Introduction
Pancreatic cancer (PC) has an extremely high degree
of malignancy, and the number of affected patients is increasing
annually (1). Currently, PC is the
fourth highest cause of cancer-related mortality among US adults
and the fifth and fourth leading cause of death among Japanese men
and women, respectively (1,2). Resection is the only form of treatment
associated with a complete cure, but only 10–20% of cases are
resectable and the majority of the cases involve metastatic or
locally advanced unresectable PC (LURPC) (3). LURPC accounts for 30–35% of all PC
cases (4), and its treatment options
include chemotherapy or chemoradiotherapy (CRT). However, its
prognosis remains poor, and there is an urgent need for novel
treatment methods (5).
The recently conducted MPACT trial verified that a
combination therapy comprising gemcitabine (GEM) and nab-paclitaxel
(nab-PTX) significantly prolonged the survival of patients with
metastatic PC compared with a therapy comprising GEM alone
(6). The results of that study
indicated that this regimen may become a new therapeutic option.
However, the responses to this form of treatment for LURPC have not
yet been fully elucidated. The aim of the present study was to
investigate the safety and efficacy of the combination of GEM and
nab-PTX for the treatment of patients with LURPC.
Patients and methods
Patients
A total of 7 patients with LURPC were treated with a
combination regimen comprising GEM and nab-PTX at the Department of
Gastroenterological Surgery of the Hirosaki University Hospital
(Hirosaki, Japan) between January and September, 2015. The
chemotherapy regimen included the administration of GEM (1,000
mg/m2 on days 1, 8 and 15, every 4 weeks) and nab-PTX (125 mg/m2 on
days 1, 8 and 15, every 4 weeks). Resectability was determined
according to the National Comprehensive Cancer Network (NCCN)
guidelines, version 2. 2015 (7). The
number of chemotherapy courses, rate of change in tumor diameter,
rate of change of serum carbohydrate CA19-9 values, incidence of
grade ≥3 adverse events, therapeutic effects and survival time were
investigated in all the cases. In an effort to determine specific
response to treatment, the Response Evaluation Criteria In Solid
Tumors guidelines, version 1.1 (8)
were used for analyzing patient computed tomography images.
Survival time was defined as the period from the date of treatment
initiation to the date on which the outcome was achieved. Overall
patient survival was analyzed using the Kaplan-Meier method. All
analyses were performed using the IBM SPSS® Statistics
version 24.0 for Windows (IBM, Armonk, NY, USA). This study was
approved by the Human Research Ethics Committee of Hirosaki
University (no. 2016-1038) and informed consent was provided by all
the participants.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median patient age was
71 years (range, 59–78 years), and the subjects comprised 1 male
and 6 female LURPC patients. The tumor was located at the body of
the pancreas in 1 and in the head of the pancreas in 6 subjects.
The criteria for diagnosing a case as unresectable were as follows:
Solid tumor in contact with the celiac axis >180° in 3 subjects,
solid tumor in contact with the common hepatic artery with
extension to the hepatic artery bifurcation in 2 subjects, an
unreconstructible portal vein due to tumor involvement in 1
subject, and an unreconstructible portal vein/superior mesenteric
vein involvement in 1 subject. The patients underwent a median of 4
(range, 2–7) courses of chemotherapy. No patients achieved a
complete response, 5 achieved a partial response, 2 had stable
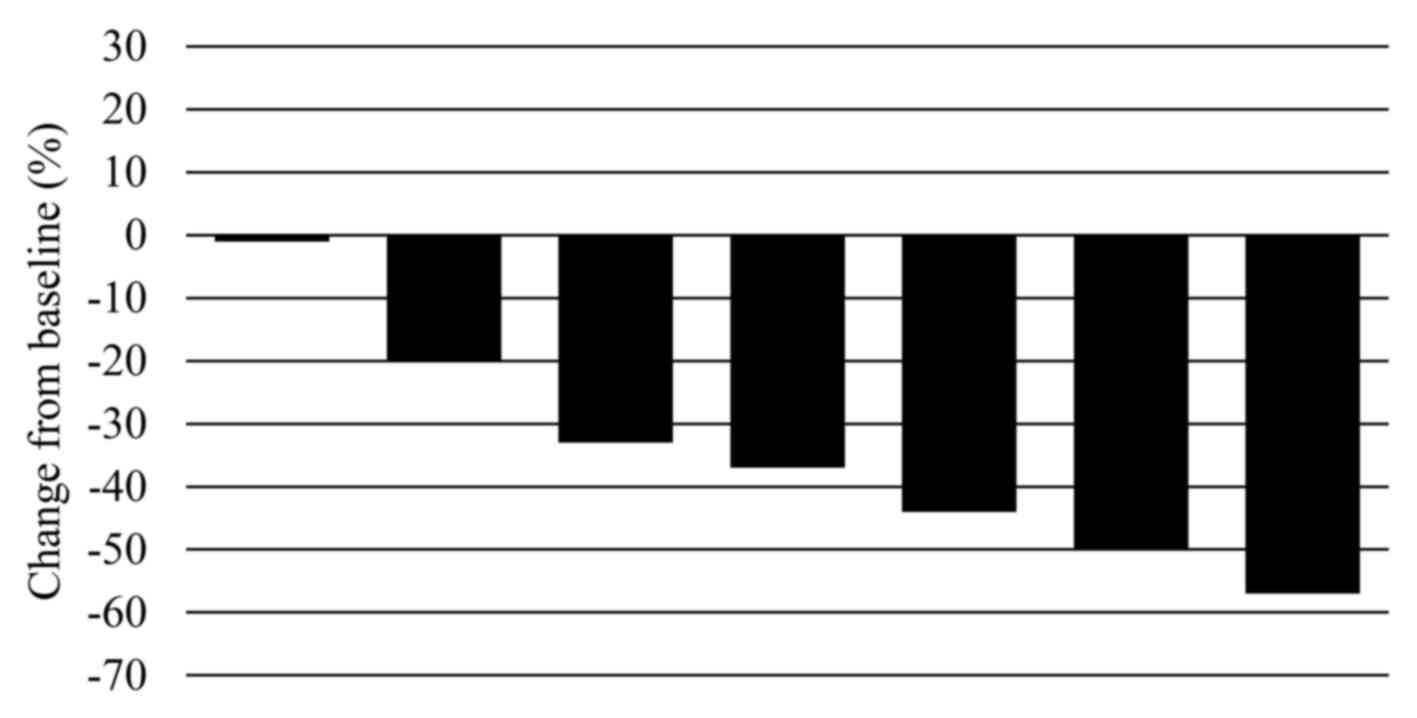
disease, and none had progressive disease. The response rate was
71% (Fig. 1).
 | Table I.Characteristics of patients with
locally advanced unresectable pancreatic cancer. |
Table I.
Characteristics of patients with
locally advanced unresectable pancreatic cancer.
| No. | Age, years | Gender | Location | Factor determining
unresectability | Number of
chemotherapy cycles | RECIST |
|---|
| 1 | 74 | F | Head | Solid tumor contact
with CA >180° | 7 | PR |
| 2 | 71 | F | Head | Unreconstructible PV
due to tumor involvement | 5 | SD |
| 3 | 66 | M | Head | Solid tumor contact
with CHA with extension to hepatic artery bifurcation | 2 | PR |
| 4 | 59 | F | Head | Solid tumor contact
with CA >180° | 4 | PR |
| 5 | 65 | F | Body | Solid tumor contact
with CA >180° | 2 | SD |
| 6 | 78 | F | Head | Unreconstructible
PV/SMV due to tumor involvement | 4 | PR |
| 7 | 77 | F | Head | Solid tumor contact
with CHA with extension to the hepatic artery bifurcation | 2 | PR |
Therapeutic effects
The therapeutic effects of the combination treatment
are summarized in Table II. The
median tumor diameter significantly decreased from 35 mm (range,
24–60 mm) prior to treatment to 22 mm (range, 20–35 mm) following
treatment (P=0.008); the median rate of tumor shrinkage was 37%
(range, 0–57%). The mean serum CA19-9 values significantly
decreased from 767 U/ml (14–1977 U/ml) prior to treatment to 35
U/ml (range, 14–123 U/ml) following treatment (P=0.038); the median
rate of reduction was 92% (range, 47–98%). When the 5 subjects who
achieved a partial response were examined, 2 were diagnosed with
resectable cancer and both underwent radical R0 resection.
Peritoneal dissemination was observed in 1 patient 2 months after
surgery; despite initiating treatment, the patient succumbed to the
disease 1 month later. Another patient was found to be
recurrence-free during the 3-month follow-up, and S-1 was
administered as ongoing postoperative adjuvant chemotherapy. The
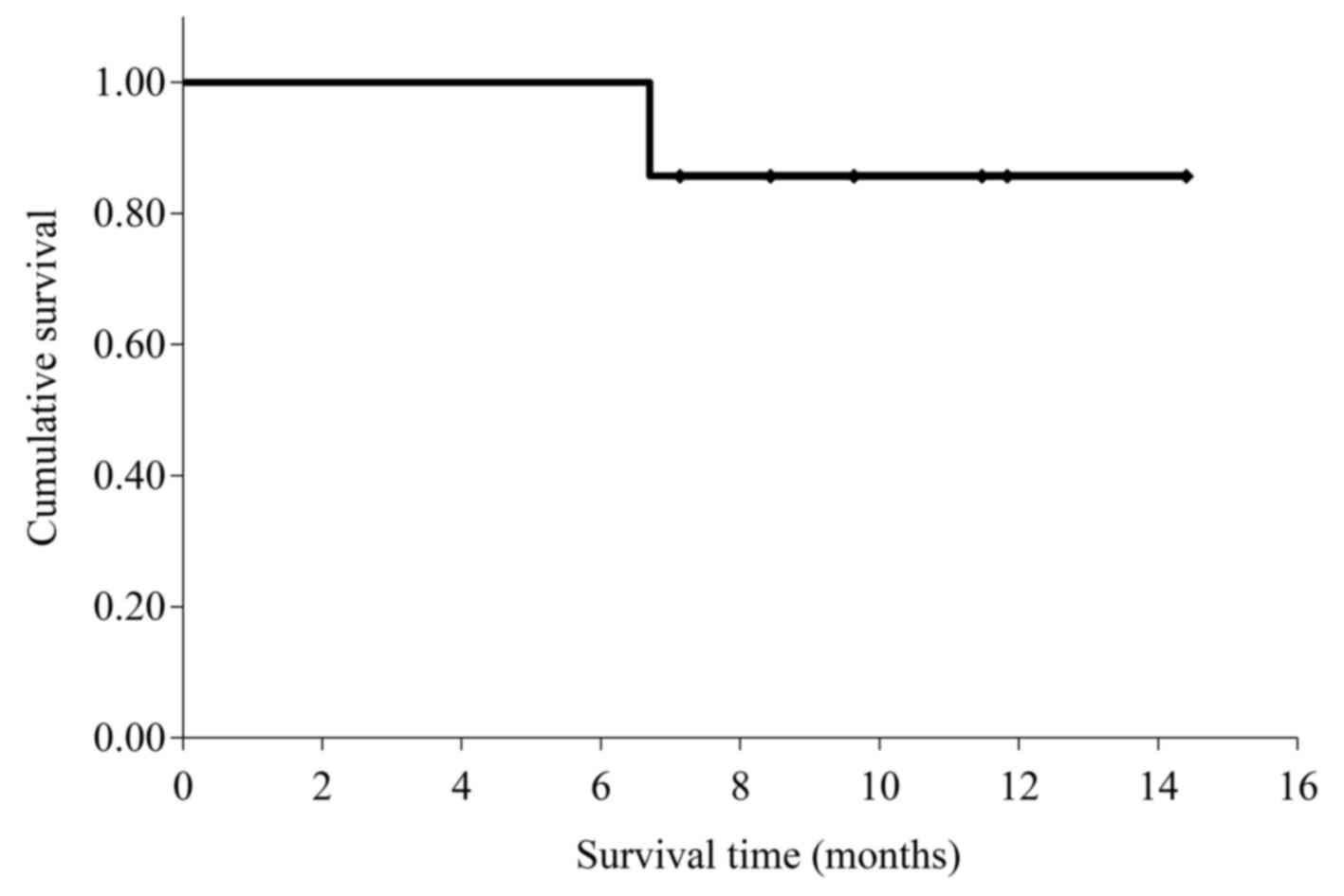
mean survival time for the 7 patients was 13.3 months [95%
confidence interval (CI): 11.3–15.3; Fig. 2].
 | Table II.Evaluation of the chemotherapy for
locally advanced unresectable pancreatic cancer. |
Table II.
Evaluation of the chemotherapy for
locally advanced unresectable pancreatic cancer.
| Variables | Values |
|---|
| Tumor diameter, mm
[median (range)] |
|
| Pre
CTx | 35 (24–60) |
| Post
CTx | 20 (15–35) |
| Reduction
rate (%) | 37 (0–57) |
| CA19-9, U/ml |
|
| Pre
CTx | 247 (14–1,977) |
| Post
CTx | 35 (4–123) |
| Reduction
rate (%) | 92 (47–98) |
| RR, % | 71 (CR, 0; PR,
5; |
|
| SD, 2; PD, 0) |
| Grade >3 adverse
events, n (%) |
|
|
Neutropenia | 3 (43) |
|
Cellulitis | 1 (14) |
| CS | 2 (29) |
| R0
resection, n/total (%) | 2/2
(100) |
Adverse events classified as grade ≥3 occurred in 4
patients (57%): 3 patients (43%) suffered from neutropenia and 1
(14%) developed bilateral cellulitis of the lower extremities. None
of these patients experienced an increase in disease severity, and
all were able to continue treatment following temporary withdrawal
or dosage reduction.
Discussion
The nab-PTX formulation comprises human
albumin-bound PTX nanoparticles and has been reported to be useful
for treating breast, gastric and non-small-cell lung cancer
(9–11). When combined with GEM for the
treatment of PC, nab-PTX acts by decreasing the interstitial
components of PC and increases the microvasculature within the
tumor. By doing so, the intratumoral concentration of GEM is
increased by ~3-fold (12). In
addition, nab-PTX decreases the plasma concentration of cytidine
deaminase, one of the enzymes that metabolizes GEM, and has also
been reported to stabilize the active form of an intratumoral GEM
metabolite, gemcitabine triphosphate (13). Therefore, the direct anticancer and
synergistic effects of nab-PTX used in combination with GEM enable
powerful tumor-reducing effects. During the MPACT trial, the
combination therapy comprising GEM and nab-PTX was more efficient
in prolonging patient survival compared with GEM alone for the
treatment of metastatic PC (8.5 months; hazard ratio=0.72)
(6) and has also been shown to be
useful in phase II/III clinical trials in Japan [median
progression-free survival: 6.5 months (95% CI: 5.1–8.3); median
overall survival: 13.5 months (95% CI: 10.6-not reached)] (14). However, these reports investigated
patients with metastatic PC, and this regimen remains to be
investigated using patients with LURPC as subjects. Accordingly, in
the present study, the safety and efficacy of a combination therapy
comprising GEM and nab-PTX for the treatment of patients with LURPC
were investigated. The median tumor regression rate was 37% (range,
0–57%), and powerful tumor-reducing effects were achieved.
Furthermore, the median survival time was 13.3 months, which is
considered to be a favorable outcome.
The NCCN guidelines recommend chemotherapy or CRT
for the treatment of LURPC when the performance status of the
patients is favorable. To date, chemotherapy using GEM,
5-fluorouracil (5-FU)/leucovorin (LV) or capecitabine, or combined
treatment comprising radiation in the form of CRT, are recommended.
In recent years, 5-FU/LV plus oxaliplatin and irinotecan
(FOLFIRINOX regimen) have been reported to be useful and
recommended as a new treatment regimen. Recent reports regarding
the treatment of LURPC are presented in Table III. Habermehl et al
investigated CRT using GEM for the treatment of LURPC and reported
a response rate (RR) of 9%, with a median survival time of 12.3
months (15). In addition, Faris
et al evaluated the FOLFIRINOX regimen for the treatment of
LURPC and found an RR of 27%, as well as favorable treatment
outcomes (median disease-free survival: 11.3 months); however, the
median survival time was not reported (3). Moreover, Nanda and Blazer et al
also reported favorable outcomes when they compared FOLFIRINOX with
CRT for the treatment of LURPC, with median survival times of 18.6
and 12.2 months, respectively (16,17).
During the present study, the RR was 71%, and although the median
survival time was not calculated, the mean survival time was 13.3
months. These findings suggest that the combination therapy
comprising GEM and nab-PTX is effective for treating patients with
LURPC.
 | Table III.Previous studies on chemotherapy for
locally advanced unresectable pancreatic cancer. |
Table III.
Previous studies on chemotherapy for
locally advanced unresectable pancreatic cancer.
| No. | First author | Year | Patient no. | Regimen | RR (%) | CS (%)/R0 resection
(%) | MST (months) | (Refs.) |
|---|
| 1 | Habermehl | 2012 | 198 | GEM/radiation | 9 | 26/39 | 12.3 | (15) |
| 2 | Faris | 2013 | 22 | FOLFIRINOX | 27.3 | 22/60 | NA | (3) |
| 3 | Nanda | 2015 | 15 | FOLFIRINOX | NA | 13/NA | 18.6 | (16) |
| 4 | Blazer | 2015 | 25 | FOLFIRINOX | 9 | 44/91 | 21.2 | (17) |
| 5 | Present study | 2015 | 7 | GEM/nab-PTX | 71 | 29/100 | (mean) 13.3 |
|
There are reports of severe adverse events during
chemotherapy and CRT treatment for LURPC. Chen et al
performed a meta-analysis comparing CRT, chemotherapy alone and
radiotherapy alone for LURPC, and reported that grade ≥3 adverse
events were common (18). In
addition, a phase III trial (FFCD-SFRO trial) compared CRT using
5-FU and cisplatin to chemotherapy using GEM alone. They found
that, compared with the chemotherapy group, the CRT group was
associated with a significantly higher incidence of hematotoxic and
non-hematotoxic adverse events, such as infection, vomiting and
diarrhea (66 vs. 40%, respectively; P=0.008) (19). The same trial also observed a
significant prolongation of median survival time in the
chemotherapy group (13.0 vs. 8.6 months; P=0.03), suggesting that
chemotherapy is useful for treating LURPC (19). Moreover, another phase III trial
(ECOG-4201 trial) compared CRT using GEM alone to chemotherapy
using GEM alone. There were no significant differences in the
incidence of grade ≥3 adverse events (79 vs. 77%, respectively;
P=0.1) and the median survival time was reported to be
significantly longer in the CRT group (11.1 vs. 9.2 months,
respectively; P=0.017) (20). There
continues to be a multitude of such contradictory reports and
discussion regarding the usefulness of CRT for the treatment of
LURPC (21). In terms of using
FOLFIRINOX to treat LURPC and metastatic PC, although some reports
have described a high response to treatment, with RRs 9–27%, other
studies have reported the presence of grade ≥3 adverse events,
including neutropenia (46%), loss of appetite (23.6%), vomiting
(14.5%), diarrhea (13%) and peripheral neuropathy (9%) (22–24).
Furthermore, reports on metastatic PC have investigated the
toxicity of the combination therapy comprising GEM and nab-PTX and
found grade ≥3 adverse events such as neutropenia (38%), loss of
appetite (17%), peripheral neuropathy (17%) and diarrhea (6%)
(6). Although the frequency of
peripheral neuropathy is marginally higher with the combination
therapy, the overall incidence of adverse events is lower compared
with that of FOLFIRINOX. In addition, 8% of the subjects were
unable to continue the study due to peripheral neuropathy, and
approximately half of them improved to grade ≤1 following dosage
reduction (6). During the present
study, grade 3 adverse events were observed in 4 of the 7 subjects
(neutropenia in 3 and cellulitis in 1 subjects); however, these
events were mild and did not increase in severity during the
clinical course in any of the 4 subjects. All the patients were
able to continue the study on an outpatient basis following
temporary treatment withdrawal or dosage reduction. Combination
therapy comprising GEM and nab-PTX is associated with a lower
incidence of adverse events compared with CRT and FOLFIRINOX.
Furthermore, as a high quality of life was maintained during our
treatment, we consider this treatment to be a highly favorable
option.
If tumors become resectable following a successful
primary course of treatment, conversion surgery (CS) may prolong
survival. A study by Satoi et al compared a group that
underwent CS following primary treatment for LURPC to a group that
did not, and found that the median survival was significantly
prolonged in the CS group (CS vs. non-CS: 39.7 vs. 20.8 months,
respectively; P<0.001) (25).
Similarly, Habermehl et al reported that median survival was
significantly prolonged in the CS group (CS vs. non-CS: 11.9 vs.
14.4 months, respectively; P=0.004) (15); they also reported an even longer
patient survival if the surgical margins were found to be clear (R0
vs. R1 vs. R2: 22.1 vs. 15.6 vs. 10.3 months, respectively)
(15). Accordingly, it is extremely
important to develop treatment methods that incorporate CS, which
is a highly successful treatment strategy for LURPC. As shown in
Table III, when Habermehl et
al investigated CRT as a treatment for LURPC, CS was performed
in 26% of the cases and the rate of clear surgical margins was 39%
(15). When Faris et al
studied the FOLFIRINOX regimen for treating LURPC, they reported
that CS was performed in 22% of the cases and the rate of clear
surgical margins was 60%, indicating more favorable treatment
outcomes compared with those observed using CRT (3). In the present study, CS was performed
in 29% of the cases and the rate of clear surgical margins was
100%. Thus, combination therapy comprising GEM and nab-PTX is a
potential treatment option associated with a higher CS rate, a
higher rate of clear surgical margins, and a longer survival time
compared with those observed with CRT or FOLFIRINOX.
Based on the abovementioned findings, this
combination therapy is associated with a low incidence of adverse
events, maintains the quality of life and markedly prolongs
survival for patients with LURPC; thus, we consider it to be a
useful form of treatment for such cases. CS is common during LURPC
and should be considered as an important and novel treatment
strategy. However, the present study was associated with the
following limitations: i) The patient sample was very small, ii)
the study design was retrospective and iii) the period of
observation was brief. Thus, the usefulness of the combination
therapy comprising GEM and nab-PTX for patients with LURPC requires
verification by future prospective studies.
Acknowledgements
The authors would like to thank Crimson Interactive
Pvt. Ltd. (Ulatus)-www.ulatus.jp
for their assistance with the manuscript translation and
editing.
References
|
1
|
Statistics and Information Department:
Vital statistics 2014. Tokyo: Ministry of Health, Labor and
Welfare. 2014.http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei14/index.htmlDecember
20–2016
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faris JE, Blaszkowsky LS, McDermott S,
Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen
JN, Dias LE, et al: FOLFIRINOX in locally advanced pancreatic
cancer: The massachusetts general hospital cancer center
experience. Oncologist. 18:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Philip PA: Locally advanced pancreatic
cancer: Where should we go from here? J Clin Oncol. 29:4066–4068.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network, .
NCCN clinical practic guideline in oncology: Pancreatic
adenocarcinoma. Fort Washington, PA: National Comprehensive Cancer
Network, Version; 2. pp. 222015
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakayama N, Ishido K, Chin K, Nishimura K,
Azuma M, Matsusaka S, Inokuchi Y, Tanabe S, Kumekawa Y and Koizumi
W: A phase I study of S-1 in combination with nab-paclitaxel in
patients with unresectable or recurrent gastric cancer. Gastric
Cancer. 20:350–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rizvi NA, Riely GJ, Azzoli CG, Miller VA,
Ng KK, Fiore J, Chia G, Brower M, Heelan R, Hawkins MJ and Kris MG:
Phase I/II trial of weekly intravenous 130-nm albumin-bound
paclitaxel as initial chemotherapy in patients with stage IV
non-small-cell lung cancer. J Clin Oncol. 26:639–643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alvarez R, Musteanu M, Garcia-Garcia E,
Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A,
Rodriguez-Pascual J, et al: Stromal disrupting effects of
nab-paclitaxel in pancreatic cancer. Br J Cancer. 109:926–933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frese KK, Neesse A, Cook N, Bapiro TE,
Lolkema MP, Jodrell DI and Tuveson DA: nab-Paclitaxel potentiates
gemcitabine activity by reducing cytidine deaminase levels in a
mouse model of pancreatic cancer. Cancer Discov. 2:260–269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T,
Omuro Y, Nakajima TE and Furuse J: Phase I/II study of
nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese
patients with metastatic pancreatic cancer. Cancer Chemother
Pharmacol. 77:595–603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Habermehl D, Kessel K, Welzel T, Hof H,
Abdollahi A, Bergmann F, Rieken S, Weitz J, Werner J, Schirmacher
P, et al: Neoadjuvant chemoradiation with Gemcitabine for locally
advanced pancreatic cancer. Radiat Oncol. 7:282012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nanda RH, El-Rayes B, Maithel SK and
Landry J: Neoadjuvant modified FOLFIRINOX and chemoradiation
therapy for locally advanced pancreatic cancer improves
resectability. J Surg Oncol. 111:1028–1034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blazer M, Wu C, Goldberg RM, Phillips G,
Schmidt C, Muscarella P, Wuthrick E, Williams TM, Reardon J,
Ellison EC, et al: Neoadjuvant modified (m) FOLFIRINOX for locally
advanced unresectable (LAPC) and borderline resectable (BRPC)
adenocarcinoma of the pancreas. Ann Surg Oncol. 22:1153–1159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Sun XJ, Jiang TH and Mao AW:
Combined radiochemotherapy in patients with locally advanced
pancreatic cancer: A meta-analysis. World J Gastroenterol.
19:7461–7471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chauffert B, Mornex F, Bonnetain F,
Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L,
Azzedine A, et al: Phase III trial comparing intensive induction
chemoradiotherapy (60 Gy, infusional 5-FU and intermittent
cisplatin) followed by maintenance gemcitabine with gemcitabine
alone for locally advanced unresectable pancreatic cancer.
Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol.
19:1592–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loehrer PJ Sr, Feng Y, Cardenes H, Wagner
L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR
and Benson AB III: Gemcitabine alone versus gemcitabine plus
radiotherapy in patients with locally advanced pancreatic cancer:
An Eastern Cooperative Oncology Group trial. J Clin Oncol.
29:4105–4112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim R and Salf MW: Is there an optimal
neoadjuvant therapy for locally advanced pancreatic cancer? JOP.
8:279–288. 2007.PubMed/NCBI
|
|
22
|
Conroy T, Paillot B, François E, Bugat R,
Jacob JH, Stein U, Nasca S, Metges JP, Rixe O, Michel P, et al:
Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil
in advanced pancreatic cancer-a Groupe Tumeurs Digestives of the
Federation Nationale des Centres de Lutte Contre le Cancer study. J
Clin Oncol. 23:1228–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gourgou-Bourgade S, Bascoul-Mollevi C,
Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A,
Raoul JL, Boige V, et al: Impact of FOLFIRINOX compared with
gemcitabine on quality of life in patients with metastatic
pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized
trial. J Clin Oncol. 31:23–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese Society of
Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013. View Article : Google Scholar : PubMed/NCBI
|