Introduction
Cancer anemia, also known as cancer-associated
malignant anemia, is a common clinical symptom of cancer, with a
rate of incidence approaching 50% of cancer cases (1,2). There
are several causes of cancer-associated malignant anemia, such as
bleeding, hemolysis, lack of nutrition, bone marrow necrosis and
fibrosis caused by tumor cell infiltration, direct inhibition of
red blood cells caused by radiotherapy and chemotherapy, relative
insufficiency of erythropoietin (EPO) secretion (3), hematopoietic inhibition and influence
of iron metabolism and reduced reactivity of EPO and cytokines from
cancerous human bone marrow (4–6).
Malignant tumor anemia is a common complication in various
malignant tumors, in which red blood cell depletion leads to
inadequate tissue oxygenation, reducing the sensitivity of tumors
to radiation and chemotherapy, thereby lowering the patient's
quality of life, decreasing survival time and affecting overall
prognosis (4,7,8). At 3
years post-cancer diagnosis, mortality among patients with anemia
is twice as high as that among those without anemia (9–12).
Clinically speaking, quality of life and adverse events are
important predictors in patients with cancer (8).
At present, there are four methods for treating
cancer-associated anemia: Blood transfusion (primarily red blood
cell suspension), iron supplements (reduced iron deposits caused by
EPO treatment), change of treatment scheme and administration of
stimulating factor (4,13). Blood transfusion is not without
risks, particularly for patients with cancer. Blood transfusions
may decrease the survival rate, and tumor recurrence may occur
following blood transfusion, which may lead to poor prognosis. The
effect of iron supplements alone is often slow, with low efficacy
(2,5). EPO is a type of high-purity active
glycoprotein produced using genetically modified technology, and it
specifically stimulates bone marrow hematopoietic cells (13–15).
Although a large body of research has demonstrated that the EPO is
able to improve the symptoms of cancer-associated malignant anemia,
this conclusion remains controversial. In order to assess the
efficacy of EPO in this setting, the current study conducted a
meta-analysis of the available research.
Materials and methods
Search strategy
A series of electronic searches of PubMed, EMBASE,
the Cochrane Library and ClinicalTrails.gov up to8 February 2016 for eligible
randomized or parallel-group design clinical trials investigating
EPO for the treatment of cancer-associated malignant anemia was
performed using the following Medical Subject Headings and text
words: ‘neoplasm*’, ‘tumor*’, ‘cancer*’, ‘erythropoietin’ and
‘anaemia*’.
Inclusion criteria
All studies were selected in accordance with
following inclusion criteria: (1)
All studies were randomized or parallel-group design clinical
trials; (2) eligible studies
included patients with cancer-associated malignant anemia patients
older than 18 years; (3) the entire
study population was patients diagnosed with cancer with malignant
solid tumors confirmed by histology or cytology; (4) the hemoglobin (HB) levels of the
patients were >8.5 and <13.5 g/dl; (5) eligible studies included at least one of
the following outcomes: Change in HB, change in hematocrit (HCT),
the ratio of transfusion and transfusion requirements during the
treatment.
Exclusion criteria
Studies were excluded in accordance with following
criteria: (1) The study reported
that the patients had received radiation, chemotherapy, or surgery
prior to or during receiving EPO; (2) anemia was not caused by cancer;
(3) the study included primary
hematologic disease, seizure disorder, uncontrolled hypertension,
recent history of thromboembolic disease (within 1 year), other
clinically significant systemic disease, an active infectious
process, pregnancy, ongoing blood loss, scheduled autologous blood
donation or blood transfusion within the previous 30 days;
(4) duplicate published research was
excluded; (5) the data could not be
extracted or obtained through contact with the original author.
Data extraction
The relevant information including study design,
patients' characteristics, the criteria of tumor staging, tumor
staging, the initial HB levels, interventions, controls and
outcomes (change in HB, change in HCT, the ratio of transfusion or
transfusion requirements) were independently extracted and entered
it into a database by two investigators. When relevant research
information was missing, particularly design or outcomes, the
original authors were contacted for clarifications. Then,
intention-to-treat (ITT) datasets were used for all outcomes
whenever available. Disagreements between the two authors on data
extraction were resolved by discussion. If the dispute persisted,
other senior investigators were consulted to reach a consensus.
Statistical analysis
For the outcomes based on dichotomous data (16,17),
relative risk (RR) as was utilized as an effect estimator, while
for continuous outcomes mean difference (MD) was used (17,18).
Subsequently, 95% confidence intervals (CIs) and P-values were
calculated using RevMan 5.3 software (Cochrane Collaboration,
London, UK) in all outcomes. A statistical test for heterogeneity
was performed and an I2>40 was adopted as evidence for
heterogeneity according to the Cochrane handbook (19). If the data were heterogeneous under
the fixed effects model, a random effects model was utilized
(20). Through observing asymmetry
of funnel plots, publication bias was evaluated qualitatively
(21). In a funnel plot, larger
studies that provide a more precise estimate of an intervention's
effect form the spout of the funnel, whereas smaller studies with
less precision form the cone end of the funnel. Asymmetry in the
funnel plot indicates potential publication bias. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Study selection and data
collection
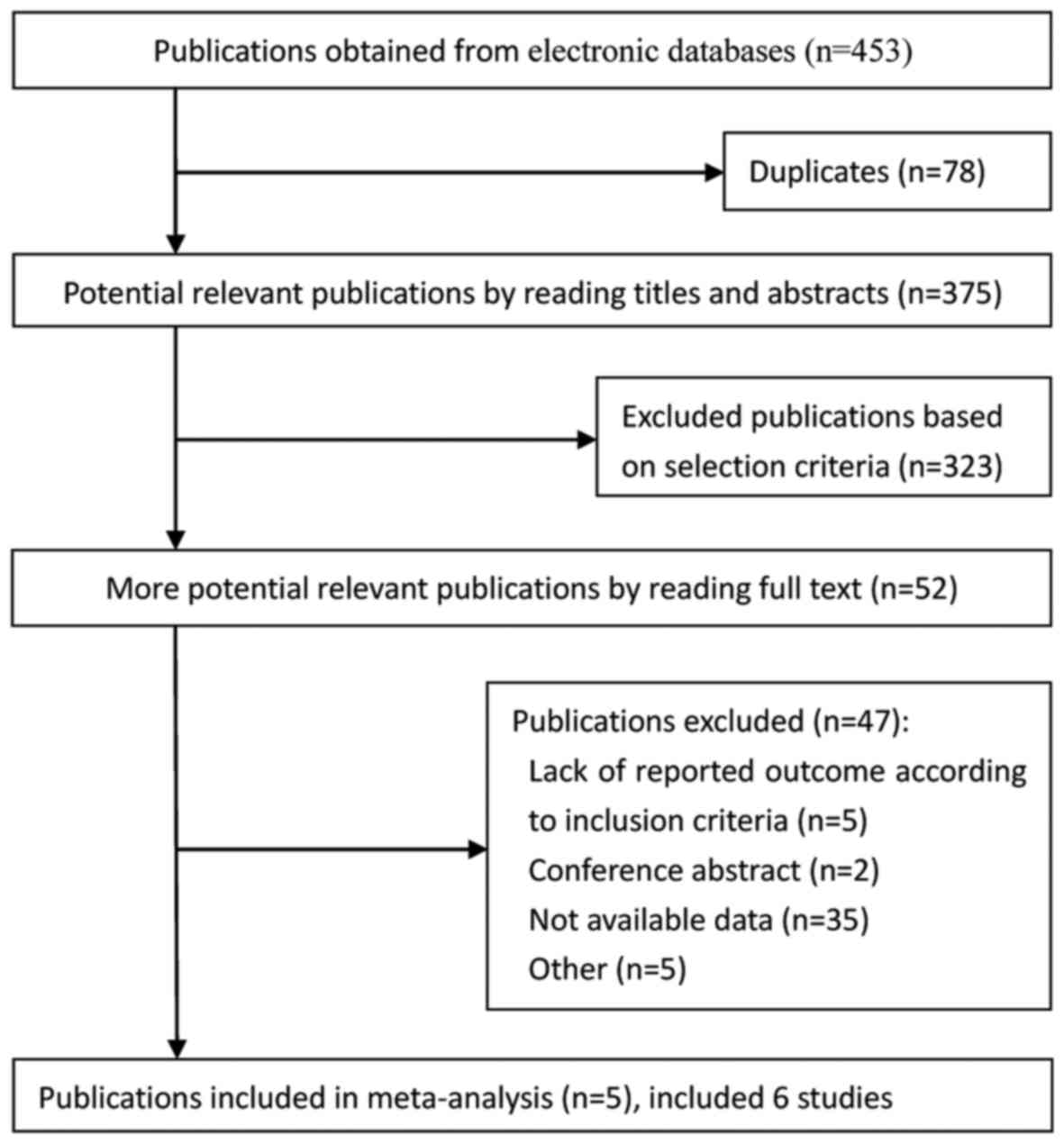
In total, 453 publications were obtained from
electronic databases (Fig. 1). Based
on the selection criteria, quantitative data was obtained for the
current meta-analysis by reading all titles, abstracts and
performing full text evaluations. Finally, 6 eligible studies from
5 articles were entered into the meta-analysis.
Study characteristics
The search obtained 6 eligible studies from 5
articles (22–26), which enrolled a total of 595
patients. The change in HB was reported in 2 studies (23,26), the
transfusion requirements were noted from 3 studies (23,25), the
ratio of transfusion was obtained from a total of 233 of 495
patients from 5 studies (22–26) and
the change in HCT was reported in 2 studies (23,26).
Table I presents the clinical
characteristics from all studies.
 | Table I.Baseline information of the studies
included. |
Table I.
Baseline information of the studies
included.
|
|
|
|
|
|
|
|
|
|
| Usage, Dosage,
Frequency |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author | Year | Country | Sample (F,
I/C) | Age (I/C) | Criteria of tumor
staging | Tumor staging
(I/C) | Tumor location
(I/C) | HB levels (I/C,
g/dl) | EPO type | Intervention | Control | ITT/PP | (Refs.) |
|---|
| Kettelhack | 1998 | Germany | 48 (27)/54
(32) | 71 (53–87)/67
(37–91) | NA | NA | CC | 11.5
(10.5–12.5)/12.0 (11.0–13.1) | EPO beta | EPO 20,000
IU/day | Placebo | ITT | (22) |
| Scott | 2002 | USA | 29 (13)/29
(11) |
67.6±11.0/61.8±10.8 | AJCC 1997 | 1/1, II5/5, III
6/2, IV 17/21 | PC, BM | 10-13.5 | EPO alfa | EPO 600 IU/kg/day,
150 mg FeSO4, BID | 300 mg/day by
FeSO4 | PP | (23) |
| Kosmadakis | 2003 | Greece | 31 (16)/32
(13) |
67.1±2.1/66.4±2 | NA | NA | Stomach 18%/19%,
Colon 53%/52%, Rectum 29%/29% | 8.5–13 | EPO alfa | EPO, 100 mg/day
iron | Placebo, 100 mg/day
iron | ITT | (24) |
|
Christodoulakis | 2005 | Greece | 67 (37)/68
(40) | 71 (36–92)/70
(44–89) | Dukes | A and B 28/26, C
and D 31/32, unknown 8/10 | CC | 9–12 | EPO alfa | EPO 300 IU/kg/day,
200 mg/day iron | 200 mg/day
iron | ITT | (25) |
|
Christodoulakis | 2005 | Greece | 69 (38)/68
(40) | 72.5 (43–91)/70
(44–89) | Dukes | A and B 35/26, C
and D 29/32, unknown 5/10 | CC | 9–12 | EPO alfa | EPO 150 IU/kg/day,
200 mg/day iron | 200 mg/day
iron | ITT | (25) |
| Mystakidou | 2005 | Greece | 50 (28)/50
(33) | 63 (32–79)/64.5
(41–79) | NA | NA | PC 36%/16%, CC
16%/16%, GS 32%/22%, C: LC 18% |
10.15±0.15/9.87±0.50 | EPO alfa | EPO 40000 IU
QD/BID, 200 mg/day iron | 200 mg/day
iron | ITT | (26) |
Change in HB
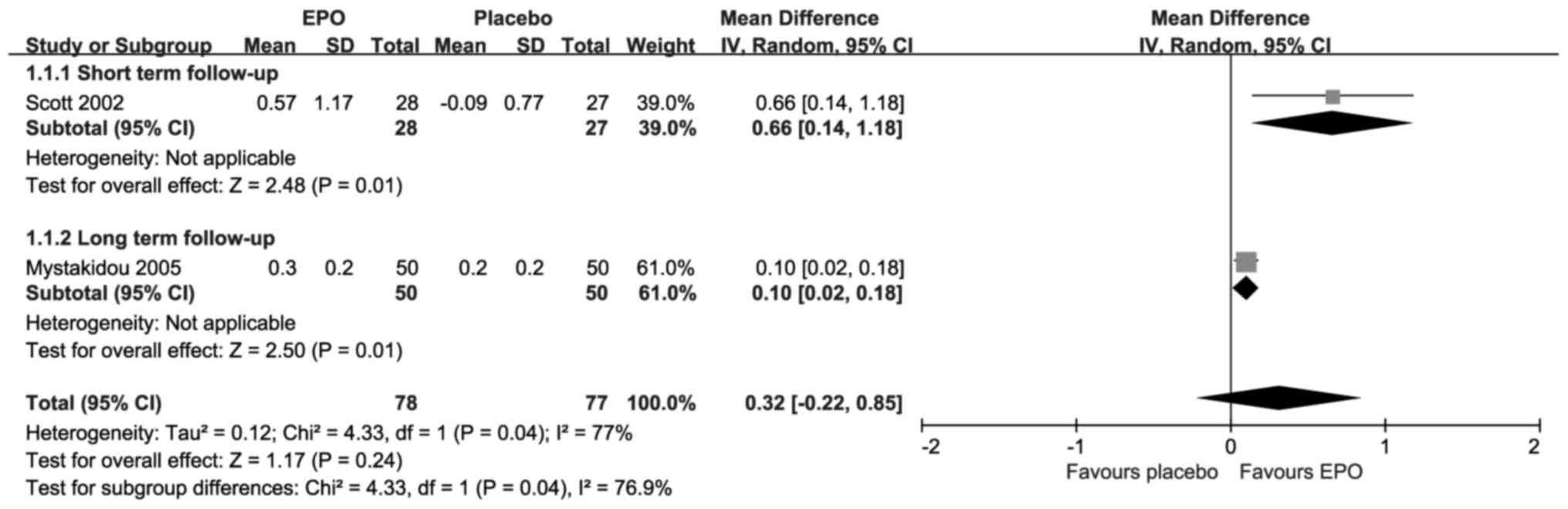
The change in HB levels is presented in Fig. 2; each included study suggested
statistically significant improvements upon treatment with EPO.
Compared with placebo, the results revealed there were significant
differences in the short-term follow-up [MD=0.66; 95% CI,
0.14–1.18; I2=Not applicable (NA); P=0.01] and the long-term
follow-up (MD=0.10; 95% CI, 0.02–0.18; I2=NA; P=0.01). However, the
overall result revealed that there was not a significant difference
(MD=0.32; 95% CI, −0.02–0.85; I2=77%; P=0.24) under substantial
heterogeneity.
Change in HCT
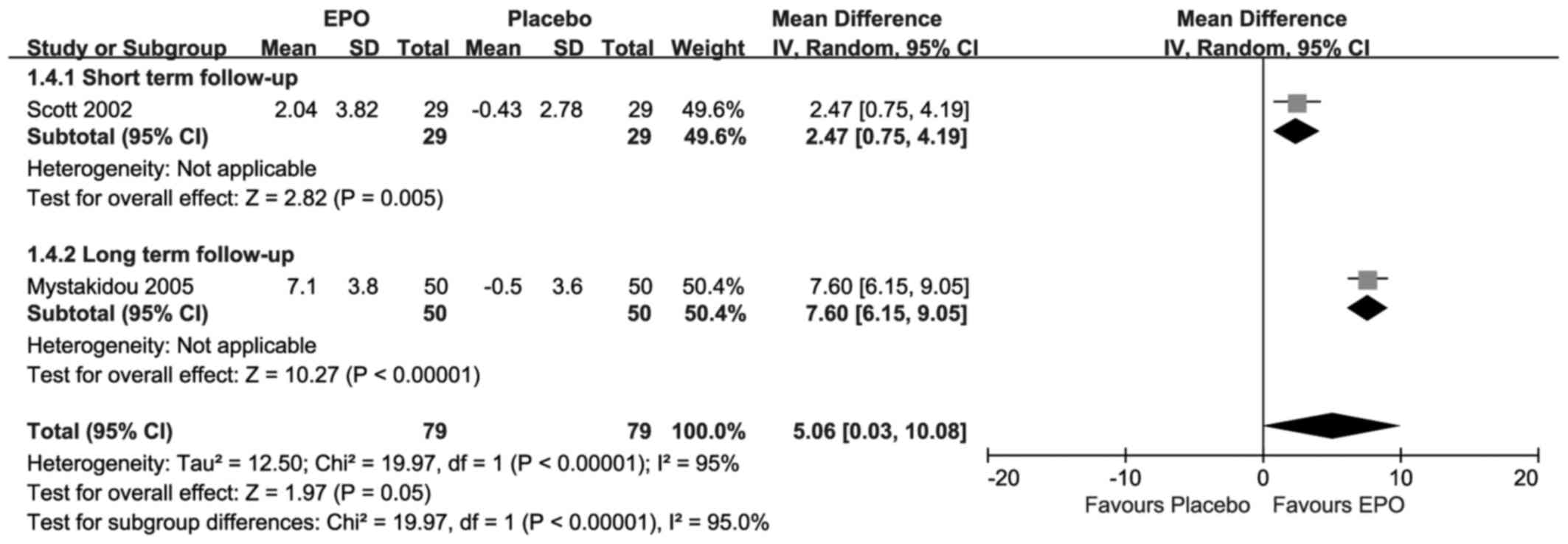
As presented in Fig.
3, each included research suggested that there were
statistically significant changes in HCT upon treatment with EPO.
Compared with placebo, the results showed there were significant
differences at both the short-term follow-up (MD=2.47, 95% CI,
0.75–4.19; I2=NA;P=0.005) and the long-term follow-up (MD=7.60; 95%
CI, 6.15–9.05; I2=NA; P<0.00001). The overall result also
revealed that there was a significant difference (MD=5.06; 95% CI,
0.03–10.08; I2=95%; P=0.05) under substantial homogeneity.
Ratio of transfusion
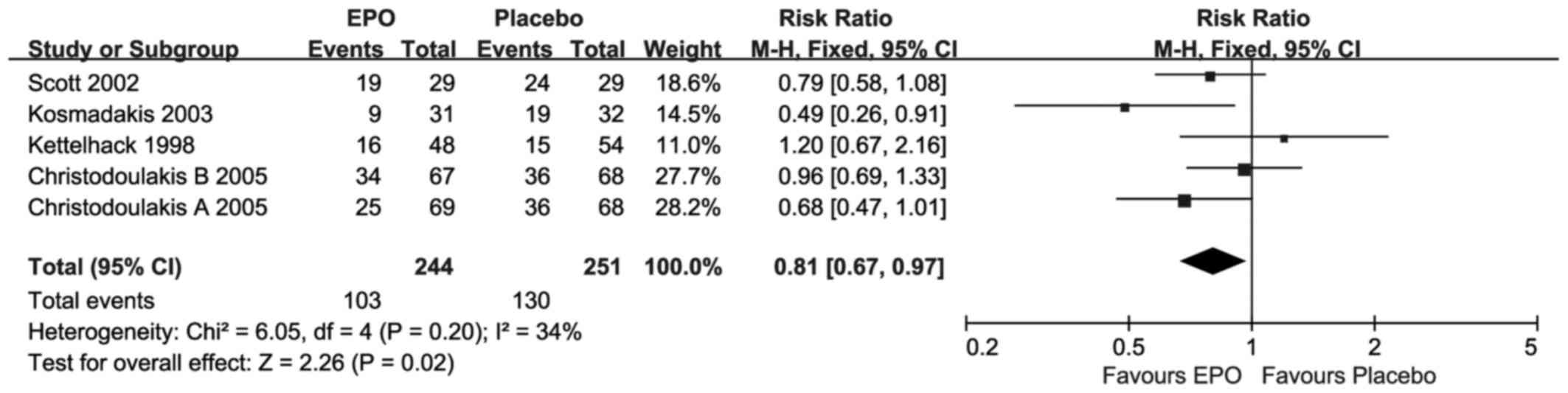
As presented in Fig.
4, for the ratio of transfusion only one study (24) suggested statistically significant
improvements, upon EPO treatment. Compared with placebo, the
overall meta-analysis result revealed that there was a significant
difference (RR=0.81; 95% CI, 0.67–0.97; I2=34%; P=0.02) at
short-term follow-up under moderate homogeneity.
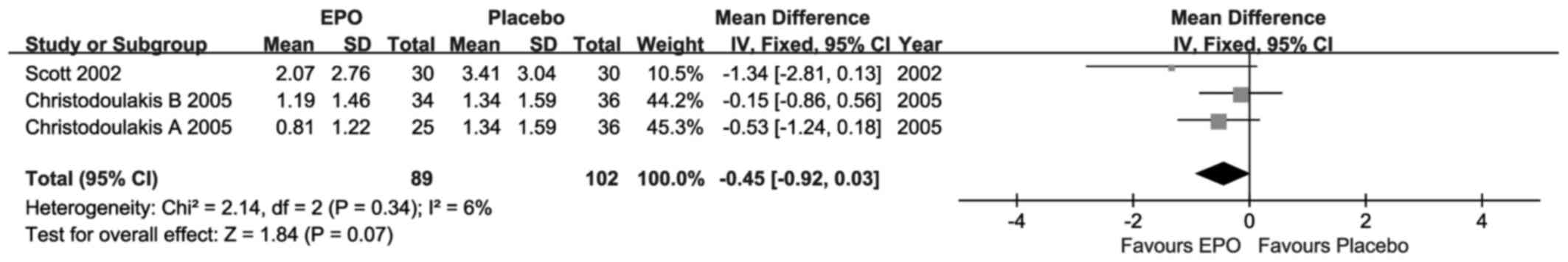
Transfusion requirements
For the transfusion requirements, as presented in
Fig. 5, each included research
suggested that there were no statistically significant differences,
although the MD value was advantageous to EPO. Compared with
placebo, the overall result revealed that there was no significant
difference (MD=−0.45; 95% CI, −0.92–0.03; I2=6%; P=0.07) at
short-term follow-up under weak homogeneity.
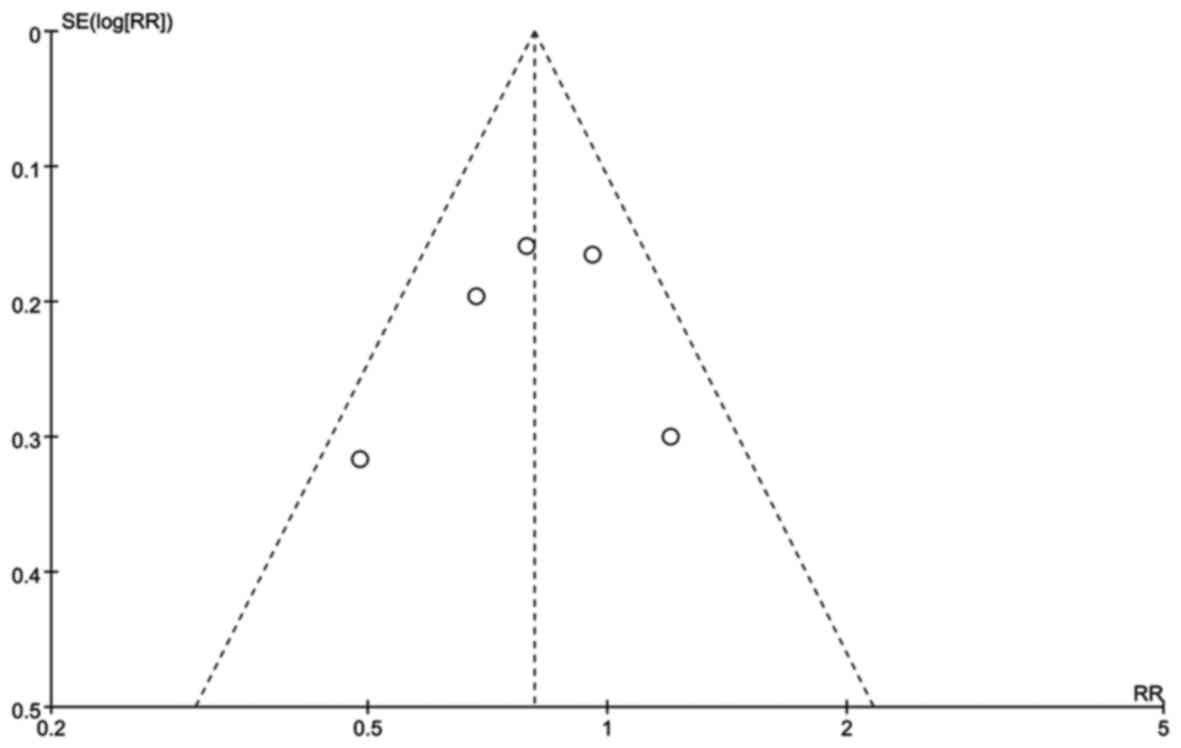
Publication bias
There was no potential publication bias based on
symmetry from a funnel plot of the ratio of transfusion outcome
(Fig. 6).
Discussion
There are several potential causes of
cancer-associated malignant anemia. These include bleeding,
hemolysis, poor nutrition, radiotherapy and chemotherapy. However,
as anemia reflects a shortage of red blood cells, which are
produced in the bone marrow, cancer-associated anemia can also be
caused by the inability of the bone marrow to produce them due to a
relative deficiency of EPO. To establish if EPO significantly
increased HB levels, and whether this was associated with improved
energy levels and a reduction in blood transfusions, the current
meta-analysis was performed with 6 eligible studies from 5 articles
that included patients with cancer-associated anemia. The efficacy
of EPO for cancer-associated malignant anemia was evaluated by
measuring four types of outcome.
The results revealed that EPO improved HB levels,
based on the change in HB, independent of the follow-up duration.
While the overall result was not statistically significant, the
finding is supported by previous studies (26,27). The
overall result did not reach statistical significance primarily due
to the small sample size. Cancer-associated anemia reduced the HCT
level below normal, which was reversed by EPO at the short-and
long-term follow-ups. With respect to transfusion requirements and
the ratio of transfusion, EPO reduced the need for transfusions.
The HB levels were increased owing to stimulation by EPO, which
improved the patients' anemic state, thereby reducing the demand
for transfusions. EPO is a highly glycosylated (40% of total
molecular weight) compound with half-life of ~5 h in the blood,
which may vary between its endogenous and various recombinant
versions (28). Additional
glycosylation or other alterations of EPO via recombinant
technology increases its stability in blood (thus reducing the
frequency of injections required) (29). EPO binds to the EPO receptor on the
red cell progenitor surface and activates a Janus kinase 2
signaling cascade. EPO receptor expression is found in a number of
tissues, such as bone marrow and peripheral/central nervous tissue.
In the bloodstream, red cells themselves do not express EPO
receptors, so they are not able to respond to EPO (30). However, indirect dependence of red
cell longevity in the blood on plasma erythropoietin levels has
been reported, a process termed neocytolysis (31). The majority of anemic patients with
cancer of the gastrointestinal tract have iron deficiency due to
subclinical blood loss; therefore, an iron supplement has been
advocated (32). In particular,
anemia is common in patients with colorectal cancer, as they are
more likely to experience a loss and malabsorption of iron
(33). However, iron supplementation
(5,34) has not been found to be able to
stimulate erythropoiesis to a sufficient degree to facilitate
autologous blood donation or to reduce the need for allogeneic
blood transfusions in patients with cancer (32). This may be due to a
reticulo-endothelial blockage of iron, characteristic of several
other conditions observed in these patients (35). The treatment scheme combining EPO
with iron resulted in a more profound HB increase with intravenous
iron, which may contribute to a superior optimization of the
patient's condition and possibly a decrease in postoperative
morbidity (33).
The advantage of the current meta-analysis was that
itincluded studies to explain the effect of EPO from four
comprehensive aspects ofblood indicators (20). Thestudy had several limitations.
Firstly, only a small number of trials met the inclusion/exclusion
criteria, and the meta-analysismay have benefitted from more
associatedclinical research to support its findings (17). Secondly, certain trials had missing
data (36), which was a source of
heterogeneity, and the current studywas unable to perform
meta-regression for confounding factors.
In conclusion, the currentstudy suggests that EPO
reduces cancer-associated anemia as well as improving relevant
blood parameters among this patient population.
Glossary
Abbreviations
Abbreviations:
|
EPO
|
erythropoietin
|
|
HB
|
hemoglobin
|
|
HCT
|
hematocrit
|
|
MeSH
|
medical subject headings
|
|
ITT
|
intention-to-treat
|
|
MD
|
mean difference
|
|
NA
|
not applicable
|
References
|
1
|
Mercadante S, Gebbia V, Marrazzo A and
Filosto S: Anaemia in cancer: Pathophysiology and treatment. Cancer
Treat Rev. 26:303–311. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henry DH: Supplemental Iron: A key to
optimizing the response of cancer-related anemia to rHuEPO?
Oncologist. 3:275–278. 1998.PubMed/NCBI
|
|
3
|
Kasper C, Terhaar A, Fosså A, Welt A,
Seeber S and Nowrousian MR: Recombinant human erythropoietin in the
treatment of cancer-related anaemia. Eur J Haematol. 58:251–216.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pronzato P: Cancer-related anaemia
management in the 21st century. Cancer Treat Rev. 32 Suppl 2:S1–S3.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ludwig H, Evstatiev R, Kornek G, Aapro M,
Bauernhofer T, Buxhofer-Ausch V, Fridrik M, Geissler D, Geissler K,
Gisslinger H, et al: Iron metabolism and iron supplementation in
cancer patients. Wien Klin Wochenschr. 127:907–919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Littlewood TJ: Erythropoietin for the
treatment of anemia associated with hematological malignancy.
Hematol Oncol. 19:19–30. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glaspy J: The impact of epoetin alfa on
quality of life during cancer chemotherapy: A fresh look at an old
problem. Semin Hematol. 34 3 Suppl 2:S20–S26. 1997.
|
|
8
|
Brandberg Y: Assessing the impact of
cancer-related anaemia on quality of life and the role of rHuEPO.
Med Oncol. 17(Suppl 1): S23–S31. 2000.PubMed/NCBI
|
|
9
|
Rice L, Alfrey CP, Driscoll T, Whitley CE,
Hachey DL and Suki W: Neocytolysis contributes to the anemia of
renal disease. Am J Kidney Dis. 33:59–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leng HM, Albrecht CF, Kidson SH and Folb
PI: Erythropoietin production in anemia associated with
experimental cancer. Exp Hematol. 27:806–810. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feelders RA, Vreugdenhil G, Eggermont AM,
Kuiper-Kramer PA, van Eijk HG and Swaak AJ: Regulation of iron
metabolism in the acute-phase response: Interferon gamma and tumour
necrosis factor alpha induce hypoferraemia, ferritin production and
a decrease in circulating transferrin receptors in cancer patients.
Eur J Clin Invest. 28:520–527. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung IJ, Dai C and Krantz SB: Stem cell
factor increases the expression of FLIP that inhibits IFNgamma
-induced apoptosis in human erythroid progenitor cells. Blood.
101:1324–1328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodriguez S and ánchez CA: Recommendation
of the scientific societies on the treatment of anaemia in cancer
patients. Clin Transl Oncol. 9:582–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garcia JM Jurado, Sánchez E Torres,
Hidalgo D Olmos and Alba Conejo E: Erythropoietin pharmacology.
Clin Transl Oncol. 9:715–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahlqvist-Rastad J, Albertsson M, Bergh J,
Birgegård G, Johansson P, Jonsson B, Kjellen E, Påhlman S,
Zackrisson B and Osterborg A: Erythropoietin therapy and cancer
related anaemia: Updated Swedish recommendations. Med Oncol.
24:267–272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deeks JJ: Issues in the selection of a
summary statistic for meta-analysis of clinical trials with binary
outcomes. Stat Med. 21:1575–1600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins J and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0 [updated
March 2011]. The Cochrane Collaboration; 2011, simplewww.cochrane-handbook.orgOctober 1–2011
|
|
18
|
Walter SD and Yao X: Effect sizes can be
calculated for studies reporting ranges for outcome variables in
systematic reviews. J Clin Epidemiol. 60:849–852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henke M, Laszig R, Rübe C, Schäfer U,
Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C and
Frommhold H: Erythropoietin to treat head and neck cancer patients
with anaemia undergoing radiotherapy: Randomised, double-blind,
placebo-controlled trial. Lancet. 362:1255–1260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Copas J and Shi JQ: Meta-analysis, funnel
plots and sensitivity analysis. Biostatistics. 1:247–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kettelhack C, Hönes C, Messinger D and
Schlag PM: Randomized multicentre trial of the influence of
recombinant human erythropoietin on intraoperative and
postoperative transfusion need in anaemic patients undergoing right
hemicolectomy for carcinoma. Br J Surg. 85:63–67. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scott SN, Boeve TJ, McCulloch TM,
Fitzpatrick KA and Karnell LH: The effects of epoetin alfa on
transfusion requirements in head and neck cancer patients: A
prospective, randomized, placebo-controlled study. Laryngoscope.
112:1221–1229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kosmadakis N, Messaris E, Maris A,
Katsaragakis S, Leandros E, Konstadoulakis MM and Androulakis G:
Perioperative erythropoietin administration in patients with
gastrointestinal tract cancer: Prospective randomized double-blind
study. Ann Surg. 237:417–421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christodoulakis M and Tsiftsis DD:
Hellenic Surgical Oncology Perioperative EPO Study Group:
Preoperative epoetin alfa in colorectal surgery: A randomized,
controlled study. Ann Surg Oncol. 12:718–725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mystakidou K, Kalaidopoulou O, Katsouda E,
Parpa E, Kouskouni E, Chondros C, Tsiatas ML and Vlahos L:
Evaluation of epoetin supplemented with oral iron in patients with
solid malignancies and chronic anemia not receiving anticancer
treatment. Anticancer Res. 25:3495–3500. 2005.PubMed/NCBI
|
|
27
|
Lacson E Jr, Ofsthun N and Lazarus JM:
Effect of variability in anemia management on hemoglobin outcomes
in ESRD. Am J Kidney Dis. 41:111–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rainville N, Jachimowicz E and Wojchowski
DM: Targeting EPO and EPO receptor pathways in anemia and
dysregulated erythropoiesis. Expert Opin Ther Targets. 20:287–301.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh PK, Devasahayam M and Devi S:
Expression of GPI anchored human recombinant erythropoietin in CHO
cells is devoid ofglycosylation heterogeneity. Indian J Exp Biol.
53:195–201. 2015.PubMed/NCBI
|
|
30
|
Aapro M: Emerging topics in anaemia and
cancer. Ann Oncol. 23 Suppl 10:x289–x293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rice L and Alfrey CP: The negative
regulation of red cell mass by neocytolysis: Physiologic and
pathophysiologic manifestations. Cell Physiol Biochem. 15:245–250.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Means RT Jr and Krantz SB: Progress in
understanding the pathogenesis of the anemia of chronic disease.
Blood. 80:1639–1647. 1992.PubMed/NCBI
|
|
33
|
Borstlap WA, Buskens CJ, Tytgat KM,
Tuynman JB, Consten EC, Tolboom RC, Heuff G, van Geloven AA, van
Wagensveld BA, Wientjes CA, et al: Erratum to: Multicentre
randomized controlled trial comparing ferric (III)carboxymaltose
infusion with oral iron supplementation in the treatment of
preoperative anaemia in colorectal cancer patients. BMC Surg.
15:1102015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laï-Tiong F, Brami C, Dubroeucq O, Scotté
F, Curé H and Jovenin N: Management of anemia and iron deficiency
in a cancer center in France. Support Care Cancer. 24:1091–1096.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Braga M, Gianotti L, Vignali A, Gentilini
O, Servida P, Bordignon C and Di Carlo V: Evaluation of recombinant
human erythropoietin to facilitate autologous blood donation before
surgery in anaemic patients with cancer of the gastrointestinal
tract. Br J Surg. 82:1637–1640. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jackson D, White IR, Mason D and Sutton S:
A general method for handling missing binary outcome data in
randomized controlled trials. Addiction. 109:1986–1993. 2014.
View Article : Google Scholar : PubMed/NCBI
|