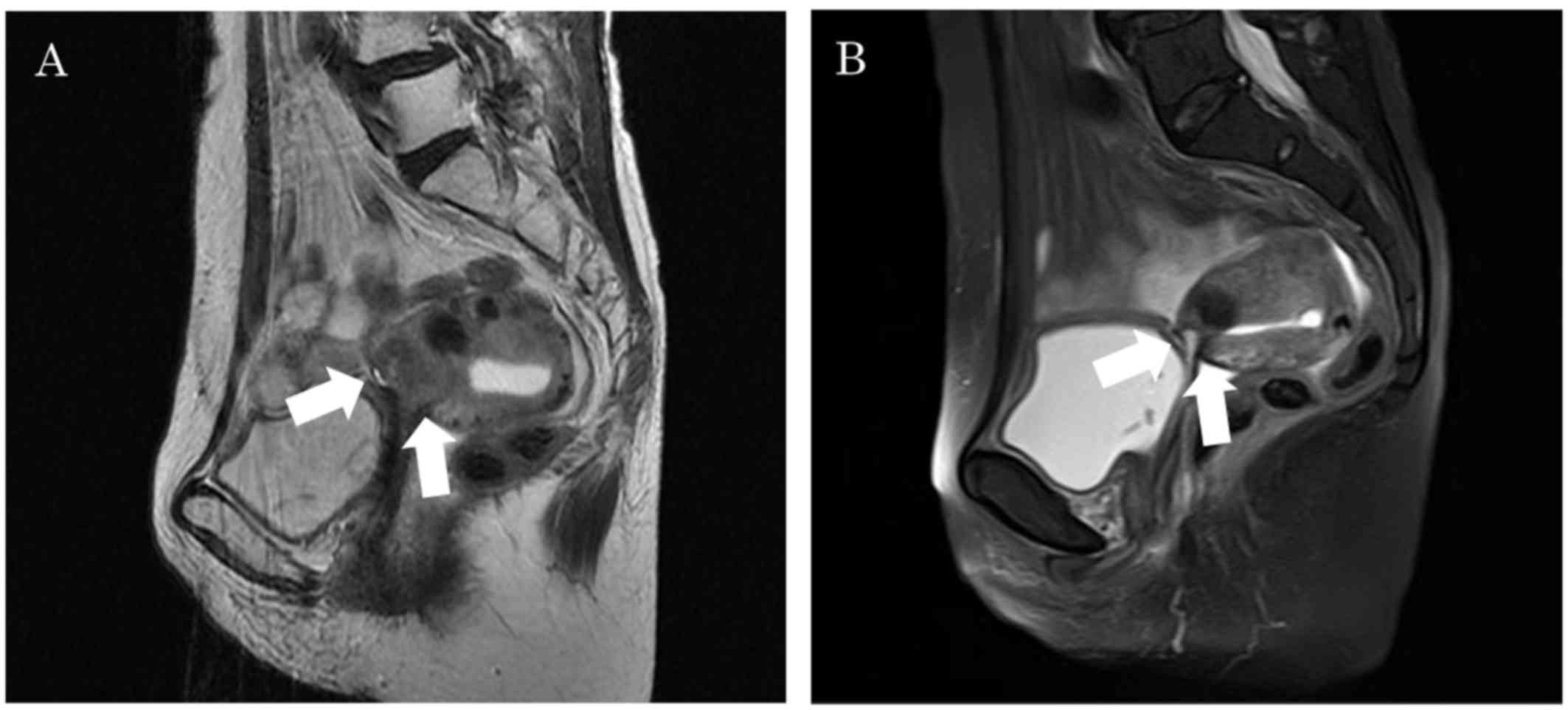
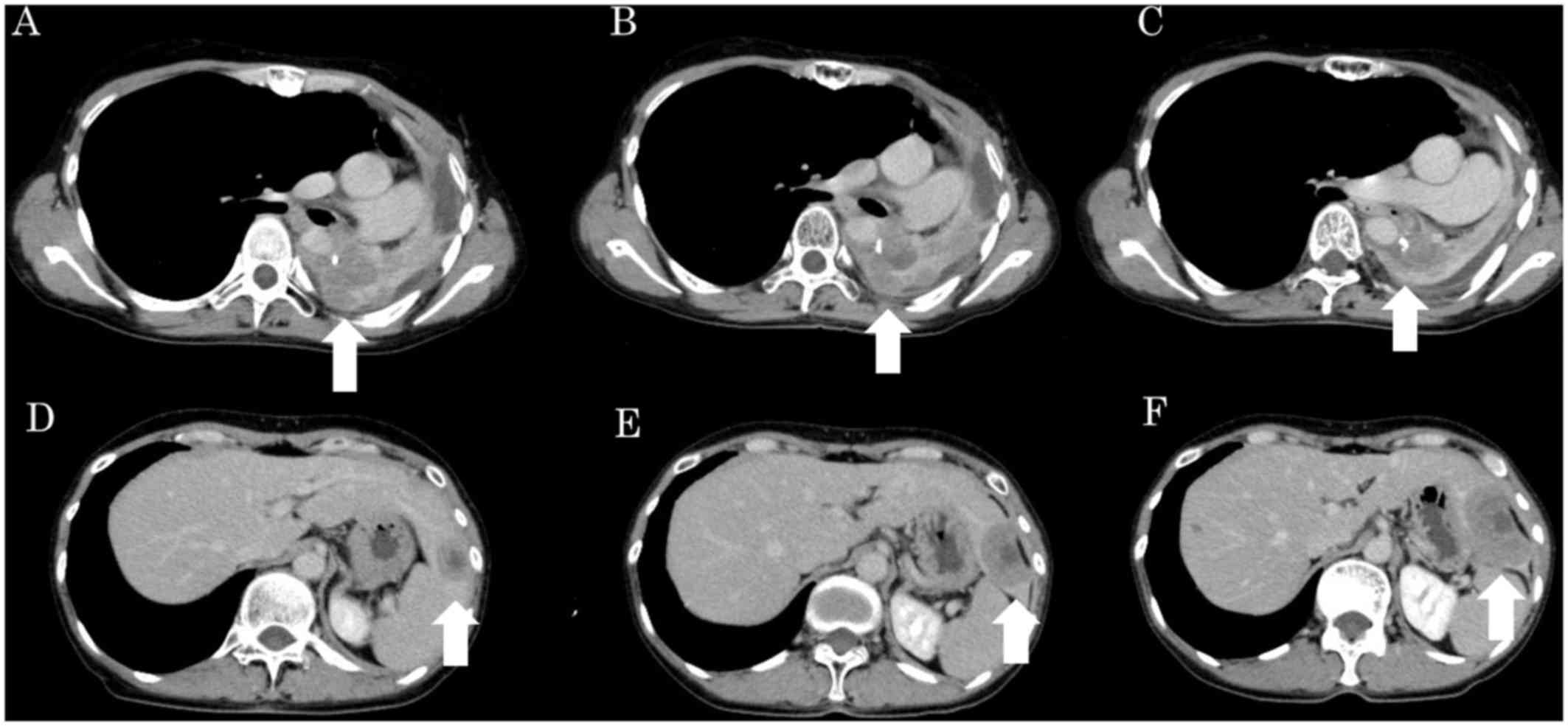
|
1
|
Sugiyama T, Nishida T, Kumagai S, Nishino
S, Fujivoshi K, Okura N, et al: Combination chemotherapy with
irinotecan and cisplatin as neoadjuvant in locally advanced
cervical cancer. Br J Cancer. 81:95–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabata T, Nishiura K, Yanoh K, Okugawa T,
Obata H, Tanaka K and Toyoda N: A pilot study of neoadjuvant
chemotherapy with mitomycin C, etoposide, cisplatin, and epirubicin
for adenocarcinoma of the cervix. Int J Clin Oncol. 9:59–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curtin JP, Blessing JA, Webster KD, Rose
PG, Mayer AR, Fowler WC Jr, Malfetano JH and Alvarez RD:
Paclitaxel, an active agent in nonsquamous carcinomas of the
uterine cervix: A Gynecologic Oncology Group Study. J Clin Oncol.
19:1275–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shoji T, Kumagai S, Yoshizaki A, Yokoyama
Y, Fujimoto T, Takano T, Yaegashi N, Nakahara K, Nishiyama H and
Sugiyama T: Efficacy of neoadjuvant chemotherapy followed by
radical hysterectomy in locally advanced non-squamous carcinoma of
the uterine cervix: A retrospective multicenter study of Tohoku
Gynecologic Cancer Unit. Eur J Gynaecol Oncol. 33:353–357.
2012.PubMed/NCBI
|
|
5
|
Monk BJ, Sill MW, McMeekin DS, Cohn DE,
Ramondetta LM, Boardman CH, Benda J and Cella D: Phase III trial of
four cisplatin-containing doublet combinations in stage IVB,
recurrent, or persistent cervical carcinoma: A Gynecologic Oncology
Group study. J Clin Oncol. 27:4649–4655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tewari KS, Sill MW, Long HJ III, Penson
RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao
MM, et al: Improved survival with bevacizumab in advanced cervical
cancer. N Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugiyama T, Mizuno M, Aoki Y, Sakurai M,
Nishikawa T, Ueda E, et al: A single-arm study evaluating
bevacizumab, cisplatin, and paclitaxel followed by single-agent
bevacizumab in Japanese patients with advanced cervical cancer. Jpn
J Clin Oncol. 46:1–8. 2016.PubMed/NCBI
|
|
8
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Gynecologic Oncology Group: Incorporation of bevacizumab in
the primary treatment of ovarian cancer. N Engl J Med.
365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: ICON7 Investigators: A phase 3
trial of bevacizumab in ovarian cancer. N Engl J Med.
365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitagawa R, Katsumata N, Shibata T, Kamura
T, Kasamatsu T, Nakanishi T, Nishimura S, Ushijima K, Takano M,
Satoh T, et al: Paclitaxel Plus Carboplatin Versus Paclitaxel Plus
Cisplatin in Metastatic or Recurrent Cervical Cancer: The
Open-Label Randomized Phase III Trial JCOG0505. J Clin Oncol.
33:2129–2135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takatori E, Shoji T, Miura Y, Nagao M,
Takada A, Nagasawa T, Omi H, Kagabu M, Honda T and Sugiyama T: A
phase II clinical trial of palonosetron for the management of
delayed vomiting in gynecological cancer patients receiving
paclitaxel/carboplatin therapy. Mol Clin Oncol. 3:281–286. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dupuis LL, Roscoe JA, Olver I, Aapro M and
Molassiotis A: 2016 updated MASCC/ESMO consensus recommendations:
Anticipatory nausea and vomiting in children and adults receiving
chemotherapy. Support Care Cancer. 25:317–321. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano T, Kato S, Ohno T, Tsujii H, Sato
S, Fukuhisa K and Arai T: Long-term results of high-dose rate
intracavitary brachytherapy for squamous cell carcinoma of the
uterine cervix. Cancer. 103:92–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Erickson B, Eifel P, Moughan J, Rownd J,
Iarocci T and Owen J: Patterns of brachytherapy practice for
patients with carcinoma of the cervix (1996–1999): A patterns of
care study. Int J Radiat Oncol Biol Phys. 63:1083–1092. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cannistra SA, Matulonis UA, Penson RT,
Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D,
Wenham R, et al: Phase II study of bevacizumab in patients with
platinum-resistant ovarian cancer or peritoneal serous cancer. J
Clin Oncol. 25:5180–5186. 2007. View Article : Google Scholar : PubMed/NCBI
|