Introduction
Conventional taxanes, such as docetaxel, are
insoluble in water. Thus, taxane formulations with surfactants such
as polysorbate 80 and ethanol have been approved for the management
of several cancers, including prostate adenocarcinoma (1–4).
However, ethanol and polysorbate 80 have the tendency to cause
infusion-related toxicities and hypersensitivity reactions
(5–7). To avoid these adverse events,
premedication with corticosteroids and antihistamines is advocated
prior to docetaxel administration. Furthermore, several new drug
delivery systems have been researched, such as liposomes, polymeric
micelles, protein and nanospheres (8–14).
Nanotechnology for drug delivery has achieved
significant improvements in the efficacy and safety of drugs
(15–17). These nanoparticles are composed of
organic substances, such as lipids, phospholipids, dextran and
chitosan. The drug particles are occasionally reduced in size to
nanoparticles in order to avoid use of other substances as carriers
(18–20).
We herein present the case of a patient with
adenocarcinoma of the prostate with bone metastasis, who developed
an allergic reaction to conventional docetaxel and was later
started on Doceaqualip, a nanosomal docetaxel lipid suspension
(NDLS). Doceaqualip, which is devoid of polysorbate 80 and ethanol,
was developed with lipids generally recognized as safe (GRAS) by
the US Food and Drug Administration. Doceaqualip has achieved a
higher systemic availability of docetaxel when compared with
conventional docetaxel in patients with solid tumors. Furthermore,
the increased efficacy of Doceaqualip compared with that of
conventional docetaxel has been proven in patients with breast
cancer without requirement for any premedication (18).
Case report
A 65-year-old male patient from Nairobi, Kenya,
initially presented with severe back pain in August, 2015. The
patient was evaluated and diagnosed with adenocarcinoma of the
prostate with bone metastasis after undergoing fluorodeoxyglucose
(FDG) positron emission tomography/computed tomography (PET/CT).
Prostate needle biopsy revealed adenocarcinoma of the prostate with
a Gleason score of 8 (4+4) and he was treated with goserelin
acetate injection and bicalutamide tablets for ~3 months. The
patient had a history of hypertension that was controlled with
treatment, and a history of transurethral resection of the prostate
(TURP). Reassessment was performed in November, 2015. The patient
had an Eastern Cooperative Oncology Group (ECOG) performance status
of <2. A Ga-68 prostate-specific membrane antigen (PSMA) PET/CT
scan revealed post-TURP changes, with small mild nodular
hypermetabolism in the left posterior peripheral zone, likely
representing residual prostatic disease. The PSMA revealed
extensive FDG-avid heterologous osteosclerotic lesions (Fig. 1), with a standardized uptake value
(SUV) of 10.5 for the prostate and 18.0 for skeletal lesions. The
serum prostate-specific antigen (PSA) level was 0.32 ng/ml. The
cardiac function was normal, with an ejection fraction of 62%. The
blood counts were within normal limits.
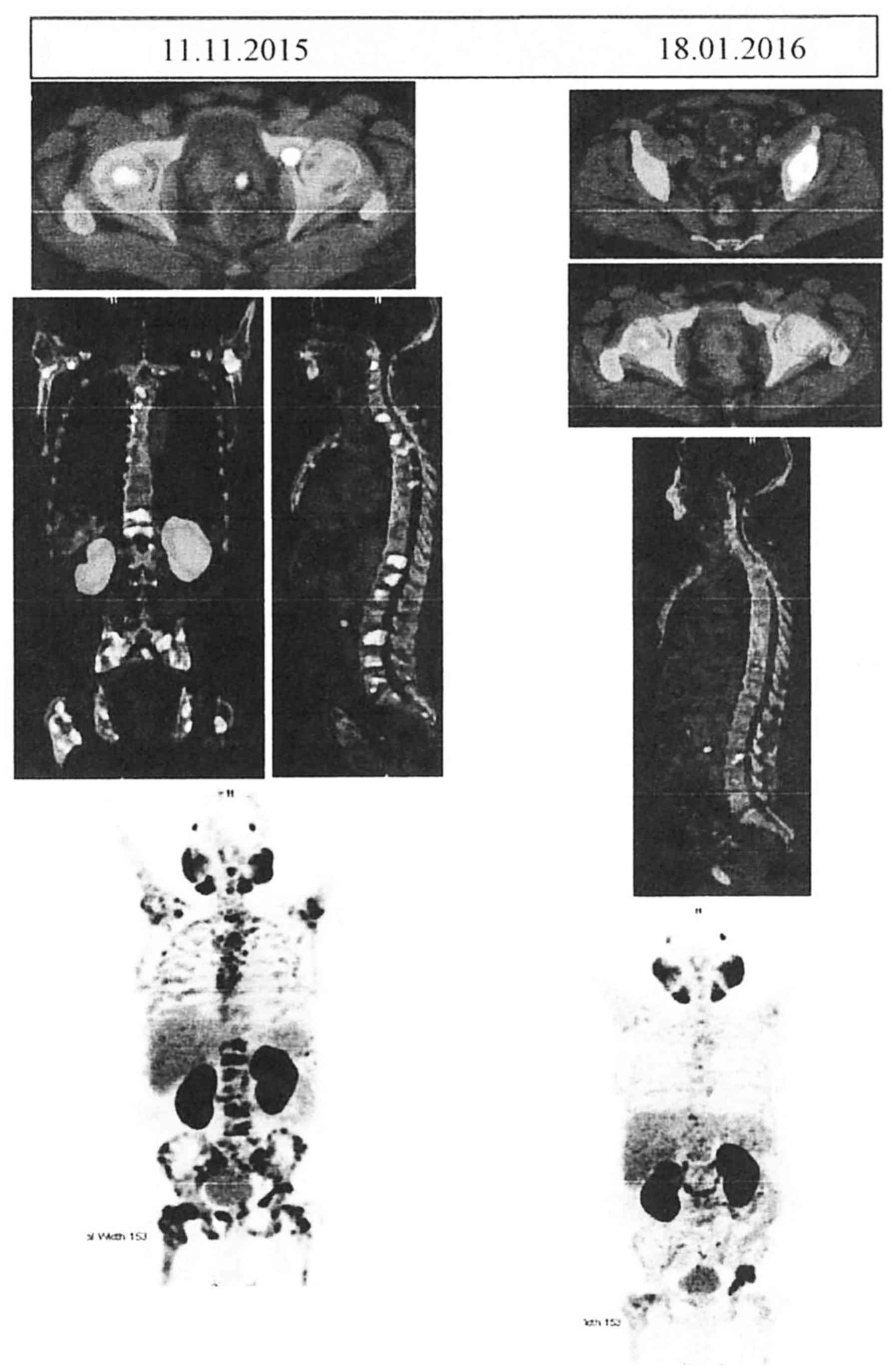 | Figure 1.Comparative Ga-68 PSMA PET CT scans
before and after the administration of Doceaqualip. Left panel:
Images obtained in November, 2015, showing post-TURP changes, small
mild nodular hypermetabolism in the left posterior peripheral zone
(likely representing residual prostatic disease) and extensive
FDG-avid heterologous osteosclerotic lesions. Right panel: Images
obtained in January, 2016, showing post-TURP changes, mild interval
regression of the nodular hypermetabolism in the left posterior
peripheral zone and morphologically stable heterogenous
osteosclerotic lesions with internal regression of the metabolic
activity. PSMA, prostate-specific membrane antigen; PET, positron
emission tomography; CT, computed tomography; TURP, transurethral
resection of the prostate; FDG, fluorodeoxyglucose. |
Chemohormonal therapy was planned and the patient
was admitted for the first cycle of chemotherapy. Injection of
docetaxel 120 mg, denosumab 120 mg and degarelix 240 mg was
scheduled, with 5 cycles planned for docetaxel. Following written
informed consent, the first cycle was initiated with conventional
docetaxel (Taxotere) 120 mg after premedication with
corticosteroids and antihistamines. The patient developed an
anaphylactic reaction (bronchospasm, hypotension and skin rash)
within 5 min; he was administered an IV injection of
chlorpheniramine maleate (Avil), hydrocortisone and paracetamol,
and recovered within 10 min. After 12 h, the patient received 120
mg of the NDLS Doceaqualip, which was well-tolerated without any
adverse reactions. The patient subsequently received the next 3
cycles of chemotherapy with Doceaqualip. A reassessment examination
performed after the third cycle of chemotherapy with a Ga-68 PSMA
PET/CT scan (Fig. 1) showed mild
interval regression of the small mild nodular hypermetabolism in
the left posterior peripheral zone and morphologically stable
heterogenous osteosclerotic lesions with internal regression of the
metabolic activity. However, mild interval progression of focal
hypermetabolism in the left acetabulum was present. No new
metastases were observed, with an SUV of 6.9 for the prostate and
19.4 for the skeletal lesions. The patient tolerated the
Doceaqualip treatment well, without any adverse events. The patient
received the fifth cycle of Doceaqualip in March, 2016, along with
degarelix injection 80 mg, denosumab 120 mg, filgrastim 300 µg and
bicalutamide tablets 50 mg. The patient was followed up 3 months
later and the adenocarcinoma of the prostate had regressed
considerably.
Discussion
The taxanes paclitaxel (Taxol) and docetaxel
(Taxotere) have been proven to be effective as first-line therapy
for metastatic cancer in several randomized clinical trials and are
widely prescribed chemotherapeutic agents (21,22).
Docetaxel is considered to be one of the more promising taxanes,
and docetaxel formulations with polysorbate 80 and ethanol
(Taxotere) have been advocated in the management of different types
of cancer, including prostate, non-small-cell lung, ovarian, breast
and head and neck cancer. Polysorbate 80 in conventional docetaxel
leaches plasticizers from polyvinylchloride infusion sets affecting
the intravenously administered drug, and also causes
hypersensitivity reactions, with the most common symptoms being
bronchospasm, urticaria and hypotension (23). To minimize these toxicities and avoid
hypersensitivity reactions, corticosteroids are used as
premedication prior to the administration of docetaxel. However,
corticosteroids are reported to cause hyperglycemia and increased
infectious episodes (24).
Fatalities due to polysorbate 80-containing docetaxel anaphylaxis
may occur even after such prophylaxis (23).
Several formulations, such as taxane analogues and
prodrugs, docetaxel-encapsulated nanoparticle-aptamer bioconjugates
albumin nanoparticles, polyglutamates, emulsions, liposomes,
docetaxel fibrinogen-coated olive oil droplets and submicronic
dispersions, have been developed to avoid the abovementioned
toxicities. Doceaqualip was primarily developed to avoid toxicities
associated with polysorbate 80 and ethanol and to avoid
premedication (18). Doceaqualip is
formulated with Nano Aqualip Technology that has nano-carriers in
suspension form, composed of GRAS lipids, and has demonstrated a
better safety profile with a higher bioavailability compared with
docetaxel (18). Moreover, even with
premedication with corticosteroids and antihistamines, minor
reactions (e.g., flushing and rash) occur in ~40% of patients
treated with conventional docetaxel (4,18,25).
Similarly, the patient described herein, who had adenocarcinoma of
the prostate with extensive bone metastasis, developed an allergic
reaction when administered conventional docetaxel with polysorbate
80 and ethanol, despite being premedicated with corticosteroids and
antihistamines. The patient was immediately administered IV
corticosteroids and antihistamines to manage the allergic reaction,
and the first cycle of NDLS Doceaqualip 120 mg (75
mg/m2) was later initiated, along with denosumab 120 mg
and filgrastim 240 mg. The treatment was well-tolerated, without
any adverse reactions. The patient received further cycles without
any incidents. The patient's condition also improved, as evidenced
with PET scans before and after the administration of Doceaqualip
(Fig. 1). In the present case, the
patient developed prostate cancer post-TURP, which is consistent
with the findings of Karlsson et al (26) who suggested a mildly increased risk
(standardized incidence ratio: 1.26; 95% confidence interval:
1.17–1.35) of prostate cancer following TURP (26).
This case report further adds to the evidence that
conventional docetaxel with polysorbate 80 and ethanol has the
propensity to result in adverse reactions, despite premedication
with corticosteroids and antihistamines. The NDLS Doceaqualip may
be an excellent tool in the management of prostate cancer with bone
metastasis.
Acknowledgements
We would like to thank Dr Kamlesh Patel (Intas
Pharmaceuticals Ltd.) for the necessary coordination during the
development of the case report and follow-up with the
journal/publisher, and Dr Venugopal Madhusudhana (Lambda
Therapeutic Research) for his support in developing the
concept/medical writing, additional editorial support and follow-up
with the journal/publisher. Dr Mujtaba Khan is an employee of Intas
Pharmaceuticals Ltd.
References
|
1
|
Valero V, Holmes FA, Walters RS, Theriault
RL, Esparza L, Fraschini G, Fonseca GA, Bellet RE, Buzdar AU and
Hortobagyi GN: Phase II trial of docetaxel: A new, highly effective
antineoplastic agent in the management of patients with
anthracycline-resistant metastatic breast cancer. J Clin Oncol.
13:2886–2894. 1995.PubMed/NCBI
|
|
2
|
Pronk LC, Stoter G and Verweij J:
Docetaxel (Taxotere): Single agent activity, development of
combination treatment and reducing side-effects. Cancer Treat Rev.
21:463–478. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hortobagyi GN and Kris MG: Expanding
horizons: An update on the use of docetaxel in non-small cell lung,
ovarian, and breast cancer. Semin Oncol. 29(3): Suppl 12. S1–S3.
2002. View Article : Google Scholar
|
|
4
|
Hennenfent KL and Govindan R: Novel
formulations of taxanes: A review. Old winein a new bottle? Ann
Oncol. 17:735–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masini E, Planchenault J, Pezziardi F,
Gautier P and Gagnol JP: Histamine-releasing properties of
Polysorbate 80 in vitro and in vivo: Correlation with its
hypotensive action in the dog. Agents Actions. 16:470–477. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loos WJ, Baker SD, Verweij J, Boonstra JG
and Sparreboom A: Clinical pharmacokinetics of unbound docetaxel:
Role of polysorbate 80 and serum proteins. Clin Pharmacol Ther.
74:364–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tije AJ, Verweij J, Loos WJ and Sparreboom
A: Pharmacological effects of formulation vehicles: Implications
for cancer chemotherapy. Clin Pharmacokinet. 42:665–685. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esmaeili F, Dinarvand R, Ghahremani MH,
Amini M, Rouhani H, Sepehri N, Ostad SN and Atyabi F:
Docetaxel-albumin conjugates: Preparation, in vitro evaluation and
biodistribution studies. J Pharm Sci. 98:2718–2730. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng SS, Mei L, Anitha P, Gan CW and Zhou
W: Poly (lactide)-vitamin E derivative/montmorillonite nanoparticle
formulations for the oral delivery of docetaxel. Biomaterials.
30:3297–3306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheikh S, Ali SM, Ahmad MU, Ahmad A,
Mushtaq M, Paithankar M, Mandal J, Saptarishi D, Sehgal A,
Maheshwari K and Ahmad I: Nanosomal Amphotericin B is an
efficacious alternative to Ambisome for fungal therapy. Int J
Pharm. 397:103–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ali SM, Ahmad A, Sheikh S, Ahmad MU, Rane
RC, Kale P, Paithankar M, Saptarishi D, Sehgal A, Maheshwari K and
Ahmad I: Polyoxyl 60 hydrogenated castor oil free nanosomal
formulation of immunosuppressant Tacrolimus: Pharmacokinetics,
safety, and tolerability in rodents and humans. Int
Immunopharmacol. 10:325–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang HY, Kim IS, Kwon IC and Kim YH:
Tumor targetability and antitumor effect of docetaxel-loaded
hydrophobically modified glycol chitosan nanoparticles. J Control
Release. 128:23–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu B, Yang M, Li R, Ding Y, Qian X, Yu L
and Jiang X: The antitumor effect of novel docetaxel-loaded
thermosensitive micelles. Eur J Pharm Biopharm. 69:527–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du W, Hong L, Yao T, Yang X, He Q, Yang B
and Hu Y: Synthesis and evaluation of water-soluble docetaxel
prodrugs-docetaxel esters of malic acid. Bioorg Med Chem.
15:6323–6330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Jong WH and Borm PJ: Drug delivery and
nanoparticles: Applications and hazards. Int J Nanomedicine.
3:133–149. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farokhzad OC and Langer R: Impact of
nanotechnology on drug delivery. ACS Nano. 3:16–20. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kipp JE: The role of solid nanoparticle
technology in the parenteral delivery of poorly water-soluble
drugs. Int J Pharm. 284:109–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmad A, Sheikh S, Taran R, Srivastav SP,
Prasad K, Rajappa SJ, Kumar V, Gopichand M, Paithankar M, Sharma M,
et al: Therapeutic efficacy of a novel nanosomal docetaxel lipid
suspension compared with taxotere in locally advanced or metastatic
breast cancer patients. Clin Breast Cancer. 14:177–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Jong WH and Borm PJ: Drug delivery and
nanoparticles: Applications and hazards. Int J Nanomedicine.
3:133–149. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kipp JE: The role of solid nanoparticle
technology in the parenteral delivery of poorly water-soluble
drugs. Int J Pharm. 284:109–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelly WK, Halabi S, Carducci M, George D,
Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, et
al: Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Urquhart LM: Taxanes as a first-line
systemic treatment in metastatic breast cancer. Clin J Oncol Nurs.
17:(Suppl). S15–S21. 2013. View Article : Google Scholar
|
|
23
|
Norris LB, Qureshi ZP, Bookstaver PB,
Raisch DW, Sartor O, Chen H, Chen F and Bennett CL: Polysorbate 80
hypersensitivity reactions: A renewed call to action. Commun Oncol.
7:425–428. 2010. View Article : Google Scholar
|
|
24
|
Yoo KE, Kang RY, Lee JY, Lee YJ, Suh SY,
Kim KS, Kim HS, Lee SH and Lee BK: Awareness of the adverse effects
associated with prophylactic corticosteroid use during docetaxel
therapy. Support Care Cancer. 23:1969–1977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sparano JA, Makhson AN, Semiglazov VF,
Tjulandin SA, Balashova OI, Bondarenko IN, Bogdanova NV, Manikhas
GM, Oliynychenko GP, Chatikhine VA, et al: Pegylated liposomal
doxorubicin plus docetaxel significantly improves time to
progression without additive cardiotoxicity compared with docetaxel
monotherapy in patients with advanced breast cancer previously
treated with neoadjuvant-adjuvant anthracycline therapy: Results
from a randomized phase III study. J Clin Oncol. 27:4522–4529.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karlsson CT, Wiklund F, Grönberg H, Bergh
A and Melin B: Risk of prostate cancer after trans urethral
resection of BPH: A cohort and nested case-control study. Cancers
(Basel). 3:4127–4138. 2011. View Article : Google Scholar : PubMed/NCBI
|















