Introduction
Malignant melanoma of the penis is extremely rare,
accounting for <1.4% of all primary penile malignant lesions and
0.1–0.2% of all extraocular melanomas (1,2).
Malignant melanoma of the penis occurs most frequently on the glans
penis (55%), followed by the prepuce (28%), penile shaft (9%) and
urethral meatus (8%) (3). As
malignant melanoma of the penis is rare, the pathogenesis and risk
factors have not been well established. At the time of the first
visit, malignant melanoma of the penis is often in an advanced
stage, and ~50% of the patients develop metastatic lesions to the
inguinal region (4). The primary
treatment of melanoma of the penis and urethra is surgical,
although there is a lack of consensus regarding the extent of
treatment that is indicated (5). We
herein present the case of a patient with malignant melanoma of the
penile foreskin who underwent circumcision for histological
diagnosis and treatment.
Case report
A 71-year-old Japanese male patient presented to the
Department of Urology, Okayama University Hospital (Okayama, Japan)
with two non-healing lesions on the penile foreskin, which had been
growing gradually for 5 years and had enlarged over the last year.
The patient's medical and family history was unremarkable.
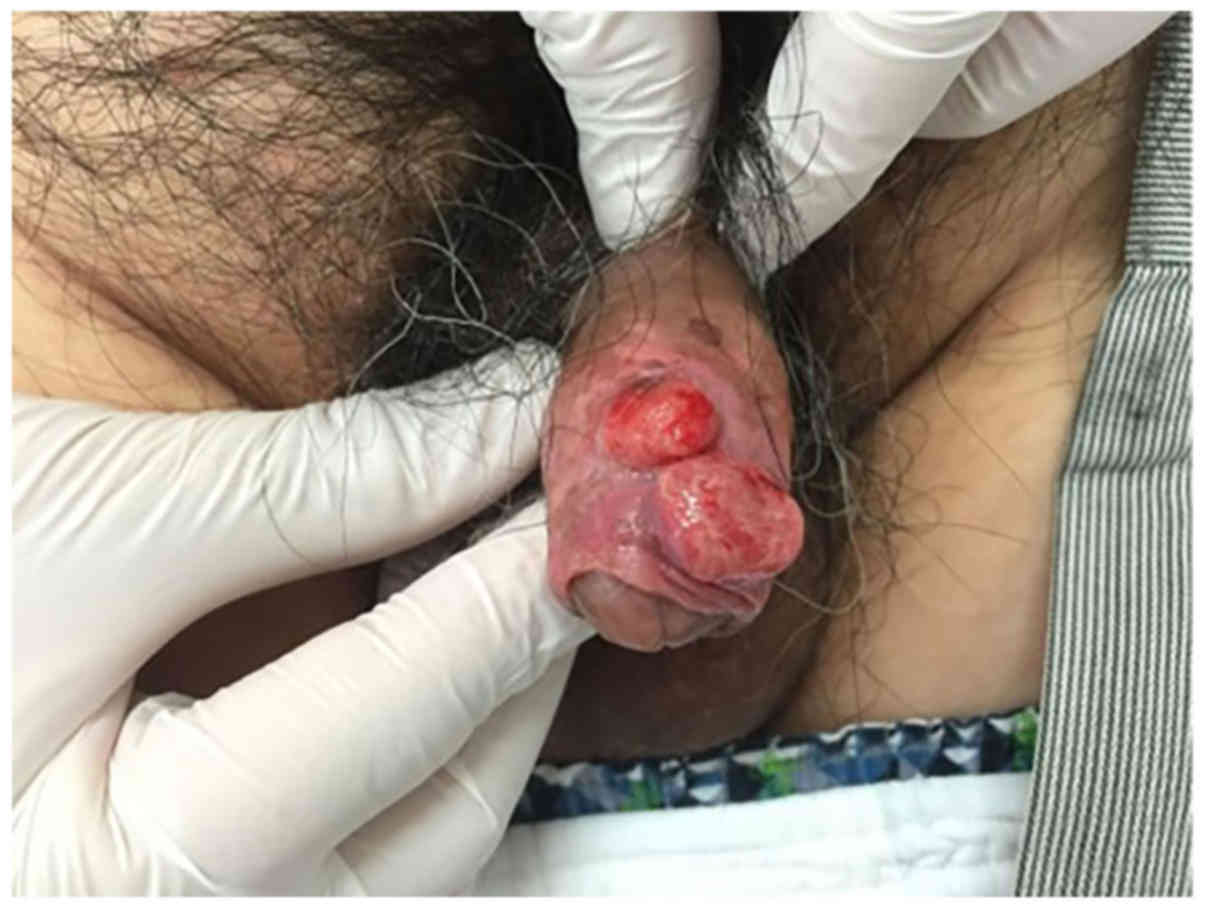
On physical examination, two hemorrhagic
red-pigmented lesions and an ulcer on the penile foreskin were
observed (Fig. 1). No metastasis was
evident on computed tomography (CT) and magnetic resonance imaging,
and the foreskin had good mobility; thus, the lesions were
considered to be confined to the foreskin.
Segmental circumcision was performed for
histological diagnosis and treatment. The histological examination
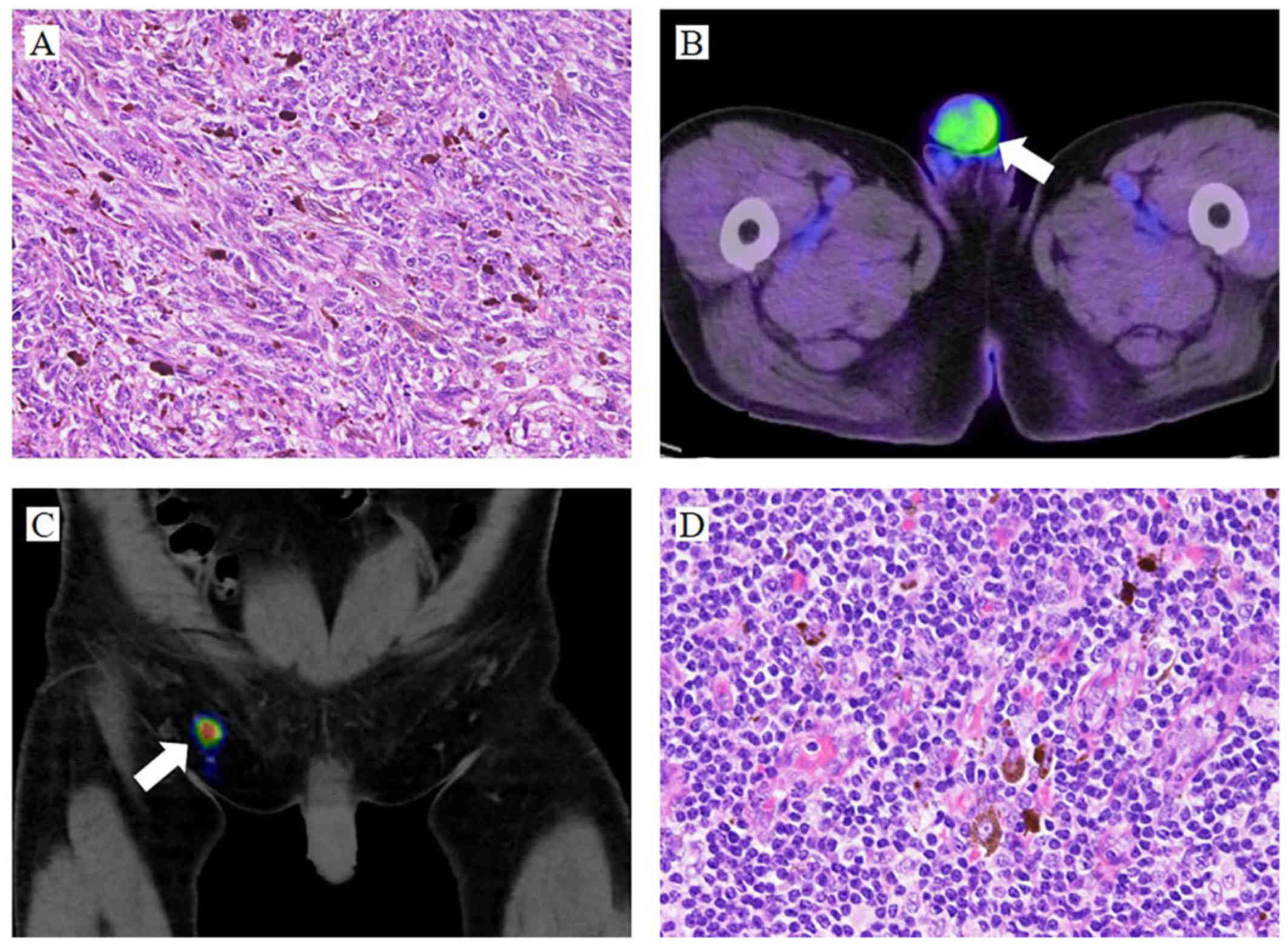
revealed a malignant melanoma. The atypical melanocytic cells
proliferated in the dermis and invaded into the basal cells of the
epidermis (Fig. 2A). The tumor
thickness was 11 mm, and the surface was ulcerated. According to
the classification of the Union for International Cancer Control,
the tumor was stage IIC (pT4bN0M0). No tumor cells were detected on
the resection margins of the specimens; however, the distance of
the lesion from the margins was <1 cm. In addition, positron
emission tomography (PET)/CT revealed increased uptake of
18F-fluorodeoxyglucose in the penis (standardized uptake
value=3.23; Fig. 2B). Considering
the possibility of the residual tumor or metastasis, an extended
circumcision and right sentinel lymph node biopsy (SLNB) were
performed following sentinel lymph node scintigraphy with
Tc-99m-phytate (Fig. 2C). There were
no histological findings consistent with residual tumor or
metastasis (Fig. 2D). Adjuvant
interferon treatment was initiated, although it was discontinued
due to intense pain associated with the subcutaneous injection. The
patient remained alive and had no recurrence at the 2-year
follow-up evaluation on January 2018.
Discussion
Primary malignant melanoma of the penis is extremely
rare. Malignant melanoma is one of the most aggressive tumors, as
it can metastasize to any tissue or organ without symptoms. The
incidence of melanoma is rapidly increasing, with an overall rate
of 33% in men and 23% in women between 2002 and 2006 (6). As a primary lesion, melanoma of the
penis accounts for <0.2% of all melanomas and 1.4% of all
primary penile malignant lesions (1,2). Penile
malignant melanoma most frequently involves the glans penis (55%),
followed by the prepuce (28%), penile shaft (9%) and urethral
meatus (8%) (3). Malignant melanoma
of the penis is mainly a disease of the elderly; specifically, men
aged 50–70 years are most frequently diagnosed with this disease.
By contrast, cutaneous melanomas in other areas of the body are
mostly diagnosed in the 40–49-year age group. The 2- and 5-year
overall survival (OS) rates for malignant melanoma of the penis are
63 and 31%, respectively, while the 5-year OS rate is ≥50% for
cutaneous melanomas (7,8). This difference in OS may be attributed
to metastasis at the initial visit. Although 84% of patients with
cutaneous melanomas initially present with localized disease,
43–62% of patients with melanoma of the penis present with lymph
node involvement. As penile lesions are a sensitive subject for
men, there is usually a delay in seeking medical advice until the
lesions enlarge (9); thus, diagnosis
is usually delayed.
The typical melanoma is black in color; however,
melanomas may occasionally have a red or brown appearance, as in
the present case. Red melanomas are frequently diagnosed as
amelanotic melanomas (AMs), the incidence of which is estimated to
be between 1.8 and 8.1% of all melanoma cases (10). AMs lack pigment. The histological
examination in the present case revealed minimal deposition of
melanin pigment in the tumor cells; therefore, the pathological
findings in our case were similar to those of an AM. Melanomas are
divided into the following four clinical subtypes: Lentigo maligna
melanoma; superficial spreading melanoma; nodular melanoma; and
acral lentiginous melanoma. Among these subtypes, acral lentiginous
melanoma is most common in Japan, and usually develops in
non-sun-damaged sites, such as the soles, palms, or subungual
areas. Our patient was diagnosed with a nodular melanoma, which is
characterized by the highest malignant potential among all
subtypes, with a large depth of invasion and frequent metastasis
(11).
Prediction of the clinical course of melanoma is
mainly based on tumor thickness; however, the assessment of tumor
thickness alone is not sufficient. Other important factors
associated with the prognosis of melanomas include tumor diameter
(≥15 mm), extent of involvement of local structures, and whether
there is evidence of metastases to the inguinal or pelvic lymph
nodes (12). The American Joint
Committee on Cancer (AJCC) staging protocol for melanoma, which is
currently the most widely accepted, confirms that tumor thickness
and ulceration are the most important predictors in the TNM
classification (8). Our patient was
diagnosed with AJCC stage IIC (pT4bM0N0) melanoma of the penis.
The primary treatment of melanoma of the glans penis
and urethra is surgery; however, the main area of controversy lies
with the extent of surgery for localized disease (13). In the 1970s and 1980s, some authors
suggested an aggressive surgical approach, with total amputation of
the penis, perineal urethrostomy, and radical inguinal, iliac and
obturator lymph node dissection (14). A recent study, however, recommends a
more conservative, wide local excision (15). Several prospective randomized trials
have been conducted to define the surgical margins for melanomas,
and it was concluded that margins >2 cm are not associated with
a superior OS, regardless of the melanoma thickness (16,17).
Based on the 2017 National Comprehensive Cancer Network (NCCN)
guidelines, a 2.0-cm clinical margin is recommended when the tumor
is >2.0 mm in thickness. In our patient, the foreskin maintained
good mobility; therefore, the tumor was considered to be confined
to the penile foreskin and a segmental circumcision was
recommended. However, the clinical margin was <1.0 cm; thus, the
resection was extended to 1.5 cm.
Elective lymph node dissection is not recommended in
patients with malignant melanoma of the penis due to the low
probability of positive findings (20%), and lack of impact on the
OS compared with expectant management (18). By contrast, SLNB is considered to be
useful in the evaluation of melanomas with a thickness of ≥1 mm
according to the NCCN guidelines. In the case of very thin
melanomas (IA or B), the positive SLNB rate is low (≤5%) and is not
associated with a significant benefit, while thicker tumors (≥4 mm)
have a 34.4% positive rate (19). To
identify the sentinel lymph node, lymph node scintigraphy prior to
biopsy is useful (20). If the SLNB
is negative, regional lymph node dissection is not necessary. As
the tumor was thicker (11 mm) in the present case, SLNB was
performed to detect metastasis following sentinel lymph node
scintigraphy with Tc-99m-phytate.
The prognosis of stage II and III disease is poor
due to the lack of effective systemic chemotherapy. It has been
reported that six cycles of combination chemotherapy (decarbazine,
carmustine, cisplatin and tamoxifen) achieve an overall response
rate of 45% and a complete response rate of 12–14% (21). It is considered that 5% of patients
with stage III melanoma may be curable (21,22).
Several trials have demonstrated that treatment with high doses of
adjuvant interferon in high-risk melanoma reduced the risk of
recurrence and prolonged the median disease-free survival (23,24). The
Eastern Cooperative Oncology Group trial E1684 demonstrated that an
adjuvant high-dose interferon regimen increased the median
relapse-free survival (RFS) from 1 to 1.7 years (P=0.0023), and the
OS from 2.8 to 3.8 years (P=00237) compared with observation alone
(23). Ipilimumab, which is a
monoclonal antibody targeting the immune checkpoint receptor
CTLA-4, has also been approved for adjuvant treatment of patients
with completely resected stage III melanoma, with reported
improvement of progression-free survival and OS (25). Adjuvant radiation therapy decreases
lymph node field recurrence; however, RFS or OS exhibited no
statistically significant differences, whereas grade 2–4 toxicities
occurred frequently (26). In our
patient, the melanoma was thicker and considered to have a high
risk of recurrence; therefore, interferon was attempted as adjuvant
chemotherapy.
As late recurrences (>10 years after diagnosis)
are well-documented, it is recommended that all melanoma patients
undergo skin examinations and surveillance at least once a year for
life according to the NCCN guidelines. Among patients with stage
IIB-IV melanomas, a comprehensive history and physical examination
with specific emphasis on the regional nodes and skin should be
undertaken every 3–12 months for 5 years, and annually thereafter,
as clinically indicated.
Malignant melanomas of the penis, particularly the
penile foreskin, are extremely rare. Early detection and diagnosis
are associated with a good prognosis, as early melanoma is curable.
Thus, early and appropriate aggressive surgical therapy following
effective adjuvant therapy is recommended. In the present case,
melanoma was not diagnosed at the initial examination due to the
red color. However, physicians should bear in mind that the
clinical presentation of malignant melanomas of the penis may vary
greatly. The present case may assist physicians reach an accurate
diagnosis in future cases of malignant melanoma of the penile
foreskin.
Acknowledgements
The authors would like to thank the clinical
laboratory technicians of Okayama University Hospital for their
technical support.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YM wrote the manuscript. TS was a major contributor
to writing the manuscript. YM collected imaging data. KW and RT
conceived and designed the study. YK performed the surgery. TW
performed the patient's examination. MW and YN critically revised
the manuscript for intellectual content. The final version of the
manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Stillwell TJ, Zincke H, Gaffey TA and
Woods JE: Malignant melanoma of the penis. J Urol. 140:72–75. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brady KL, Mercurio MG and Brown MD:
Malignant tumors of the penis. Dermatol Surg. 39:527–547. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tallerman A: Malignant melanoma of the
penis. Urol Int. 27:66–80. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rogers RS III and Gibson LE: Mucosal,
genital, and unusual clinical variants of melanoma. Mayo Clin Proc.
72:362–366. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hankins CL, Kotwal S, Majumder S, Weston
P, Phipps A and Anathhanam AJ: Multifocal melanoma of the glans
penis. Plast Reconstr Surg. 118:33e–38e. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Saraiya M, Patel P, Cherala SS,
Barnholtz-Sloan J, Kim J, Wiggins CL and Wingo PA: Recent trends in
cutaneous melanoma incidence and death rates in the United States,
1992–2006. J Am Acad Dermatol. 65 5 Suppl 1:S17–S25.e1-3. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Geel AN, den Bakker MA, Kirkels W,
Horenblas S, Kroon BB, de Wilt JH, Eggermont AM, Mooi WJ and van
der Aa MN: Prognosis of primary mucosal penile melanoma: A series
of 19 Dutch patients and 47 patients from the literature. Urology.
70:143–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ortega FT, Kondo RN, Belinetti FM, Okamura
MO and Tuma B: Primary cutaneous amelanotic melanoma and
gastrointestinal stromal tumor in synchronous evolution. An Bras
Dermatol. 92:707–710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kibbi N, Kluger H and Choi JN: Melanoma:
Clinical presentations. Cancer Treat Res. 167:107–129. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Giorgi V, Grazzini M, Massi D, Rossari
S, Gori A, Janowska A, Bruscino N and Lotti T: Melanoma of the
penis: A clinical dermoscopic case study. Acta Derm Venereol.
90:87–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manivel JC and Fraley EE: Malignant
melanoma of the penis and male urethra: 4 case reports and
literature review. J Urol. 139:813–816. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bracken RB and Diokno AC: Melanoma of the
penis and the urethra: 2 case reports and review of the literature.
J Urol. 111:198–200. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fenn NJ, Johnson RC, Sharma AK, Attanoos
RL and Horgan K: Malignant melanoma of the penis. Eur J Surg Oncol.
22:548–549. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayes AJ, Maynard L, Coombes G,
Newton-Bishop J, Timmons M, Cook M, Theaker J, Bliss JM, Thomas JM;
UK Melanoma Study Group, ; et al: Wide versus narrow excision
margins for high-risk, primary cutaneous melanomas: Long-term
follow-up of survival in a randomised trial. Lancet Oncol.
17:184–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
>Thomas JM, Newton-Bishop J, A'Hern R,
Coombes G, Timmons M, Evans J, Cook M, Theaker J, Fallowfield M,
O'Neill T, et al: Excision margins in high-risk malignant melanoma.
N Engl J Med. 350:757–766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
>Cascinelli N, Morabito A, Santinami M,
MacKie RM and Belli F: Immediate or delayed dissection of regional
nodes in patients with melanoma of the trunk: A randomised trial.
WHO melanoma programme. Lancet. 351:793–796. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
>Han D, Zager JS, Shyr Y, Chen H, Berry
LD, Iyengar S, Djulbegovic M, Weber JL, Marzban SS, Sondak VK, et
al: Clinicopathologic predictors of sentinel lymph node metastasis
in thin melanoma. J Clin Oncol. 31:4387–4393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
>Leijte JA, Kroon BK, Valdés Olmos RA,
Nieweg OE and Horenblas S: Reliability and safety of current
dynamic sentinel node biopsy for penile carcinoma. Eur Urol.
52:170–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reintgen D and Saba H: Chemotherapy for
stage 4 melanoma: A three-year experience with cisplatin, DTIC,
BCNU, and tamoxifen. Semin Surg Oncol. 9:251–255. 1993.PubMed/NCBI
|
|
22
|
Li Y, Yuan H, Wang A, Zhang Z, Wu J and
Wei Q: Malignant melanoma of the penis and urethra: One case
report. World J Surg Oncol. 12:3402014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kirkwood JM, Ibrahim JG, Sondak VK,
Richards J, Flaherty LE, Ernstoff MS, Smith TJ, Rao U, Steele M and
Blum RH: High- and low-dose interferon alfa-2b in high-risk
melanoma: First analysis of intergroup trial E1690/S9111/C9190. J
Clin Oncol. 18:2444–2458. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirkwood JM, Ibrahim JG, Sosman JA, Sondak
VK, Agarwala SS, Ernstoff MS and Rao U: High-dose interferon
alfa-2b significantly prolongs relapse-free and overall survival
compared with the GM2-KLH/QS-21 vaccine in patients with resected
stage IIB-III melanoma: Results of intergroup trial
E1694/S9512/C509801. J Clin Oncol. 19:2370–2380. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Adjuvant ipilimumab versus placebo after
complete resection of high-risk stage III melanoma (EORTC 18071): A
randomised, double-blind, phase 3 trial. Lancet Oncol. 16:522–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Henderson MA, Burmeister BH, Ainslie J,
Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon K, Scolyer RA,
Carruthers S, et al: Adjuvant lymph-node field radiotherapy versus
observation only in patients with melanoma at high risk of further
lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG
02.01): 6-year follow-up of a phase 3, randomised controlled trial.
Lancet Oncol. 16:1049–1060. 2015. View Article : Google Scholar : PubMed/NCBI
|