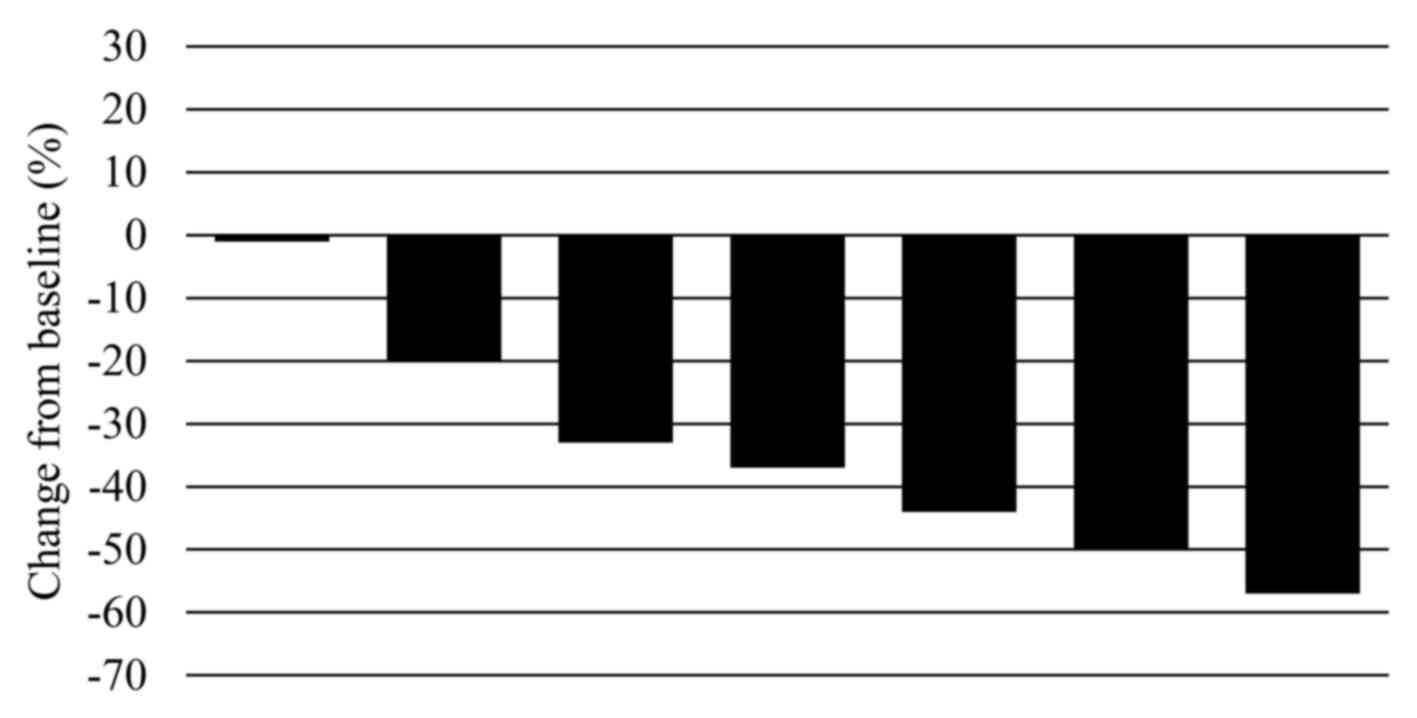
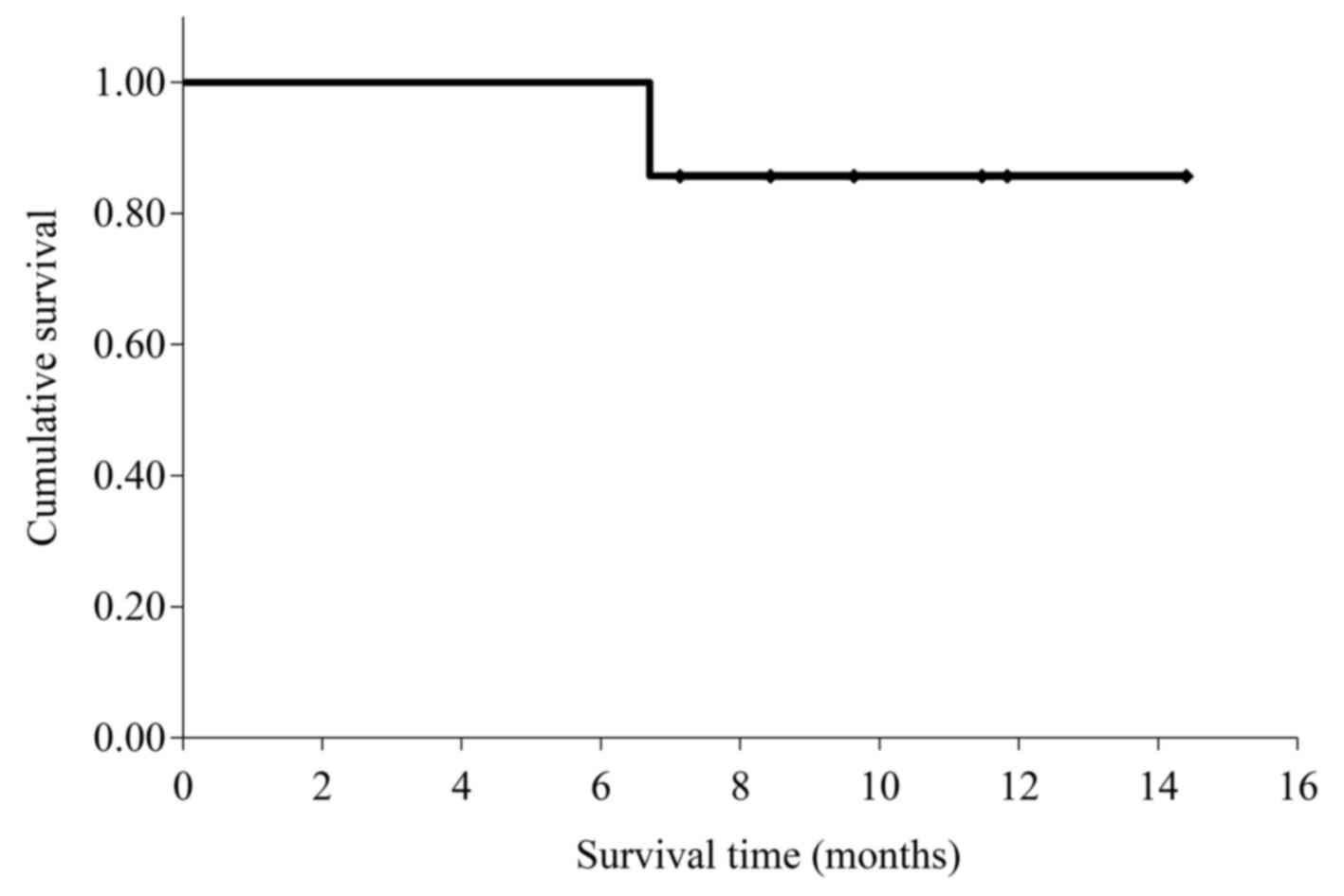
|
1
|
Statistics and Information Department:
Vital statistics 2014. Tokyo: Ministry of Health, Labor and
Welfare. 2014.http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei14/index.htmlDecember
20–2016
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faris JE, Blaszkowsky LS, McDermott S,
Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen
JN, Dias LE, et al: FOLFIRINOX in locally advanced pancreatic
cancer: The massachusetts general hospital cancer center
experience. Oncologist. 18:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Philip PA: Locally advanced pancreatic
cancer: Where should we go from here? J Clin Oncol. 29:4066–4068.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network, .
NCCN clinical practic guideline in oncology: Pancreatic
adenocarcinoma. Fort Washington, PA: National Comprehensive Cancer
Network, Version; 2. pp. 222015
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakayama N, Ishido K, Chin K, Nishimura K,
Azuma M, Matsusaka S, Inokuchi Y, Tanabe S, Kumekawa Y and Koizumi
W: A phase I study of S-1 in combination with nab-paclitaxel in
patients with unresectable or recurrent gastric cancer. Gastric
Cancer. 20:350–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rizvi NA, Riely GJ, Azzoli CG, Miller VA,
Ng KK, Fiore J, Chia G, Brower M, Heelan R, Hawkins MJ and Kris MG:
Phase I/II trial of weekly intravenous 130-nm albumin-bound
paclitaxel as initial chemotherapy in patients with stage IV
non-small-cell lung cancer. J Clin Oncol. 26:639–643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alvarez R, Musteanu M, Garcia-Garcia E,
Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A,
Rodriguez-Pascual J, et al: Stromal disrupting effects of
nab-paclitaxel in pancreatic cancer. Br J Cancer. 109:926–933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frese KK, Neesse A, Cook N, Bapiro TE,
Lolkema MP, Jodrell DI and Tuveson DA: nab-Paclitaxel potentiates
gemcitabine activity by reducing cytidine deaminase levels in a
mouse model of pancreatic cancer. Cancer Discov. 2:260–269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T,
Omuro Y, Nakajima TE and Furuse J: Phase I/II study of
nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese
patients with metastatic pancreatic cancer. Cancer Chemother
Pharmacol. 77:595–603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Habermehl D, Kessel K, Welzel T, Hof H,
Abdollahi A, Bergmann F, Rieken S, Weitz J, Werner J, Schirmacher
P, et al: Neoadjuvant chemoradiation with Gemcitabine for locally
advanced pancreatic cancer. Radiat Oncol. 7:282012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nanda RH, El-Rayes B, Maithel SK and
Landry J: Neoadjuvant modified FOLFIRINOX and chemoradiation
therapy for locally advanced pancreatic cancer improves
resectability. J Surg Oncol. 111:1028–1034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blazer M, Wu C, Goldberg RM, Phillips G,
Schmidt C, Muscarella P, Wuthrick E, Williams TM, Reardon J,
Ellison EC, et al: Neoadjuvant modified (m) FOLFIRINOX for locally
advanced unresectable (LAPC) and borderline resectable (BRPC)
adenocarcinoma of the pancreas. Ann Surg Oncol. 22:1153–1159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Sun XJ, Jiang TH and Mao AW:
Combined radiochemotherapy in patients with locally advanced
pancreatic cancer: A meta-analysis. World J Gastroenterol.
19:7461–7471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chauffert B, Mornex F, Bonnetain F,
Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L,
Azzedine A, et al: Phase III trial comparing intensive induction
chemoradiotherapy (60 Gy, infusional 5-FU and intermittent
cisplatin) followed by maintenance gemcitabine with gemcitabine
alone for locally advanced unresectable pancreatic cancer.
Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol.
19:1592–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loehrer PJ Sr, Feng Y, Cardenes H, Wagner
L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR
and Benson AB III: Gemcitabine alone versus gemcitabine plus
radiotherapy in patients with locally advanced pancreatic cancer:
An Eastern Cooperative Oncology Group trial. J Clin Oncol.
29:4105–4112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim R and Salf MW: Is there an optimal
neoadjuvant therapy for locally advanced pancreatic cancer? JOP.
8:279–288. 2007.PubMed/NCBI
|
|
22
|
Conroy T, Paillot B, François E, Bugat R,
Jacob JH, Stein U, Nasca S, Metges JP, Rixe O, Michel P, et al:
Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil
in advanced pancreatic cancer-a Groupe Tumeurs Digestives of the
Federation Nationale des Centres de Lutte Contre le Cancer study. J
Clin Oncol. 23:1228–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gourgou-Bourgade S, Bascoul-Mollevi C,
Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A,
Raoul JL, Boige V, et al: Impact of FOLFIRINOX compared with
gemcitabine on quality of life in patients with metastatic
pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized
trial. J Clin Oncol. 31:23–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese Society of
Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013. View Article : Google Scholar : PubMed/NCBI
|