Introduction
The coexistence of myeloproliferative neoplasms
(MPN) and lymphoproliferative neoplasms (LPN) is a rare finding. In
particular, essential thrombocythemia (ET) and chronic lymphocytic
leukemia (CLL) rarely coexist in the same patient. Patients with a
Philadelphia chromosome (Ph)-negative MPN may develop a
lymphoproliferative disorder (LPD); however, the clinical and
molecular determinants and the chronological onset of the two
events remains unknown (1).
Monoclonal B lymphocytosis (MBL) is defined as the
presence of a clonal B-cell population in the peripheral blood of
<5×109/l and no other signs of an LPD. Based on the
number of clonal B cells, MBL is divided into ‘low-count’
(<0.5×109/l) and ‘high-count’ MBL
(>0.5×109/l). MBL that precedes CLL, has a similar
immunophenotype. It has been demonstrated that the natural history
of CLL is preceded by MBL; however, despite its prevalence of 12%
in the healthy population, MBL progresses to overt CLL/small
lymphocytic lymphoma (SLL) in only 1-2% of the cases annually
(2,3).
CLL/SLL is a disorder of morphologically mature but
immunologically incompetent B lymphocytes. It is defined as
>5×103/µl circulating B lymphocytes with a specific
phenotype expressing CD19, CD5, CD23, CD43 and CD200, and a weak
expression of CD20, CD79b and surface immunoglobulin (4). Key pathways promoting CLL cell
proliferation and survival are activation of B-cell receptor and
nuclear factor-κB pathways (5). CLL,
a mature B-cell neoplasm, represents one of the most frequent LPNs,
and the most common type of leukemia in the elderly (6).
MPNs are known to be clonal hematopoietic stem cell
diseases characterized by overproduction of one or more blood cell
lines, albeit with normal maturation and hematopoiesis. MPNs
represent a group of heterogeneous chronic conditions, in which the
main picture is characterized by medullary proliferation of at
least one myeloid lineage, and increased number of mostly mature
elements in the peripheral blood (7).
According to the literature, concurrent
manifestation of two chronic-stage myeloid and lymphoid neoplasms
in the same patient is a rare condition that appears to account for
<1% of the cases (8). The most
frequent combination appears to be found in patients with
Ph-negative MPN with concurrent B-cell CLL (8).
We herein report the case of a patient with two
hematological malignancies of both lymphoid and myeloid origin.
Case report
We herein report the case of a 64-year-old man with
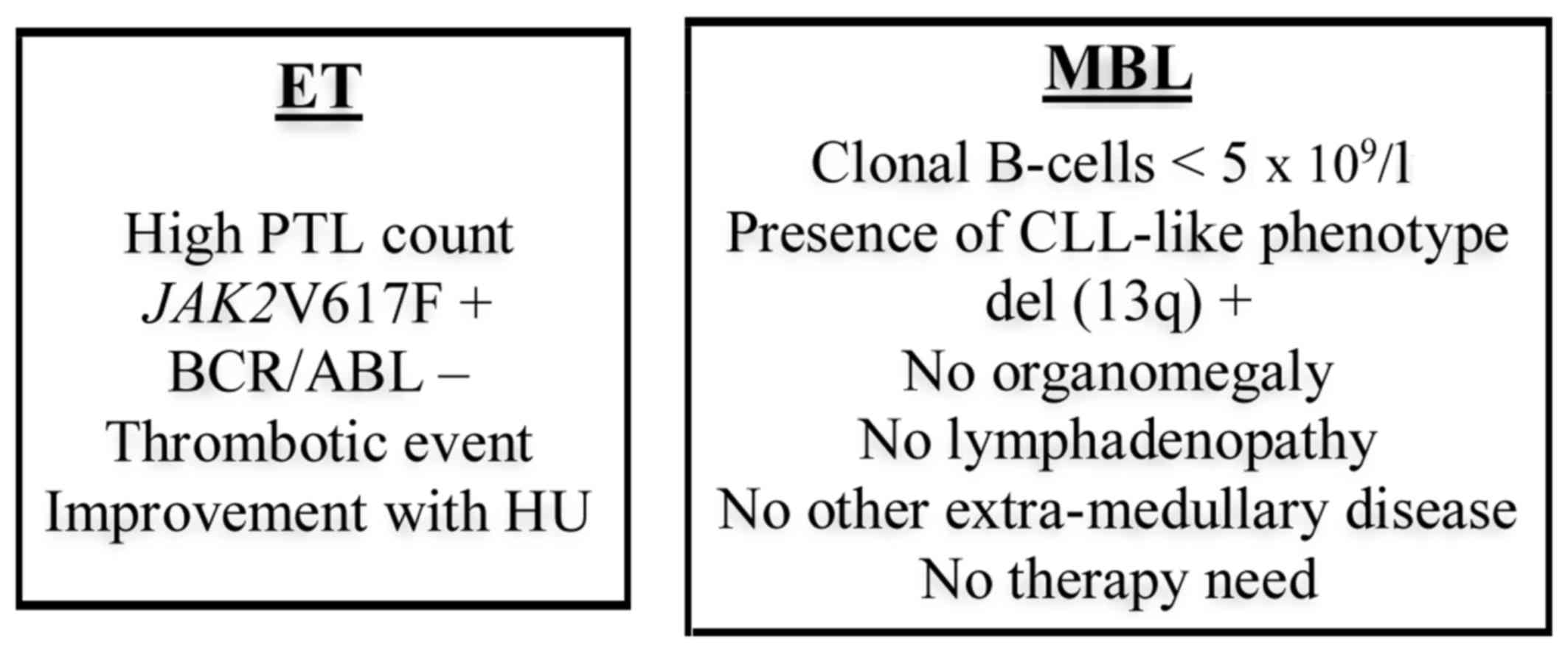
concomitant diagnosis of high-risk ET and MBL/CLL (Fig. 1).
The patient was referred to the Department of
Hematology, Hospital of São Francisco Xavier, West Lisbon Hospital
Centre (Portugal) for a consultation due to thrombocytosis of
700×109/l detected on routine examination. The patient
had a good Eastern Cooperative Oncology Group performance status
(score: 1), but had a personal history of diabetes mellitus type 2
and chronic renal disease, and was followed at the Nephrology
Department. The patient had no history of previous exposure to
myelotoxic drugs or radiation.
During the diagnostic evaluation, in addition to
thrombocytosis, the peripheral blood smear revealed the presence of
lymphocytosis (6.6×109/l), with monomorphic small mature
lymphocytes and smudge cells. The patient refused bone marrow
aspiration and biopsy, so all the tests were performed using
peripheral blood.
The clinical presentation was suggestive of ET
diagnosis, and both JAK2V617F mutation and BCR/ABL
translocation were tested, the former being positive and the latter
negative, which was in agreement with Ph-negative MPN (Fig. 1).
The findings on flow cytometry were consistent with
a typical CLL-like phenotype; however, the absolute number of
clonal B cells was <5×109/l (25.9% pathological
lymphocytes of a total of 16.1×109/l leukocytes).
Moreover, cytogenetic examination was performed, in order to detect
abnormalities characteristic of CLL/SLL (del 11q, del 13q, TP53
gene mutation, del 17p, trisomy 12), and revealed the presence of
del 13q (24%), without other rearrangements, and a normal karyotype
(Fig. 1).
A whole-body computed tomography scan revealed a
massive thrombosis of the left iliac artery. No hepatomegaly,
splenomegaly or lymphadenopathy were identified (Fig. 1).
The patient was started on cytoreductive therapy
with hydroxyurea 500 mg 3 times/week, achieving an absolute
platelet count drop to values within the normal range
(380×109/l).
Concomitantly, the patient was started on
hypocoagulation treatment with a vitamin K antagonist (warfarin
with a 5 mg/day initial dose, regularly adjusted according to
international normalized ratio), for ≥6 months and until complete
resolution of the thrombi.
This patient is currently on surveillance and did
not develop other thrombotic events, exhibiting clinical
improvement with moderately reduced claudication during the course
of treatment.
Discussion
It remains unclear whether there is a specific
mechanism connecting the two events described herein, in terms of
whether there is a molecular association between the two, or if
they are two independent events developing simultaneously in
genetically predisposed individuals.
There is no clear evidence of the pathogenetic
association between myeloproliferative and lymphoproliferative
diseases. However, given the higher risk of LPN development in MPN
patients reported in larger studies, the genomic instability
characteristic of MPNs may play a role in the subsequent LPN
occurrence (9).
Since both groups of diseases usually have a slowly
progressive, indolent course, which may continue for decades, it
may be difficult to establish the chronological onset of the
occurrence of the two diseases in the same patient.
One hypothesis includes the possibility of the two
diseases originating from common progenitors; this hypothesis would
be supported by the presence of the JAK2V617F mutation in
both myeloid cells and B lymphocytes (10).
Another hypothesis is that these are two independent
diseases occurring from different progenitors, and having different
etiopathogenetic routes. To support this hypothesis, the criteria
should include absence of the JAK2V617F mutation from the
lymphoid cells, as reported previously (11,12).
It is generally accepted that myeloid and lymphoid
neoplasms emerge and progress independently. However, there are
difficulties when trying to identify a biclonal origin of the two
lymphoid and myeloid clones, evidence of which is still lacking
(4).
Although an association may be found incidentally,
the hypothesis that the two neoplasms may be related requires a
close look at the two clones involved.
There is evidence of lymphoproliferative and
myeloproliferative events running consistently through the same
family. Such evidence includes the occurrence of B-cell
malignancies and ET in different generations of the same family
(13).
There are already some references in the literature
supporting a common progenitor, as in Tabaczewski et al
(14), which have reported the
hypothesis that there may be an initial ‘trigger hit’ occurring in
a pre-JAK2 common early progenitor multipotential
hematopoietic stem cell that can differentiate into both lymphoid
and myeloid pathways, and subsequent additional molecular events
that could promote myeloid and lymphoid differentiation, leading to
the development of two diseases of likely identical origin, but
different lineages.
In-depth knowledge of the origin and nature of the
concomitance of these two events is currently lacking. To date,
there is no evidence supporting the presence of a common and unique
stem cell capable of giving rise to both leukemic and myeloid
clones.
The sparse number of cases of simultaneous diagnoses
of MPNs and LPNs explains the difficulty in finding consistent
explanations that may apply to all cases.
Further studies are required to elucidate the
molecular pathogenesis of these underlying events. There remains
the question of how to treat efficiently these patients when the
two conditions progress simultaneously, and whether there should be
different indications regarding the timing of the treatment.
The occasional reports of ET coexisting with MBL/CLL
in the same patient do not allow a precise understanding of the
origin or proof of whether the two malignancies share a common
etiopathogenesis.
In conclusion, it would be interesting to compare
genetic biomarkers of the two diseases in both identified clones,
in order to report whether they share any common pathways. More
studies are required to evaluate the genomic link between these two
diseases and to elucidate whether their concomitance is
coincidental, or if there is an association between these two
entities.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FM contributed to the conception and design of the
study, drafted and wrote the manuscript, and revised it critically
for important intellectual content; PSS and APA contributed to the
conception and design of the study, analysis and interpretation of
the data, and revised the paper; JMP, RL, SM, JFV and FL analyzed
the data and revised the paper. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Patient anonymity and consent were guaranteed, in
accordance with the Declaration of Helsinki.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marchetti M, Carobbio A, Capitoni E and
Barbui T: Lymphoproliferative disorders in patients with chronic
myeloproliferative neoplasms: A systematic review. Am J Hematol.
93:698–703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strati P and Shanafelt TD: Monoclonal
B-cell lymphocytosis and early-stage chronic lymphocytic leukemia:
Diagnosis, natural history and risk stratification. Blood.
126:454–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duarte S, Pereira SC, Rodrigues É and
Pereira A: Concomitant chronic myeloid leukemia and monoclonal B
cell lymphocytosis-a very rare condition. Rev Bras Hematol Hemoter.
39:167–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herishanu Y, Pérez-Galán P, Liu D,
Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E,
Stennett L, et al: The lymph node microenvironment promotes B-cell
receptor signaling, NF-kappaB activation and tumor proliferation in
chronic lymphocytic leukemia. Blood. 117:563–574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shanafelt TD, Byrd JC, Call TG, Zent CS
and Kay NE: Narrative review: Initial management of newly
diagnosed, early-stage chronic lymphocytic leukemia. Ann Intern
Med. 145:435–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones AV, Kreil S, Zoi K, Waghorn K,
Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, et al:
Widespread occurrence of the JAK2 V617F mutation in chronic
myeloproliferative disorders. Blood. 106:2162–2168. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hauck G, Jonigk D, Kreipe H and Hussein K:
Simultaneous and sequential concurrent myeloproliferative and
lymphoproliferative neoplasms. Acta Haematol. 129:187–196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trifa AP, Cucuianu A, Popp RA, Paţiu M,
Selicean C, Militaru MS and Pop IV: Concomitant myeloproliferative
and lymphoid neoplasms in two patients positive for JAK2 V617F
mutation. Case report and literature review. Indian J Hematol Blood
Transfus. 30 Suppl 1:S120–S123. 2014. View Article : Google Scholar
|
|
10
|
Kodali S, Chen C, Rathnasabapathy C and
Wang JC: JAK2 mutation in a patient with CLL with coexistent
myeloproliferative neoplasm (MPN). Leuk Res. 33:e236–e239. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henry L, Carillo S, Jourdan E, Arnaud A,
Brun S and Lavabre-Bertrand T: Association of essential
thrombocythemia and chronic lymphocytic leukemia: Absence of the
V617F JAK2 mutation in the lymphoid compartment. Am J Hematol.
82:500–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hussein K, Brakensiek K, Ballmaier M,
Göhring G, Buhr T, Bock O and Kreipe H: B-CLL developing in a
patient with PV is not affected by V617F mutation of the Janus
kinase 2. Eur J Haematol. 77:539–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gaillard JB, Carillo S, Henry L, Jourdan E
and Lavabre-Bertrand T: Association of myeloproliferative and
lymphoproliferative disorders. Clin Adv Hematol Oncol. 10:756–757.
2012.PubMed/NCBI
|
|
14
|
Tabaczewski P, Nadesan S and Lim SH:
Zap-70 positive chronic lymphocytic leukemia co-existing with Jak 2
V671F positive essential thrombocythemia: A common defective stem
cell? Leuk Res. 33:854–855. 2009. View Article : Google Scholar : PubMed/NCBI
|