Introduction
Malignant odontogenic neoplasms account for a small
percentage of all odontogenic tumors and may be classified as
carcinomas, sarcomas or carcinosarcomas (1). Ameloblastic fibro-odontosarcoma (AFOS)
is an extremely rare odontogenic sarcoma, composed of benign
odontogenic epithelium, a malignant mesenchymal component, and
dental hard tissue (1). AFOS may
either arise as a de novo lesion of the jaws (2,3), or
following the malignant transformation of a pre-existing
ameloblastic fibro-odontoma (AFO) (4–6). AFOS
usually occurs in the second and third decades of life, with a
predilection for the mandible (2–6). Due to
its rarity and the lack of clinicopathological information, the
diagnosis of AFOS is challenging. We herein report a de novo
case of AFOS of the left mandible in a 31-year-old female patient,
exhibiting active epithelial proliferation, which is considered as
an uncommon finding in AFOS. In addition, the clinicopathological
characteristics, clinical management and prognosis were also
discussed, combined with a review of the relevant literature.
Case report
In September 2006, a 31-year-old woman was referred
to the West China Hospital of Stomatology (Chengdu, China) due to a
6-month history of a swelling in the left mandible. The patient
complained that the mass had expanded over the last 2 months, with
associated pain.
On extraoral evaluation, the patient's face was
asymmetrical due to a sizeable swelling over the left mandible,
accompanied by limitation in opening the mouth. Intraorally, an
exophytic neoplasm was observed, extending from the left lower
premolar to the ascending ramus of the left mandible, measuring
~8×6×4 cm3, with migration of teeth 43–44 and missing
teeth 45–47. No evidence of regional lymph node or distant
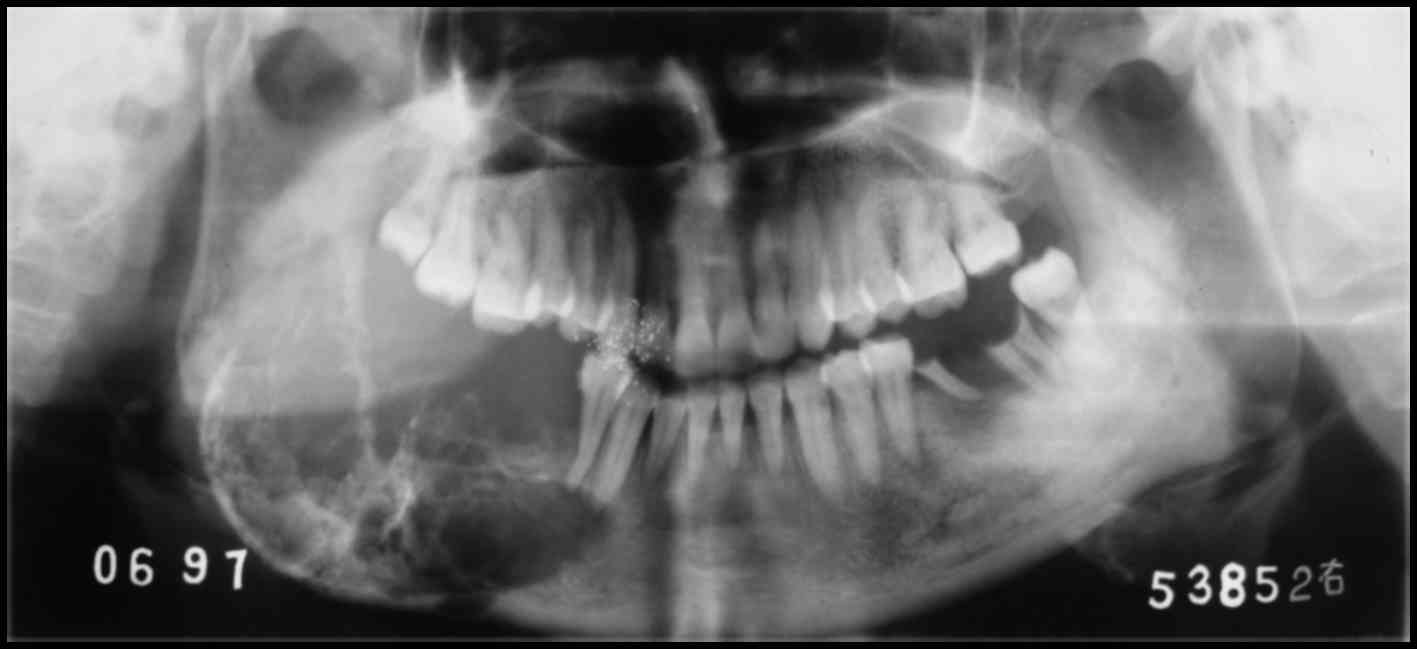
metastasis was detected. The panoramic radiographic examination
revealed a sizeable multilocular radiolucent lesion in the left
mandible. The neoplasm extended from the left canine to the ramus
of the left mandible, with indistinct margins and local perforation
of the cortical plate. In addition, irregular radiopaque foci were
observed within the lesion (Fig.
1).
The initial preoperative diagnosis was
ameloblastoma, with local invasion and the possibility of malignant
transformation. A left hemimandibular resection was performed, with
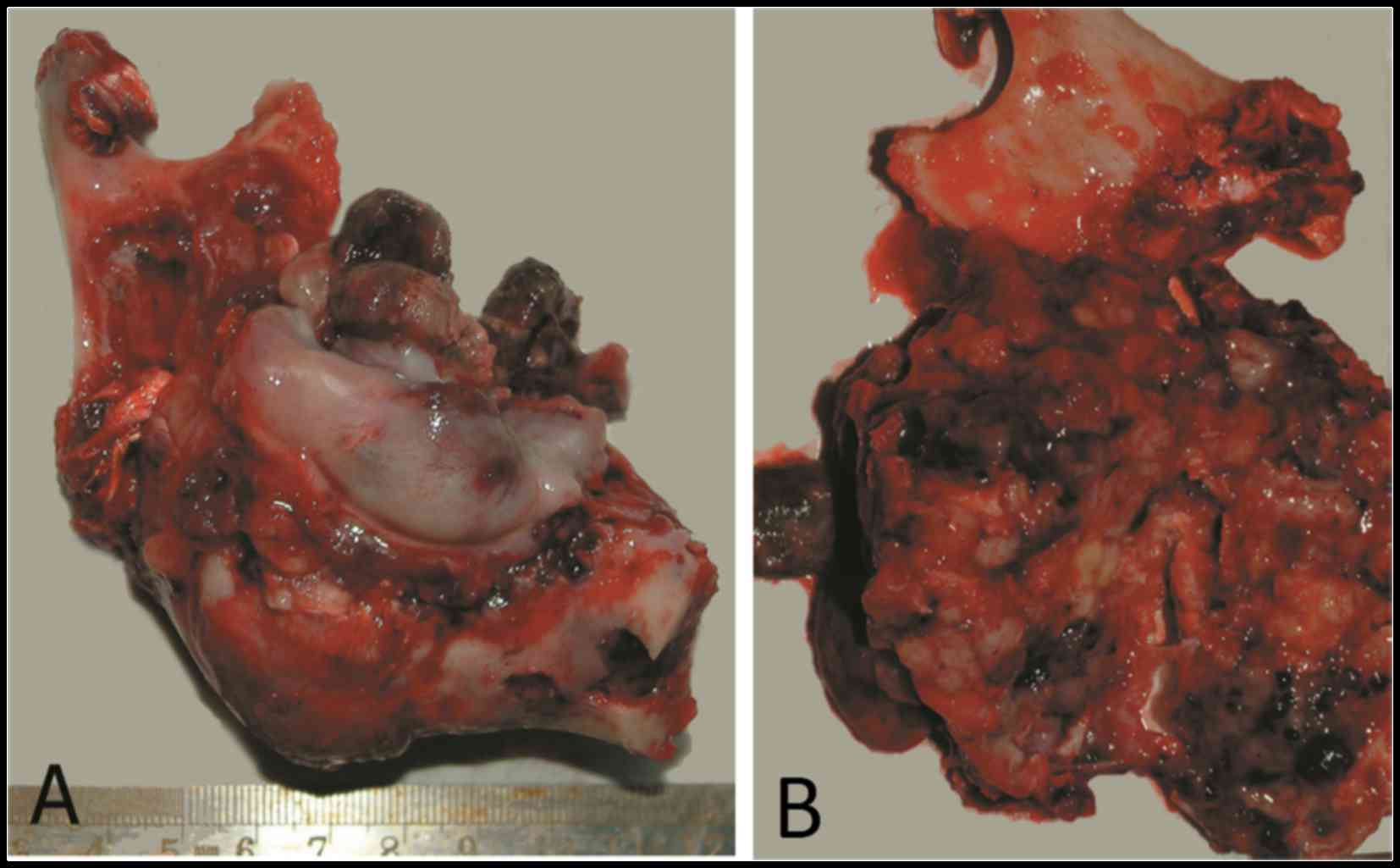
tumor-free margins. Grossly, the tumor was exophytic and fleshy,
with considerable destruction and replacement of the left mandible.
The cut surface was grayish brown, with scattered firm tissue and
hemorrhagic areas (Fig. 2).
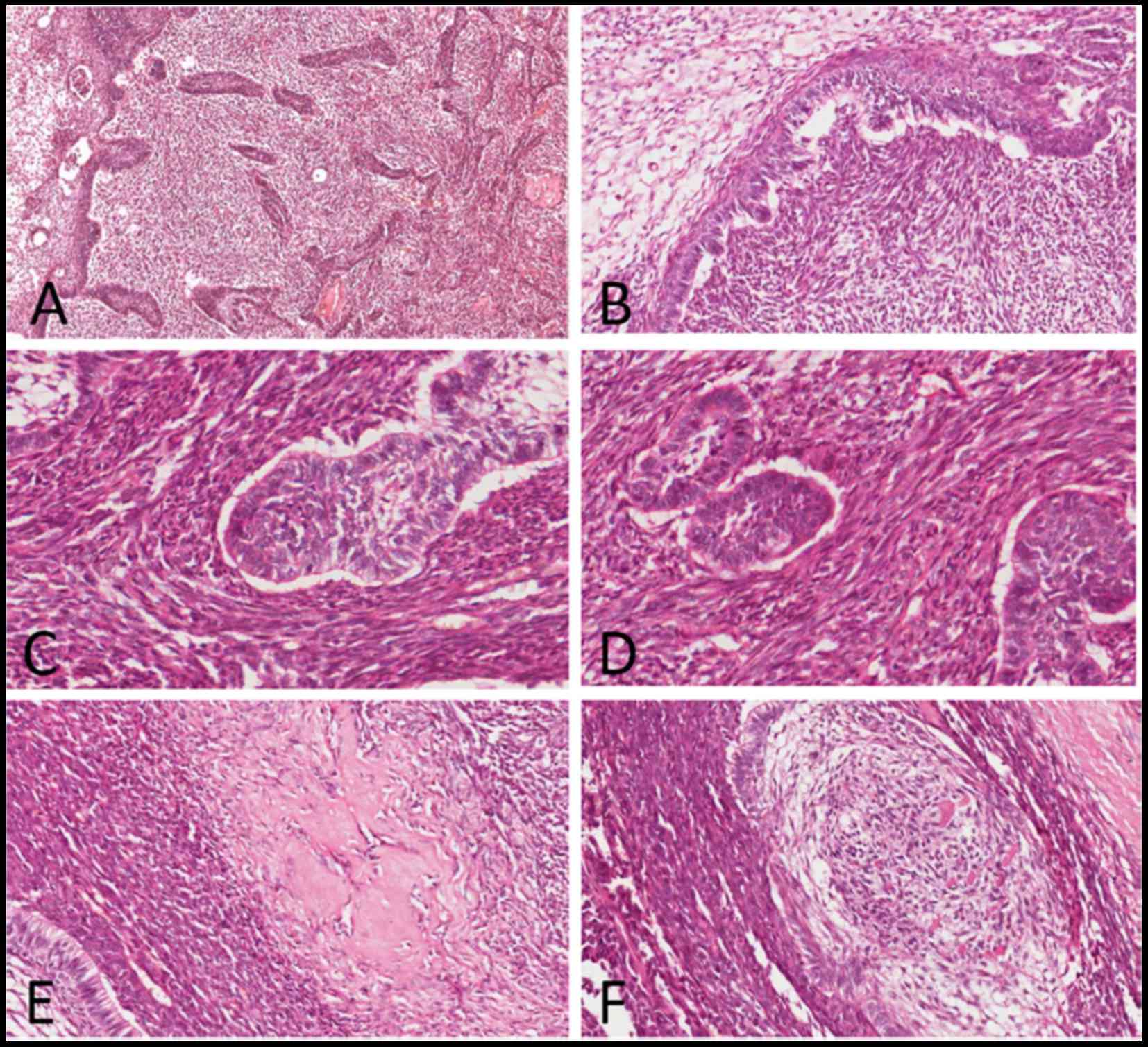
Microscopic examination of the hematoxylin and
eosin-stained slides showed a biphasic tumor with two major
components, namely the benign odontogenic epithelial islands and
the surrounding anaplastic mesenchymal component, with hyalinized
areas at the epithelial-mesenchymal interface (Fig. 3A). The structure of the epithelial
islands resembled that of the enamel organ, with a central area
simulating the stellate reticulum cells outlined by a single layer
of cuboidal or columnar ameloblast-like cells arrayed in a
palisading pattern (Fig. 3B). The
majority of the epithelial component displayed benign
characteristics, but a few epithelial cells proliferated actively,
with hyperchromatic cytoblasts and mitotic figures [mean, 2–4
mitoses/10 high-power fields (HPFs)] (Fig. 3C). The mesenchymal component
exhibited cytological characteristics typical of malignancy, with
spindle-to-ovoid shaped cells arranged in a fascicular pattern. The
cells exhibited obvious nuclear pleomorphism and active mitoses
(mean, 20 mitoses/10 HPFs) (Fig.
3D). In addition, irregular dysplastic dentin and enamel matrix
were identified adjacent to the epithelial structures (Fig. 3E and F).
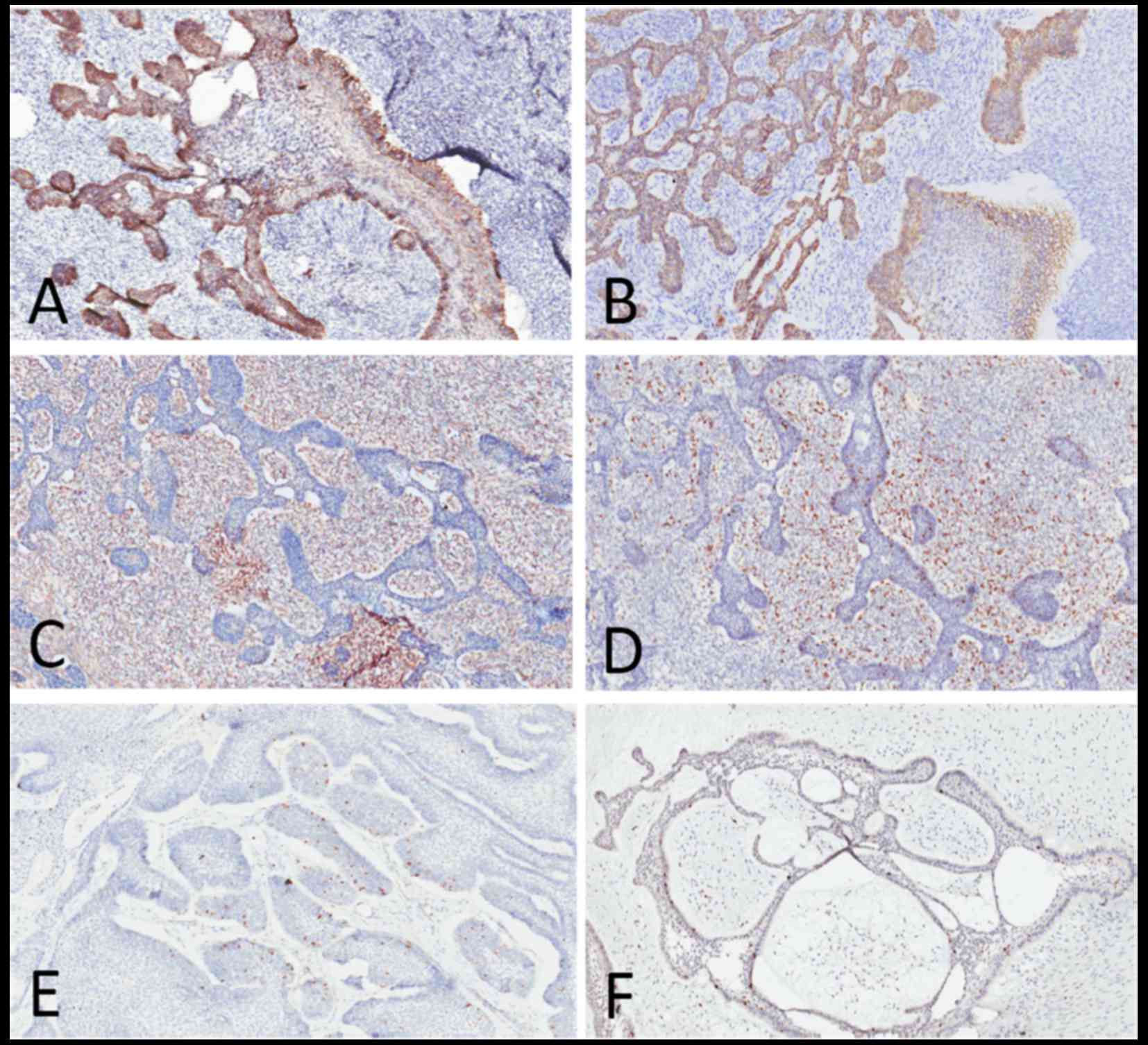
Immunohistochemical (IHC) staining was performed for
cytokeratin (CK)14 and CK19, vimentin and Ki-67. Intense membranous
and cytoplasmic staining for CK14 and CK19 were observed in the
epithelium, with negative expression in the mesenchymal background
(Fig. 4A and B). The mesenchymal
cells were strongly positive for vimentin, which was negative in
the epithelial component (Fig. 4C).
The Ki-67 labeling index was considerably higher in the mesenchymal
component (mean, 40%) compared with the epithelial component (mean,
5–8%) (Fig. 4D). One ameloblastoma
(AB) and one ameloblastic fibroma (AF) case with the same markers
were used for comparison, and were found to share the same staining
pattern as AFOS regarding CK14, CK19 and vimentin; however, the
Ki-67 labeling index in those two cases was very low compared with
AFOS (mean, 3% in AB and <2% in AF) (Fig. 4E and F; Table I).
 | Table I.Comparison of immunohistochemical
staining characteristics of AFOS, AB and AF. |
Table I.
Comparison of immunohistochemical
staining characteristics of AFOS, AB and AF.
|
| AFOS | AB | AF |
|---|
|
|
|
|
|
|---|
| Staining | Epithelial | Mesenchymal | Epithelial | Mesenchymal | Epithelial | Mesenchymal |
|---|
| CK14/19 | + | − | + | − | + | − |
| Vimentin | − | + | − | + | − | + |
| Ki67 | 5–8% | 40% | 3% | − | <2% | <2% |
Finally, combined with the abovementioned
examinations, a final diagnosis of AFOS was made. At 3 months
postoperatively, clinical and radiographic follow-up of the patient
revealed no recurrence or indication of metastasis. The patient was
then lost to follow-up.
Discussion
AFOS is an extremely rare subtype of odontogenic
sarcoma. The 2005 WHO classification (1) divided odontogenic sarcomas into two
categories according to the presence of dentin and enamel matrix
(without clinical or prognostic significance) as follows:
Ameloblastic fibrosarcoma (AFS) and ameloblastic
fibro-odontosarcoma/fibrodentino-sarcoma (AFOS/AFDS). The relative
information is sparse, with no more than 73 cases (7,8) of AFS
and 19 cases of AFOS/AFDS (8 females) (2–6) reported
in the English literature to date. In addition, AFOS has also been
reported in a bovine mandible (9).
Due to its rarity, the diagnosis of AFOS may be challenging, and it
mainly relies on clinicopathological findings and radiological
examination.
Clinically, a painful swelling is the most common
complaint, with a reported duration of symptoms from 2 months
(2) to 12 years (10). AFOS occurs at a mean age of 23.5
years (3), with a wide age range of
4–83 years (2,3). The male:female ratio is 1.2:1 according
to the cases published to date (2–6). The
majority of the cases involve the mandible, with a predilection for
the retromolar region and the mandibular ramus, with only one
reported case located in the maxilla (6). AFOS may either arise as a de
novo lesion (2,3) or from the malignant transformation of a
pre-existing AFO (4–6). The mechanism underlying this conversion
has not been fully elucidated. Radiographically, AFOS usually
presents as a uni- or multilocular expansile radiolucent lesion,
with ill-defined borders and focal dense opacities. When a tumor
exhibits a poorly circumscribed outline and perforation of the
cortex, the possibility of a malignant odontogenic tumor should be
taken into consideration (2–6).
The case presented herein occurred in a 31-year-old
female in the left mandible as a de novo lesion with a
history of 6 months. The clinical and radiological examination
suggested the possibility of malignancy and the patient underwent
hemimandibular resection. Based on the abovementioned assessments
alone, the risk of a false diagnosis is high. However, preoperative
incisional biopsy and intraoperative frozen section biopsy may
improve the accuracy of the diagnosis, preventing surgical
overtreatment. The definitive diagnosis of AFOS is based on the
postoperative histopathological examination.
Microscopically, this lesion exhibits the
characteristic architecture of benign epithelial islands/cords
enmeshed in the malignant mesenchymal component, with focal
deposits of dentin and enamel matrix. The odontogenic epithelium
elements are composed of columnar or cuboidal cells at the
periphery arranged in a palisading pattern, exhibiting reverse
polarization of the nuclei away from the basal membrane. The
central areas of the epithelial islands/cords are less cellular,
edematous, mimicking the stellate reticulum of the enamel organ.
The mesenchymal component includes spindle-to-ovoid cells
exhibiting the characteristics of malignancy, including cell
pleomorphism, nuclear atypia and hyperchromatism, with numerous
mitotic figures. Occasionally, bizarre giant cells may be
identified (2–6). In addition, IHC examination is a useful
method for accurate diagnosis and prognosis evaluation. Vimentin is
strongly positive in the mesenchymal component, and intense
staining of CK14, CK19 and pan-CK may be detected in the
epithelium. The malignant mesenchymal component highly expresses
proliferating cell nuclear antigen and Ki-67, which are negative or
poorly expressed in the epithelial element (2–6).
In the present case, part of the odontogenic
epithelium exhibited active proliferation, accompanied by deposits
of immature dentinoid and enameloid matrix. The same appearance may
be observed in AFO, but not in AB. Thus, we hypothesized that the
odontogenic epithelium may induce the formation of dentin and
enamel matrix under the influence of the benign or malignant
mesenchymal component in biphasic odontogenic tumors. This may be
an important finding in subsequent experiments on the induction of
dental hard tissue.
AFO, a benign biphasic neoplasm with the same
epithelial characteristics and hard dental tissue, is the main
differential diagnosis in AFOS. Unlike AFOS, the mesenchymal
component of AFO exhibits a benign appearance. The IHC staining of
Ki-67 is of great significance in distinguishing AFO from AFOS. As
approximately one-third of AFOS cases are transformed from a
pre-existing AFO, serial extensive sampling of the resected AFO
specimen is crucial for elucidating the possible stepwise
progression of AFO to AFOS. In addition, AFOS should be
differentiated from ameloblastic carcinosarcoma, which is a
biphasic malignant tumor with both epithelial and mesenchymal
malignant components (2–6).
AFOS is usually considered as a
low-to-intermediate-grade malignant tumor, with locally aggressive
behavior and a recurrence rate of 10%. Two cases with recurrence
(6,11) have been reported, one of which
involved the base of the skull (11). By contrast, regional lymph node and
distant metastases are rare. Only 1 of 20 cases developed local
metastasis (10).
Due to the rarity of AFOS and the overall lack of
experience with the treatment of this lesion, a standard of
treatment has not been established. To date, radical resection with
clear margins is the preferred treatment strategy. Long-term
follow-up is crucial. Adjuvant radiotherapy and chemotherapy have
been successfully used in some odontogenic sarcoma cases (12,13).
Chemotherapy with ifosfamide and doxorubicin and corresponding
consolidated treatment by re-irradiation has been recommended for
AFOS by Gatz et al (6).
However, lung metastases were found in a patient 20 months after
surgery and postoperative chemotherapy (14). Therefore, due to its uncertain
benefits, the use of adjuvant radiochemotherapy remains a subject
of debate.
As AFOS is a rare subtype of odontogenic sarcoma,
its diagnosis and treatment may be challenging. Clinical assessment
and X-ray examination play an important role, but the definitive
diagnosis is based on histopathological examination. Radical
resection is currently considered the optimal treatment and
long-term follow-up is crucial. The aim of the present case report
was to provide detailed information in order to enrich the database
and improve our understanding of this rare tumor.
Acknowledgements
The present study was supported by the Chengdu
Huimin Project of Science and Technology (2015-HM01-00412-SF).
References
|
1
|
Barnes L, Eveson J, Reichart P and
Sidransky D: World Health Organization classification of
tumorsPathology and genetics of head and neck tumors. Lyon: IARC
Press; pp. 294–295. 2005
|
|
2
|
Wang S, Shi H, Wang P and Yu Q:
Ameloblastic fibro-odontosarcoma of the mandible: Imaging findings.
Dentomaxillofac Radiol. 40:324–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen SJ, Zheng XW, Lin X and Liu H:
Ameloblastic fibro-odontosarcoma of the mandible in a pediatric
patient. Eur Ann Otorhinolaryngol Head Neck Dis. 133:419–421. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mainenti P, Oliveira GS, Valério JB,
Daroda LS, Daroda RF, Brandão G and Rosa LE: Ameloblastic
fibro-odontosarcoma: A case report. Int J Oral Maxillofac Surg.
38:289–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reiser V, Alterman M, Shuster A and Kaplan
I: Pediatric ameloblastic fibro-odontosarcoma of the mandible: A
challenge of diagnosis and treatment. J Oral Maxillofac Surg.
71:e45–e57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gatz SA, Thway K, Mandeville H, Kerawala
C, MacVicar D and Chisholm J: Chemotherapy responsiveness in a
patient with multiply relapsed ameloblastic fibro-odontosarcoma of
the maxilla. Pediatr Blood Cancer. 62:2029–2032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohsenifar Z, Behrad S and Abbas FM:
Epithelial dysplasia in ameloblastic fibrosarcoma arising from
recurrent ameloblastic fibroma in a 26-year-old iranian man. Am J
Case Rep. 16:548–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loya-Solis A, González-Colunga KJ,
Pérez-Rodríguez CM, Ramírez-Ochoa NS, Ceceñas-Falcón L and
Barboza-Quintana O: Ameloblastic fibrosarcoma of the mandible: A
case report and brief review of the literature. Case Rep Pathol.
2015:2450262015.PubMed/NCBI
|
|
9
|
Binnington JA and Adkins KF: Ameloblastic
odontosarcoma in a bovine mandible. J Pathol. 108:169–172. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herzog U, Putzke HP, Bienengräber V and
Radke C: The ameloblastic fibro-odontoma-an odontogenic mixed tumor
progressing into an odontogenic sarcoma. Dtsch Z Mund Kiefer
Gesichtschir. 15:90–93. 1991.(In German). PubMed/NCBI
|
|
11
|
Takeda Y, Kuroda M and Suzuki A:
Ameloblastic odontosarcoma (ameloblastic fibro-odontosarcoma) in
the mandible. Acta Pathol Jpn. 40:832–837. 1990.PubMed/NCBI
|
|
12
|
Huguet P, Castellvi J, Avila M, Alejo M,
Autonell F, Basas C and Bescos MS: Ameloblastic fibrosarcoma:
Report of a case. Immunohistochemical study and review of the
literature. Med Oral. 6:173–179. 2001.(In English, Spanish).
PubMed/NCBI
|
|
13
|
Demoor-Goldschmidt C, Minard-Colin V,
Cassagneau E, Supiot S, Oberlin O, D'hautuille C and Corradini N:
Ameloblastic fibrosarcoma of the mandible: Report of 2
chemosensitive pediatric cases. J Pediatr Hematol Oncol.
34:e72–e76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pourdanesh F, Mohamadi M, Moshref M and
Soltaninia O: Ameloblastic fibrosarcoma of the mandible with
distant metastases. J Oral Maxillofac Surg. 73:2067.e1–7. 2015.
View Article : Google Scholar
|