Introduction
Regorafenib (Stivarga, Bayer HealthCare
Pharmaceuticals) is an orally administered multikinase inhibitor.
Preclinical studies demonstrated that regorafenib targets cell
signaling pathways involved in tumor formation and progression,
including inhibition of protein kinases associated with
angiogenesis [e.g., vascular endothelial growth factor receptor
(VEGFR) 1–3 and TIE2], oncogenesis (e.g., KIT and RET), and
maintainance of the tumor microenvironment (e.g., platelet-derived
growth factor receptor and fibroblast growth factor receptor)
(1,2).
At present, regorafenib has been approved for use
following 3rd-line therapy for metastatic colorectal cancer (CRC)
(3). As shown by computed tomography
(CT) imaging, last-line chemotherapy is often ineffective. In the
present case, treatment with regorafenib was indicated based on the
presence of synchronous multiple lung and liver metastases from
colon cancer and CT imaging revealed a significant change in tumor
size. We herein report the patient's clinical course and CT imaging
findings.
Case presentation
A 74-year-old male patient with sigmoid colon cancer
and synchronous lung and liver metastases (stage IV) was treated
with high anterior resection and D3 lymphadenectomy. The patient
had undergone 16 courses of FOLFOX + bevacizumab (BV) as
postoperative first-line therapy. Due to disease progression,
follow-up treatment with 19 courses of BV + FOLFIRI was
administered as a second-line therapy. The patient's performance
status (PS) worsened to 2 due to the side effects of chemotherapy
(general fatigue and edema). The disease continued to progress and
the patient was administered 14 courses of panitumumab (an agent
with anti-epidermal growth factor receptor activity in patients
with wild-type Kras) as third-line chemotherapy, and the PS
improved from 2 to 1. The disease progressed further and the
patient underwent fourth-line chemotherapy comprising three courses
of panitumumab plus irinotecan; however disease progression
continued. Regorafenib was then administered as the last-line
(fifth-line) therapy, the patient's side effects gradually subsided
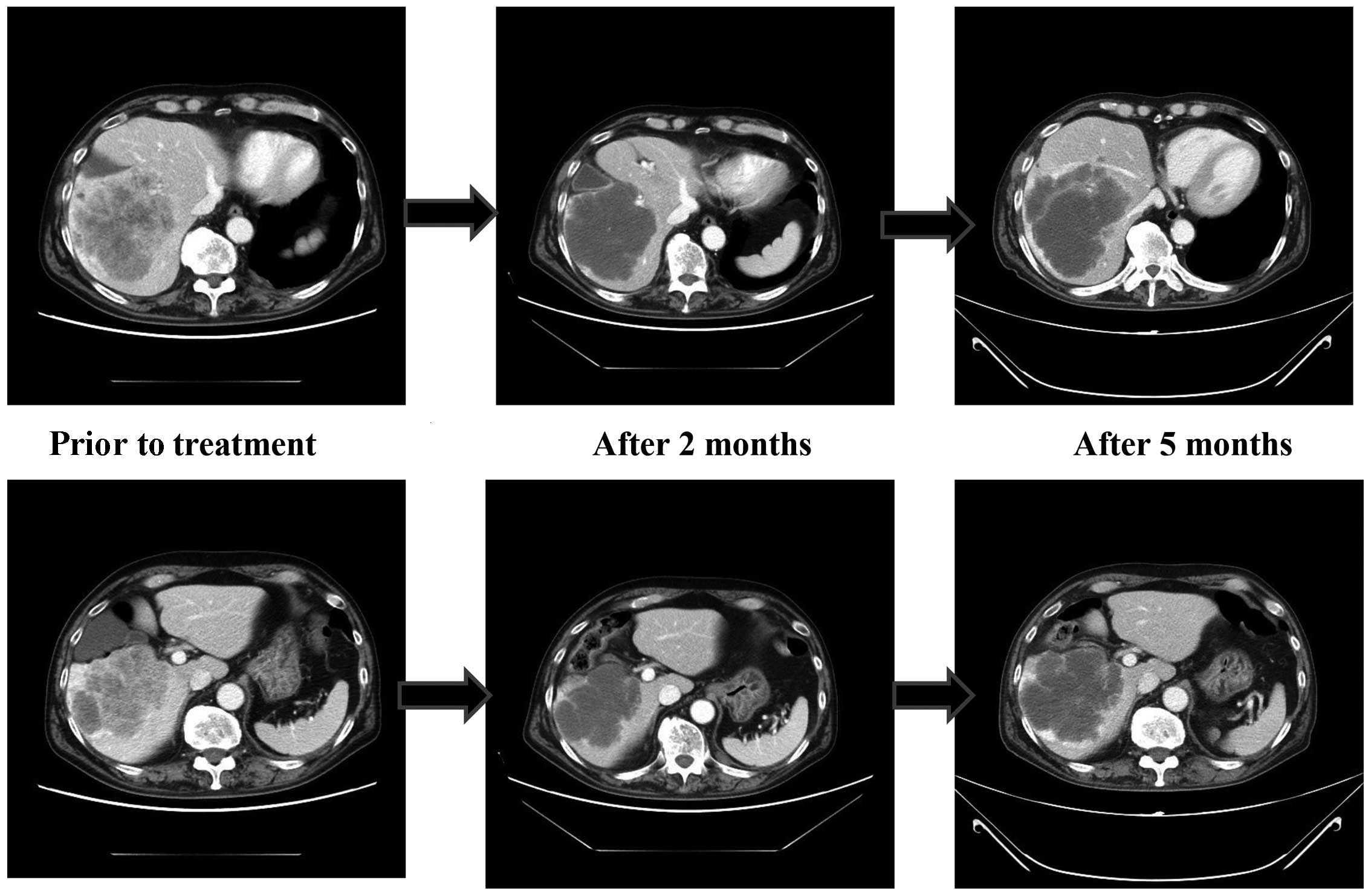
and the disease stabilized. The tumor size (liver and lung)
decreased and morphological changes were revealed by CT imaging. In
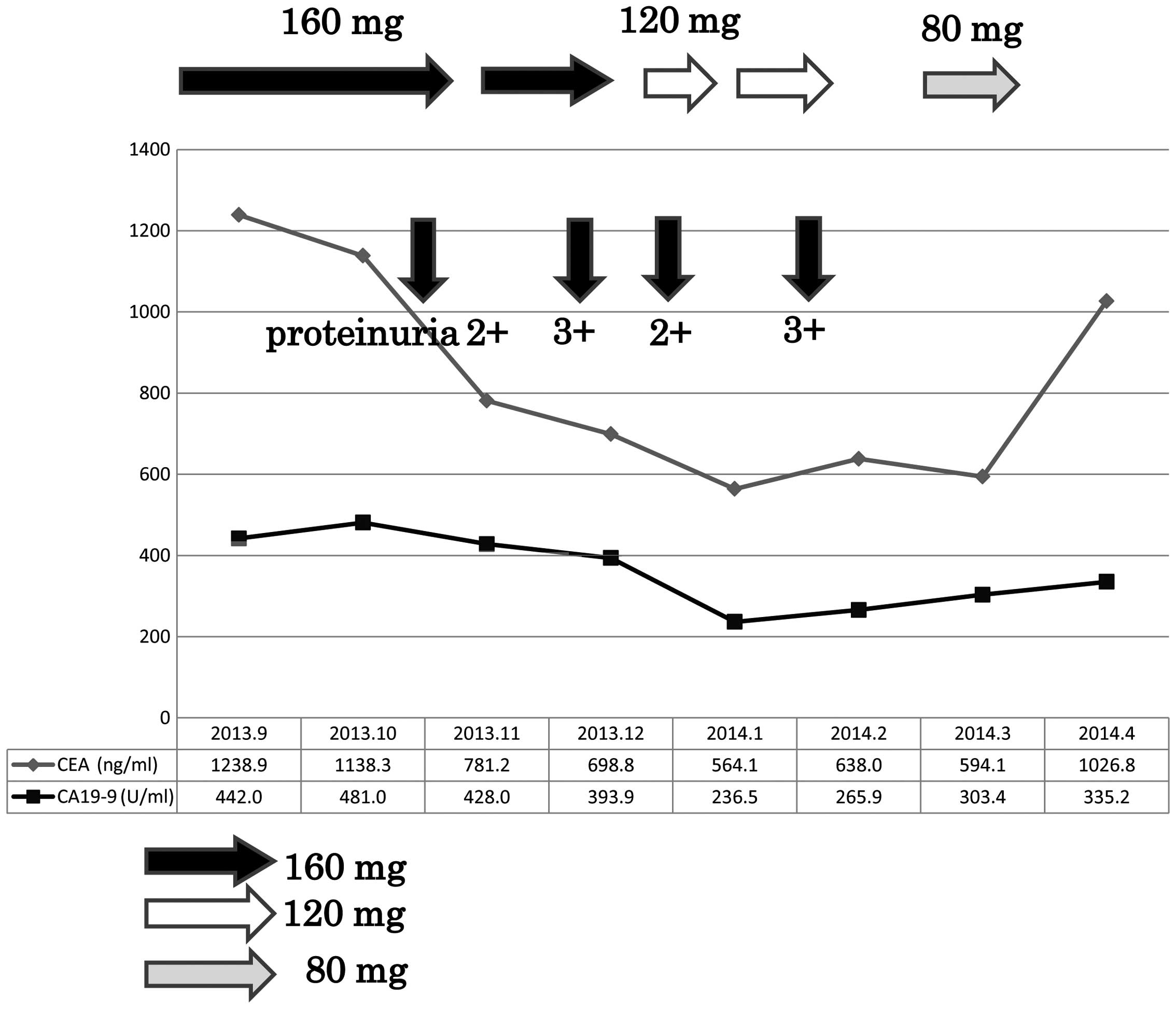
addition, the levels of the tumor markers carcinoembryonic antigen
(CEA) and carbohydrate antigen (CA) 19-9 decreased in parallel with
the changes in tumor morphology (Figs.
1–3). The patient continued to
receive regorafenib for 8 months. As proteinuria (+++) appeared
during the clinical course, the dose of regorafenib was gradually
reduced from 160 to 80 mg, while repeating stop-and-go therapy.
Although the condition improved with the dose reduction and the
stop-and-go therapy, nephrotic syndrome ultimately developed
(Fig. 3). The patient eventually
succumbed to nephrotic syndrome and cardiac failure from the
original disease at 3 years and 10 months after the initiation of
first-line chemotherapy.
Written informed consent was obtained from the
patient for the publication of this case report and accompanying
images.
Discussion
Chemotherapy has played a pivotal role in the
multidisciplinary management of colorectal liver metastases (CLM).
Systemic chemotherapy may reduce the size of metastases, increase
their resectability, and may also help select patients who are most
likely to benefit from surgery by assessing tumor response to
chemotherapy (4,5). However, the conventional tumor
size-based radiological Response Evaluation Criteria in Solid
Tumours (RECIST) may be inadequate for assessing the response to
chemotherapy (6), particularly in
patients treated with a regimen such as BV, which interferes with
angiogenesis. The pathological response to preoperative
chemotherapy was recently found to be correlated with improved
survival and has been proposed as a new outcome end-point following
resection of CLM (7,8). In particular, Shindoh et al
(9) reported that, following therapy
containing BV, the CLM tended to decrease in size and also
underwent distinct morphological changes on CT, namely homogeneous
attenuation and sharp tumor-liver interface. Recently, chemotherapy
for CRC has markedly progressed. In particular, the treatment for
advanced or metastatic CRC has significantly improved due to the
development of the FOLFOX and FOLFIRI regimens. Furthermore, the
introduction of targeted therapy has made the treatment of CRC
patients more effective (10).
Regorafenib is the first small-molecule multikinase
inhibitor to offer a survival benefit in metastatic CRC that has
progressed after all standard therapies. In the CORRECT study, no
patients achieved a complete response; however, 5 patients
receiving regorafenib and 1 patient assigned to placebo exhibited a
partial response, with objective response rates of 1.0 and 0.4%,
respectively (P=0.19) (3). As
complete and partial responses were obtained in only a few
patients, regorafenib is unlikely to achieve complete or partial
disease response. However, disease control was achieved in 41% of
the patients assigned regorafenib and 15% of the patients assigned
a placebo (P<0.0001). The median duration of stable disease was
2.0 months in the regorafenib group and 1.7 months in the placebo
group. These data indicate that regorafenib as last-line
chemotherapy is unlikely to produce effective results on imaging
(RECIST, version1.1) (6). However,
the results of the present study indicated that evaluation of liver
metastases by CT may be effective (10).
The end-point in cancer research is overall
survival; however, tumor response and time to progression are
considered pivotal for surrogate assessment of treatment efficacy.
Tumor response was initially assessed according to the World Health
Organization (WHO) criteria, and later according to the RECIST
guidelines (11). WHO and RECIST
define standard measurement methods for converting radiological
observations into a quantitative and statistically tractable
framework for measuring the response of tumor size to therapy. Both
methods offer simple approaches to determining anatomical size and
changes during treatment as indicators of response (12,13).
Target lesions may be measured with either the bilinear product
approach (WHO) or the single linear summation (RECIST) (14–16). Of
note, following anti-VEGF antigen-containing therapy, CRC
metastases not only tend to decrease in size, but also undergo
distinct morphological changes on CT (9).
Initially, the morphological changes on CT in our
patient indicated a response to regorafenib. These changes may
prolong overall survival and improve the quality of life. However,
in a number of patients, morphological changes do not result in any
change in tumor size; therefore, they are not suitable for
evaluation according to RECIST. Evaluation of chemotherapeutic
response following regorafenib and anti-VEGF antigen therapy were
included in the modified RECIST (mRECIST) guidelines, which were
developed to assess response in patients with hepatocellular
carcinoma based on the measurement of viable tumor with arterial
enhancement on CT. The guidelines of mRECIST were evaluated and
compared with those in RECIST for patients who received sorafenib
for advanced hepatocellular carcinoma. The mRECIST guidelines for
hepatocellular carcinoma introduced amendments to RECIST for the
determination of tumor response in target lesions. The majority of
the patients with stable disease according to RECIST had a
different prognosis according to mRECIST. We recommend that a
suitable CT scan evaluation according to mRECIST guidelines is
performed in patients with CLM. mRECIST should be used for the
standard assessment of treatment efficacy, particularly in patients
receiving regorafenib.
In conclusion, regorafenib appeared to be effective
as a last-line chemotherapy treatment in a patient with sigmoid
colon cancer and synchronous lung and liver metastases. Identifying
unique morphological changes of metastatic lesions on CT imaging
may be a suitable method for evaluating the effects of treatment in
CRC.
References
|
1
|
Strumberg D, Scheulen ME, Schultheis B,
Richly H, Frost A, Büchert M, Christensen O, Jeffers M, Heinig R,
Boix O and Mross K: Regorafenib (BAY 73–4506) in advanced
colorectal cancer: A phase I study. Br J Cancer. 106:1722–1727.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mross K, Frost A, Steinbild S, Hedbom S,
Büchert M, Fasol U, Unger C, Krätzschmar J, Heinig R, Boix O and
Christensen O: A phase I dose-escalation study of regorafenib (BAY
73–4506), an inhibitor of oncogenic, angiogenic, and stromal
kinases, in patients with advanced solid tumors. Clin Cancer Res.
18:2658–2667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sorbye H, Mauer M, Gruenberger T,
Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO,
Primrose JN, Walpole ET, et al: Predictive factors for the benefit
of perioperative FOLFOX for resectable liver metastasis in
colorectal cancer patients (EORTC Intergroup Trial 40983). Ann
Surg. 255:534–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwarz L, Michel P and Scotté M:
Predictive factors for the benefit of perioperative FOLFOX for
resectable liver metastasis in colorectal cancer patients (EORTC
Intergroup Trial 40983). Ann Surg. 261:e28–e29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New Response Evaluation Criteria in Solid Tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adam R, Wicherts DA, de Haas RJ, Aloia T,
Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D
and Castaing D: Complete pathologic response after preoperative
chemotherapy for colorectal liver metastases: Myth or reality? J
Clin Oncol. 26:1635–1641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubbia-Brandt L, Giostra E, Brezault C,
Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE,
Soubrane O, et al: Importance of histological tumor response
assessment in predicting the outcome in patients with colorectal
liver metastases treated with neo-adjuvant chemotherapy followed by
liver surgery. Ann Oncol. 18:299–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shindoh J, Loyer EM, Kopetz S,
Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA,
Charnsangavej C, Aloia TA and Vauthey JN: Optimal morphologic
response to preoperative chemotherapy: An alternate outcome end
point before resection of hepatic colorectal metastases. J Clin
Oncol. 30:4566–4572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chun YS, Vauthey JN, Boonsirikamchai P,
Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H,
Charnsangavej C and Loyer EM: Association of computed tomography
morphologic criteria with pathologic response and survival in
patients treated with bevacizumab for colorectal liver metastases.
JAMA. 302:2338–2344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuhashi N, Takahashi T, Tanahashi T,
Matsui S, Sasaki Y, Tanaka Y, Okumura N, Yamaguchi K, Osada S and
Yoshida K: The efficacy of ‘Abound™’, a nutritional supplement
containing L-glutamine, L-arginine, citric acid, and calcium HMB,
for skin disorders that developed as adverse drug reactions to
anti-EGFR antibody preparation administration: Pilot study. Int J
Colorectal Dis. 31:1055–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuhashi N, Takahashi T, Nonaka K,
Ichikawa K, Yawata K, Tanahashi T, Imai H, Sasaki Y, Tanaka Y,
Okumura N, et al: A case report on efficacy of Abound™ for
anti-EGFR antibody-associated skin disorder in metastatic colon
cancer. World J Surg Oncol. 12:352014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ronot M, Bouattour M, Wassermann J, Bruno
O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond
E and Faivre S: Alternative Response Criteria (Choi, European
Association for the Study of the Liver, and modified Response
Evaluation Criteria in Solid Tumors [RECIST]) versus RECIST 1.1 in
patients with advanced hepatocellular carcinoma treated with
sorafenib. Oncologist. 19:394–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edeline J, Boucher E, Rolland Y, Vauléon
E, Pracht M, Perrin C, Le Roux C and Raoul JL: Comparison of tumor
response by Response Evaluation Criteria in Solid Tumors (RECIST)
and modified RECIST in patients treated with sorafenib for
hepatocellular carcinoma. Cancer. 118:147–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawaoka T, Aikata H, Murakami E, Nakahara
T, Naeshiro N, Tanaka M, Honda Y, Miyaki D, Nagaoki Y, Takaki S, et
al: Evaluation of the mRECIST and α-fetoprotein ratio for
stratification of the prognosis of
advanced-hepatocellular-carcinoma patients treated with sorafenib.
Oncology. 83:192–200. 2012. View Article : Google Scholar : PubMed/NCBI
|