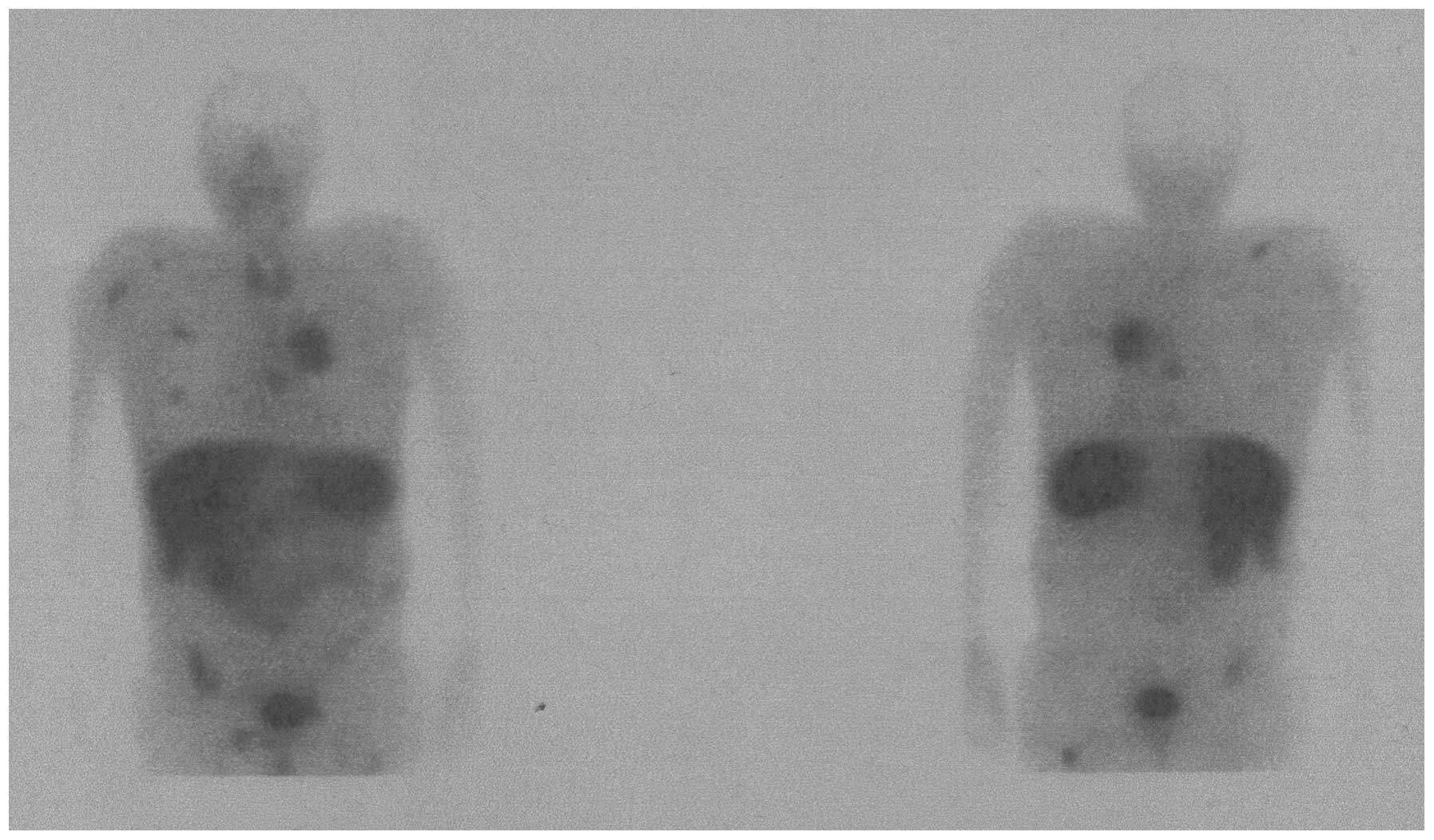
somatostatin receptor scintigraphy with
99mTc-octreotide, there was high uptake in various
locations, including almost all known lung and osseous metastases
(Fig. 1), thus confirming a global
neuroendocrine differentiation of the recurrent metastatic disease.
According to these findings, the selected treatment consisted of
chemotherapy with docetaxel and octreotide injections. In the
follow-up chest CT 6 months later, the size of the lung metastasis
had been reduced to 4.7 cm, there were no enlarged mediastinal
lymph nodes, while the PSA level had decreased to 3.09 ng/ml.
Discussion
According to the American Society of Clinical
Oncology and the Cancer Care Ontario clinical practice guideline,
the main option for patients with metastatic CRPC is systemic
therapy with docetaxel, with moderate benefit, which is however
supported by strong evidence (7).
Additionally, the guideline suggests continuation of androgen
deprivation indefinitely (7). Another
viable option may be the therapeutic targeting of the somatostatin
receptors in the surface of the neuroendocrine tumor cells with a
somatostatin analogue. This option is supported by the relatively
limited but persuasive literature, to which we aim to add our own
experience (8–10).
Somatostatin receptor scintigraphy with
111In-pentetreotide (Octreoscan) or with
99mTc-labelled octreotide analogue is mandatory to
establish the adequate presence of somatostatin receptors in the
metastatic lesions (11). The first
to identify somatostatin receptors in the metastatic lesions of
CRPC patients were Nilsson et al (12) in 1995, who also implemented the first
therapy with octreotide in a patient with CRPC and bone metastases.
The result of this therapeutic intervention was a reduction in the
metabolic activity of a major bone metastasis on
11C-methionine positron emission tomography, accompanied
by symptomatic benefit in the form of pain relief.
Somatostatin receptor scintigraphy is only used to
assess the feasibility of somatostatin analogue therapy and should
not be used for diagnosis or as a surrogate of histological
confirmation of neuroendocrine differentiation, since it has been
found to be positive in 37% of patients with CRPC, with only 11%
exhibiting bone metastases and only 15% visceral metastases on
Octreoscan (11). In particular, when
a lung tumor is identified, as in our case, histological
confirmation of the prostatic origin of the lesion is crucial, as
somatostatin receptor scintigraphy is usually positive in
non-neuroendocrine lung cancers, such as non-small-cell lung cancer
(13,14).
The antitumor effects of somatostatin and its
analogues include inhibition of cell proliferation, invasion and
tumor angiogenesis, and induction of apoptosis through complex
pathways mediated by the somatostatin receptor subtypes on tumor
cells and on cells in their microenvironment (8). Specifically, in prostate cancer,
somatostatin analogues appear to exert a limited effect as
monotherapy (9). However, the
combination of somatostatin analogues with various chemotherapeutic
and other agents has been investigated in clinical studies, with
favorable results in terms of progression-free survival with the
addition of the somatostatin analogue (9). Due to the limited number of patients in
those studies, further randomized studies, including a higher
number of patients, are required to demonstrate the role of
somatostatin analogue-containing combination regimens in prostate
cancer (9).
Recently, the synergistic antitumor activities of
docetaxel and octreotide, the regimen that was administered to our
patient, were demonstrated in vitro in a study by Zhu et
al (10). That study demonstrated
that the combination of docetaxel and octreotide was more efficient
in inducing apoptosis and reducing migration compared with either
drug alone, and that the addition of octreotide increased docetaxel
sensitivity and cytotoxicity (10).
In conclusion, the results presented above, also
supported by other studies on the molecular mechanism of the
docetaxel-octreotide combination (15), suggest that, in carefully selected
cases, somatostatin analogues such as octreotide may be added to
standard chemotherapy with docetaxel. The present study also
recommends that the selection of the cases includes histological
confirmation of the neuroendocrine nature of CRPC, as well as
somatostatin receptor scintigraphy. The outcome of this approach in
our case prompts further investigation in this direction.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Humphrey PA: Histological variants of
prostatic carcinoma and their significance. Histopathology.
60:59–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Terry S and Beltran H: The many faces of
neuroendocrine differentiation in prostate cancer progression.
Front Oncol. 4:602014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nouri M, Ratther E, Stylianou N, Nelson
CC, Hollier BG and Williams ED: Androgen-targeted therapy-induced
epithelial mesenchymal plasticity and neuroendocrine
transdifferentiation in prostate cancer: An opportunity for
intervention. Front Oncol. 4:3702014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matei DV, Renne G, Pimentel M, Sandri MT,
Zorzino L, Botteri E, De Cicco C, Musi G, Brescia A, Mazzoleni F,
et al: Neuroendocrine differentiation in castration-resistant
prostate cancer: A systematic diagnostic attempt. Clin Genitourin
Cancer. 10:164–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beltran H, Tagawa ST, Park K, MacDonald T,
Milowsky MI, Mosquera JM, Rubin MA and Nanus DM: Challenges in
recognizing treatment-related neuroendocrine prostate cancer. J
Clin Oncol. 30:e386–e389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Basch E, Loblaw DA, Oliver TK, Carducci M,
Chen RC, Frame JN, Garrels K, Hotte S, Kattan MW, Raghavan D, et
al: Systemic therapy in men with metastatic castration-resistant
prostate cancer: American Society of Clinical Oncology and Cancer
Care Ontario clinical practice guideline. J Clin Oncol.
32:3436–3448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bousquet C, Lasfargues C, Chalabi M,
Billah SM, Susini C, Vezzosi D, Caron P and Pyronnet S: Clinical
review: Current scientific rationale for the use of somatostatin
analogs and mTOR inhibitors in neuroendocrine tumor therapy. J Clin
Endocrinol Metab. 97:727–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keskin O and Yalcin S: A review of the use
of somatostatin analogs in oncology. Onco Targets Ther. 6:471–483.
2013.PubMed/NCBI
|
|
10
|
Zhu S, Oremo JA, Li S, Zhen M, Tang Y and
Du Y: Synergistic antitumor activities of docetaxel and octreotide
associated with apoptotic-upregulation in castration-resistant
prostate cancer. PLoS One. 9:e918172014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mencobonii M, Tredici S, Rebella L,
Bergaglio M, Galbusera V, Manzara A, Claudiani F, Malcangi B and
Varaldo M: Effect of chemotherapy on somatostatin receptor
detection with octreotide scintigraphy in hormone-refractory
prostate cancer patients. Anticancer Res. 26:2233–2235.
2006.PubMed/NCBI
|
|
12
|
Nilsson S, Reubi JC, Kalkner KM, Laissue
JA, Horisberger U, Olerud C and Westlin JE: Metastatic
hormone-refractory prostatic adenocarcinoma expresses somatostatin
receptors and is visualized in vivo by (111In)-labeled
DTPA-D-(Phe1)-octreotide scintigraphy. Cancer Res.
55(Suppl 23): 5805s–5810s. 1995.PubMed/NCBI
|
|
13
|
Krenning EP, Kwekkeboom DJ, Bakker WH,
Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M,
Reubi JC, et al: Somatostatin receptor scintigraphy with
(111In-DTPA-D-Phe1)-and
(123I-Tyr3)-octreotide: The Rotterdam
experience with more than 1,000 patients. Eur J Nucl Med.
20:716–731. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herlin G, Kölbeck KG, Menzel PL, Svensson
L, Aspelin P, Capitanio A and Axelsson R: Quantitative assessment
of 99mTc-depreotide uptake in patients with
non-small-cell lung cancer: Immunohistochemical correlations. Acta
Radiol. 50:902–908. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lattanzio L, Tonissi F, Monteverde M,
Milano G, Merlano MC and Lo Nigro C: Differential molecular
mechanism of docetaxel-octreotide combined treatment according to
the docetaxel-resistance status in PC3 prostate cancer cells.
Anticancer Drugs. 24:120–130. 2013. View Article : Google Scholar : PubMed/NCBI
|