Introduction
Cisplatin has been used as monotherapy or in
combination with other drugs as chemotherapy for treating cervical
cancer (1,2). Chemotherapy with taxanes has been
studied in recent years (3,4). In the GOG204 study, which was a
randomized controlled study involving 435 patients with stage-IVB
or recurrent carcinoma of the uterine cervix, including 54 with
adenocarcinoma and 36 with adenosquamous carcinoma,
vinorelbine+cisplatin therapy, gemcitabine+cisplatin therapy, and
topotecan+cisplatin therapy failed to show a therapeutic effect
superior to that of paclitaxel+cisplatin therapy (5). Therefore, at present, combination
chemotherapy with taxane plus a platinum agent is generally
considered to be the most effective regimen for the treatment of
cervical cancer. However, there is no established second-line
chemotherapy for patients who have recurrent disease after
receiving the first-line therapy with taxane plus a platinum agent.
Bevacizumab, a recombinant humanized monoclonal antibody that
limits angiogenesis by inhibiting vascular endothelial growth
factor (VEGF) and the first molecular-target agent introduced in
the gynecological field, has been established as a standard therapy
for patients with cervical cancer based on the prolongation of PFS
shown in previous studies. The Gynecologic Oncology Group (GOG) 240
trial demonstrated that bevacizumab combination chemotherapy
improved the prognosis of advanced/recurrent cervical cancer
(6). In Japan, after the
tolerability of this chemotherapy regimen was demonstrated in a
company-led clinical study on seven patients with
advanced/recurrent cervical cancer (JO29569 study) (7), bevacizumab combination chemotherapy was
approved for National Health Insurance reimbursement in May 2016.
However, tolerability was assessed based on administration of one
cycle in the Japanese study. The safety of bevacizumab combination
chemotherapy for patients with recurrent cervical cancer after
pelvic radiotherapy has still not been proven in Japan. We
experienced two cases in which bevacizumab combination chemotherapy
was safely administered for six cycles for recurrent cervical
cancer after pelvic radiotherapy. These cases are described along
with a literature review.
Case reports
Case 1
The patient was a 62-year-old woman with stage IIIB
recurrent squamous cell carcinoma (SCC) of the cervix (gravida 3,
para 3), with a history of cataract surgery at the age of 59 years
and an unremarkable family history.
At another hospital in May 2015, she received
radiotherapy [whole pelvic external beam radiotherapy (WPEBRT) at a
total dose of 50.4 Gy in 28 fractions and high dose rate
intra-cavitary brachytherapy (HDR-ICBT) at a dose of 24 Gy per 4
fractions], which resulted in complete remission. She had
subsequently been followed up. In May 2016, a recurrent tumor was
detected in the uterine cervix, and she was referred to our
hospital for treatment. At our department, SCC was detected by
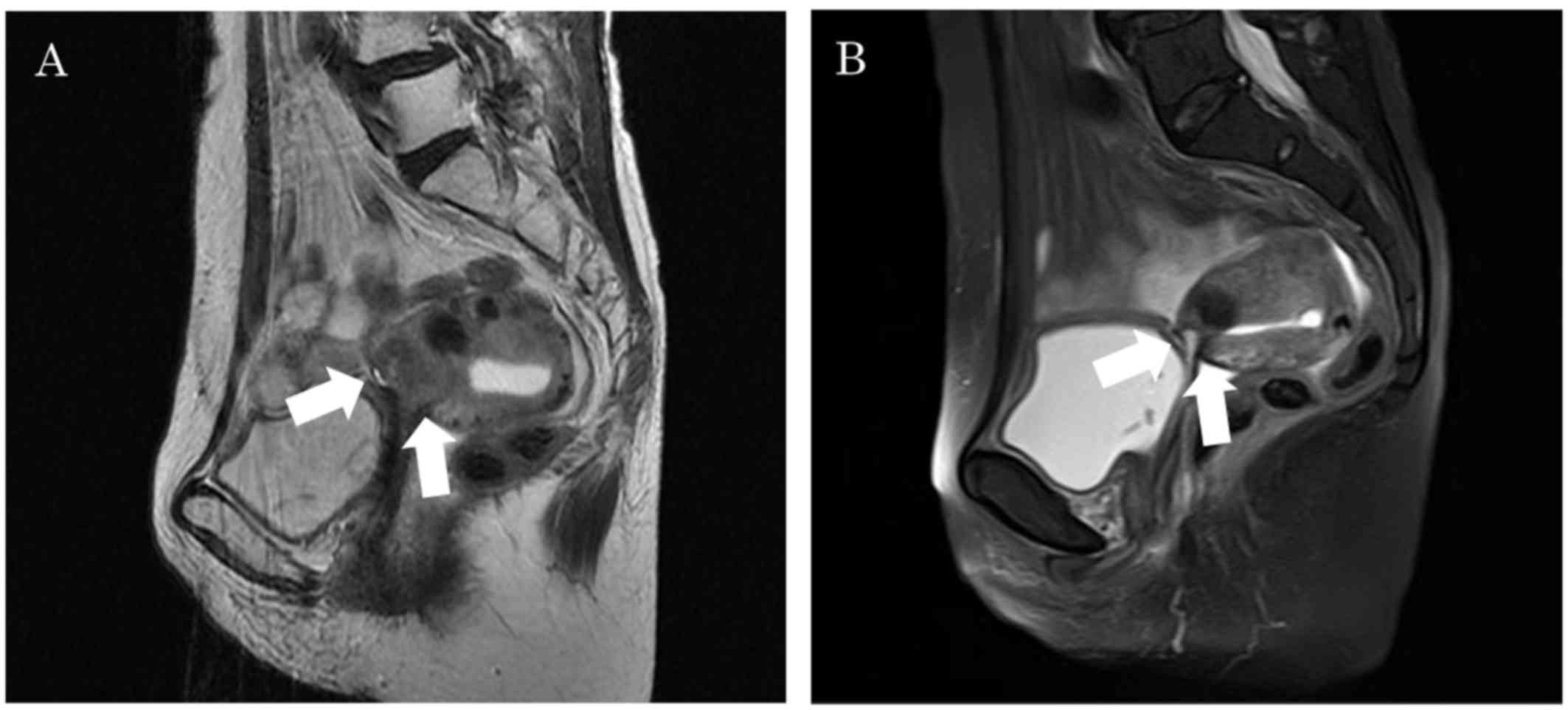
cervical biopsy. Magnetic resonance imaging (MRI) revealed a 2-cm
recurrent mass in the uterine cervix (Fig. 1A). Treatment consisted of paclitaxel
at 175 mg/m2, carboplatin at an area under the curve of
6, and bevacizumab at 15 mg/kg. This regimen was administered every
three weeks for six cycles. There was neither treatment delay nor
dose modification. On MRI obtained at the end of the sixth cycle,
the antitumor effect was determined to be complete response (CR)
(Fig. 1B). Moreover, the serum SCC
antigen level decreased from 2.5 ng/ml before treatment initiation
to 1.2 ng/ml at the end of the sixth cycle. In addition cytological
examination from the uterine cervix showed no evidence of disease.
The observed adverse events are shown in Table I. During the two months since the end
of treatment, no recurrence has been observed.
 | Table I.Adverse events. |
Table I.
Adverse events.
| Adverse event | Case 1 | Case 2 |
|---|
| Leucopenia | Grade 3 | Grade 3 |
| Neutropenia | Grade 4 | Grade 3 |
| Anemia | Grade 1 | Grade 3 |
| Thrombocytopenia | Grade 2 | Grade 1 |
| Nausea | Grade 1 | Grade 1 |
| Vomiting | Grade 0 | Grade 0 |
| Diarrhea | Grade 0 | Grade 0 |
| Fatigue | Grade 1 | Grade 1 |
| Sensory
neuropathy | Grade 0 | Grade 2 |
| Myalgia | Grade 1 | Grade 2 |
| Mucositis | Grade 1 | Grade 0 |
| Hypertension | Grade 0 | Grade 0 |
| Proteinuria | Grade 2 | Grade 0 |
| GI perforation | Grade 0 | Grade 0 |
| Fistula | Grade 0 | Grade 0 |
Case 2
A 52-year-old woman with stage IIB recurrent SCC of
the cervix (unmarried), whose family and medical histories were
unremarkable, underwent radical hysterectomy in June 2005. Because
pelvic lymph node metastasis was detected, postoperative
radiotherapy (WPEBRT at a total dose of 50.4 Gy in 28 fractions)
was added. In 2010, metastatic lesions in the left lung were
resected thoracoscopically. In 2011, she was diagnosed again as
having left lung metastasis, for which left lower lobectomy and
hilar/mediastinal lymphadenectomy were performed. After surgery,
irinotecan (CPT-11) + etoposide therapy was added. In November
2012, metastasis recurred again in the left hilar region, and
paclitaxel + carboplatin (TC) therapy was administered for six
cycles, resulting in complete remission. In April 2013, she was
diagnosed as having brain metastasis involving the left frontal
lobe, for which whole brain radiotherapy (WBRT) was administered at
a dose of 40 Gy in 20 fractions, resulting in complete remission.
In August 2015, liver and para-aortic lymph node metastases were
detected, and she was referred to our department for palliative
treatment. However, because she strongly desired curative
treatment, paclitaxel + cisplatin (TP) therapy was administered for
nine cycles at our hospital. Subsequently, this therapy failed to
induce remission and was switched to CPT-11 + cisplatin therapy.
The administration of three cycles resulted in progressive disease
(PD). Because she requested treatment with bevacizumab, the regimen
was changed to paclitaxel at 135 mg/m2, cisplatin at 50
mg/m2, and bevacizumab at 15 mg/kg. Six cycles were
administered without treatment delay or dose modification. The
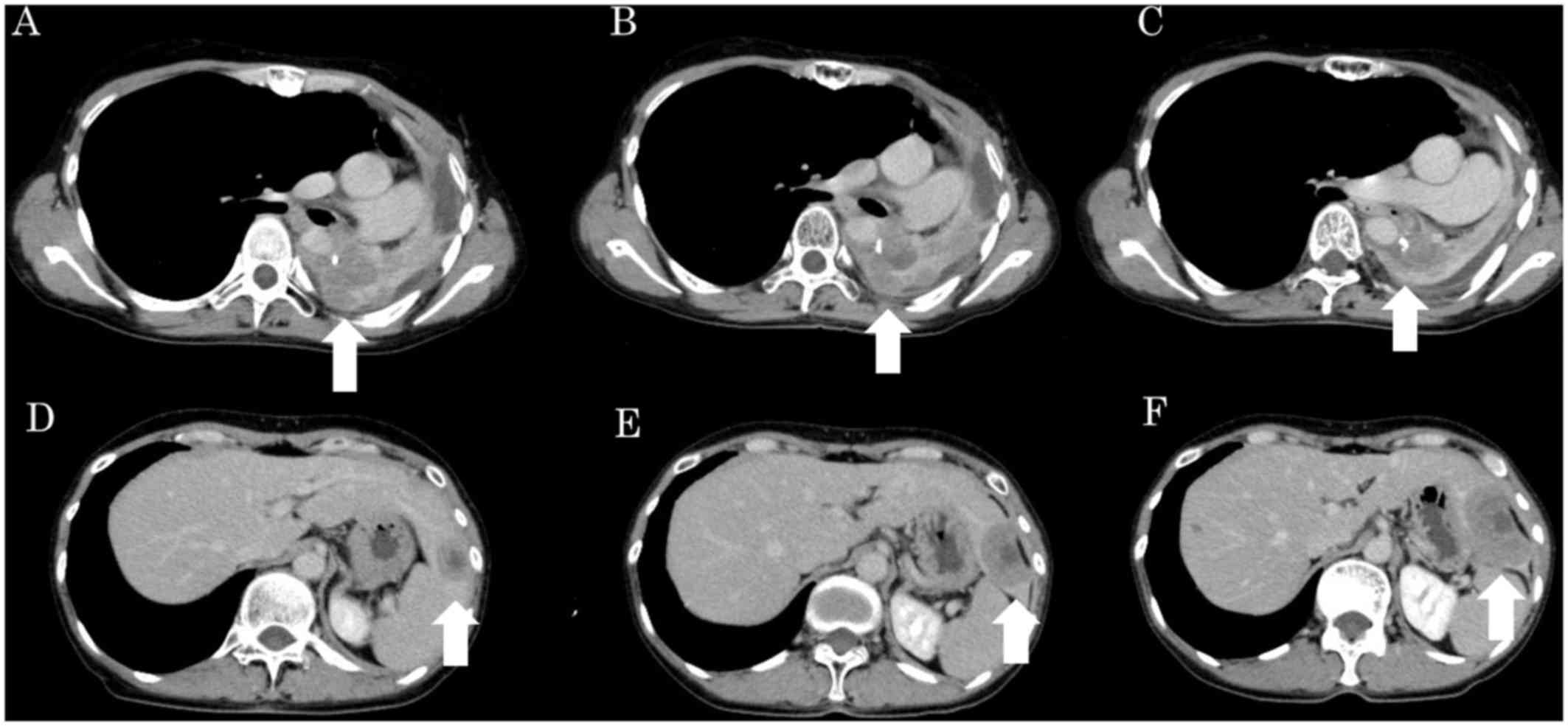
computed tomography (CT) images taken before treatment initiation
and at the end of the third and sixth cycles are shown in Fig. 2. At the end of the third cycle, the
tumor growth rate was 5%, and the antitumor effect was determined
to be stable disease (SD). Although the chemotherapy was continued,
the tumor growth rate was 37% at the end of the sixth cycle, and
the antitumor effect was determined to be PD. Meanwhile, SCC grew
from 1.9 ng/ml before treatment initiation to 1.5 ng/ml at the end
of the third cycle and 3.1 ng/ml at the end of the sixth cycle. The
observed adverse events are shown in Table I. Pegfilgrastim was prophylactically
administered on day 3 of each cycle. As a result, febrile
neutropenia did not occur, although grade 3 neutropenia did
develop. Moreover, celecoxib had been orally administered for
sensory neuropathy and myalgia. When both conditions progressed to
grade 2, daily oral administration of goshajinkigan (TJ107) was
initiated in the fifth cycle. At present, a change in the
chemotherapeutic regimen is required and we are seeking an
effective treatment alternative. Written informed consent was
provided by the two patients prior to publication of the present
case report.
Discussion
One of the serious adverse events caused by
bevacizumab is gastrointestinal perforation (GIP). In clinical
studies on ovarian cancer, namely GOG 218, the International
Cooperative Group for Ovarian Neoplasia (ICON) 7, and Avastin Use
in Platinum-Resistant Epithelial Ovarian Cancer (AURELIA) trials,
the incidences of grade 2 or higher GIP were 1.7, 1.3 and 2.2%,
respectively (8–10). Meanwhile, the incidence in the GOG
240 trial was 2.3% (6). Because no
evidence has been obtained, to date, proving that bevacizumab can
be safely administered to Japanese patients with cervical cancer
who have previously been treated with pelvic radiotherapy, we,
Japanese gynecologists, hesitate to make the decision as to whether
or not bevacizumab should be administered.
In our two cases, bevacizumab combination
chemotherapy was initiated after we provided the patients with a
sufficient explanation that the safety of bevacizumab therapy for
cervical cancer has not been established in Japanese patients
previously treated with radiotherapy and that the antitumor effect
of bevacizumab combination chemotherapy has not been proven. For
recurrent cervical cancer, TP therapy has been demonstrated to be
effective (5). However, the Japan
Clinical Oncology Group (JCOG) 505 trial proved that TC therapy was
non-inferior to TP therapy (11).
Thus, in Case 1, TC therapy combined with bevacizumab was
administered. Case 2 had been treated with five chemotherapeutic
regimens, and each regimen had caused severe hematotoxicity. The
utility of bevacizumab monotherapy for recurrent cervical cancer
has not yet been proven. Therefore, for the treatment of the
patients, we did not administer bevacizumab monotherapy, but
instead administered a combination of paclitaxel, cisplatin and
bevacizumab. We expected that another regimen would also cause
severe hematotoxicity, cisplatin was selected instead of
carboplatin. The dose of paclitaxel was reduced by one level to 135
mg/m3 (3 h) in consideration of previous incidences of
hematotoxicity and peripheral neuropathy due to chemotherapy. In
Case 2, despite the occurrence of grade 3 neutropenia, febrile
neutropenia did not develop due to prophylactic administration of
pegfilgrastim. In Case 1, no granulocyte colony-stimulating factor
preparations were administered. As for antiemetic drugs,
palonosetron and dexamethasone were administered in Case 1 as
described by Takatori et al (12), while a three-drug combination of
aprepitant, palonosetron, and dexamethasone was administered in
Case 2 according to the Multinational Association of Supportive
Care in Cancer/European Society for Medical Oncology Antiemetic
Guidelines (13). As a result,
neither grade 2 or higher nausea nor vomiting was observed. In both
cases, adverse events were manageable despite the administration of
six cycles of this chemotherapy. Although bevacizumab was added,
adequate supportive therapy, as described above, prevented serious
adverse events and allowed six cycles of bevacizumab combination
chemotherapy to be administered as safely as TC or TP therapy.
Because there is as yet no evidence proving that continued
administration of bevacizumab alone after six cycles is effective,
we completed treatment with six cycles.
The most worrisome adverse events associated with
bevacizumab treatment for recurrent cervical cancer are
gastrointestinal and genitourinary fistulas. In the GOG240 trial,
the incidence rates of grade 2 or higher gastrointestinal fistula,
GIP, and genitourinary fistula were 5.0, 2.3, and 3.6%,
respectively. Despite their history of receiving radiotherapy, two
patients in our study did not develop a fistula or GIP.
In Case 1, although she developed grade 2
proteinuria at the start of the second cycle, the chemotherapy was
administered as planned because the urinary protein creatinine
ratio was £3.5. The antitumor effect was determined to be CR in
this patient. Although it was determined to be PD in Case 2 at the
end of the sixth cycle, the best effect achieved was determined to
be SD at the third cycle.
The radiotherapeutic procedure used in Japan is
different from those used in Europe and the United States. In
Japan, a midline block is used when 30 to 40 Gy of external beam
radiation is administered, whereas no midline block is used in
Europe or the United States. As for pelvic radiotherapy, HDR-ICBT
is administered at a dose of 24 Gy in Japan, whereas low dose rate
intra-cavitary brachytherapy (LDR-ICBT) is administered at a dose
of 28 to 30 Gy in Europe and the United States (14,15).
Thus, although radiation doses to the intestinal tract are lower in
Japanese than in Europeans and Americans, the incidence of GIP in
Japan is not necessarily lower than 2.3%, the rate observed in the
GOG 240 trial, because HDR-ICBT is performed.
Furthermore, bevacizumab also reportedly increases
the incidence of GIP in patients with ovarian cancer who have
previously received three or more chemotherapy regimens (16). The safety of bevacizumb with respect
to GIP has not been established for patients with a history of
three or more chemotherapy regimens. Currently, there is no such
concern over cervical cancer. In Case 2, although she had received
four regimens, the bevacizumab combination chemotherapy was
initiated after she had been confirmed to not meet any of the
exclusion criteria for receiving bevacizumab therapy for ovarian
cancer.
In our two cases, bevacizumab combination
chemotherapy was administered for six cycles without causing GIP
and fistula. This indicates that TP or TC therapy combined with
bevacizumab may be an effective treatment strategy for cervical
cancer. To verify that bevacizumab can be administered without
causing GIP and fistula in Japanese patients with cervical cancer
who have been treated with radiotherapy, phase II clinical studies
need to be conducted. Although the Japanese Gynecologic Oncology
Group (JGOG) has designed a clinical study to assess this issue,
the study has not yet been started. The JO29569 study included only
three patients who had previously been treated with pelvic
radiotherapy (7). Although our
report presents only two cases, it is the first description of
Japanese patients with recurrent cervical cancer who had been
treated with radiotherapy and then received six cycles of
bevacizumab combination chemotherapy, with assessment for adverse
events.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TSh and TSu conceived and designed this study. RT,
RM, ET, TN and HO acquired the data and TSh, RT, and ET performed
all the analysis. All authors read and approved the final
manuscript.
Ethics approval and consent for
publication
Not applicable.
Consent for publication
Written informed consent was provided by the two
patients prior to publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sugiyama T, Nishida T, Kumagai S, Nishino
S, Fujivoshi K, Okura N, et al: Combination chemotherapy with
irinotecan and cisplatin as neoadjuvant in locally advanced
cervical cancer. Br J Cancer. 81:95–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabata T, Nishiura K, Yanoh K, Okugawa T,
Obata H, Tanaka K and Toyoda N: A pilot study of neoadjuvant
chemotherapy with mitomycin C, etoposide, cisplatin, and epirubicin
for adenocarcinoma of the cervix. Int J Clin Oncol. 9:59–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curtin JP, Blessing JA, Webster KD, Rose
PG, Mayer AR, Fowler WC Jr, Malfetano JH and Alvarez RD:
Paclitaxel, an active agent in nonsquamous carcinomas of the
uterine cervix: A Gynecologic Oncology Group Study. J Clin Oncol.
19:1275–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shoji T, Kumagai S, Yoshizaki A, Yokoyama
Y, Fujimoto T, Takano T, Yaegashi N, Nakahara K, Nishiyama H and
Sugiyama T: Efficacy of neoadjuvant chemotherapy followed by
radical hysterectomy in locally advanced non-squamous carcinoma of
the uterine cervix: A retrospective multicenter study of Tohoku
Gynecologic Cancer Unit. Eur J Gynaecol Oncol. 33:353–357.
2012.PubMed/NCBI
|
|
5
|
Monk BJ, Sill MW, McMeekin DS, Cohn DE,
Ramondetta LM, Boardman CH, Benda J and Cella D: Phase III trial of
four cisplatin-containing doublet combinations in stage IVB,
recurrent, or persistent cervical carcinoma: A Gynecologic Oncology
Group study. J Clin Oncol. 27:4649–4655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tewari KS, Sill MW, Long HJ III, Penson
RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao
MM, et al: Improved survival with bevacizumab in advanced cervical
cancer. N Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugiyama T, Mizuno M, Aoki Y, Sakurai M,
Nishikawa T, Ueda E, et al: A single-arm study evaluating
bevacizumab, cisplatin, and paclitaxel followed by single-agent
bevacizumab in Japanese patients with advanced cervical cancer. Jpn
J Clin Oncol. 46:1–8. 2016.PubMed/NCBI
|
|
8
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Gynecologic Oncology Group: Incorporation of bevacizumab in
the primary treatment of ovarian cancer. N Engl J Med.
365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: ICON7 Investigators: A phase 3
trial of bevacizumab in ovarian cancer. N Engl J Med.
365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitagawa R, Katsumata N, Shibata T, Kamura
T, Kasamatsu T, Nakanishi T, Nishimura S, Ushijima K, Takano M,
Satoh T, et al: Paclitaxel Plus Carboplatin Versus Paclitaxel Plus
Cisplatin in Metastatic or Recurrent Cervical Cancer: The
Open-Label Randomized Phase III Trial JCOG0505. J Clin Oncol.
33:2129–2135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takatori E, Shoji T, Miura Y, Nagao M,
Takada A, Nagasawa T, Omi H, Kagabu M, Honda T and Sugiyama T: A
phase II clinical trial of palonosetron for the management of
delayed vomiting in gynecological cancer patients receiving
paclitaxel/carboplatin therapy. Mol Clin Oncol. 3:281–286. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dupuis LL, Roscoe JA, Olver I, Aapro M and
Molassiotis A: 2016 updated MASCC/ESMO consensus recommendations:
Anticipatory nausea and vomiting in children and adults receiving
chemotherapy. Support Care Cancer. 25:317–321. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano T, Kato S, Ohno T, Tsujii H, Sato
S, Fukuhisa K and Arai T: Long-term results of high-dose rate
intracavitary brachytherapy for squamous cell carcinoma of the
uterine cervix. Cancer. 103:92–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Erickson B, Eifel P, Moughan J, Rownd J,
Iarocci T and Owen J: Patterns of brachytherapy practice for
patients with carcinoma of the cervix (1996–1999): A patterns of
care study. Int J Radiat Oncol Biol Phys. 63:1083–1092. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cannistra SA, Matulonis UA, Penson RT,
Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D,
Wenham R, et al: Phase II study of bevacizumab in patients with
platinum-resistant ovarian cancer or peritoneal serous cancer. J
Clin Oncol. 25:5180–5186. 2007. View Article : Google Scholar : PubMed/NCBI
|