Introduction
Psoriasis is a chronic immune-mediated disease
characterized by thick red cutaneous lesions that are covered by
gray or silvery white patches that tend to peel. Plaque psoriasis
is the most common form, affecting ~90% of patients with psoriasis.
Psoriatic lesions typically present as raised areas of erythematous
skin covered with white scales. The lesions can appear throughout
the body, although the scalp, elbows, lower back, navel and knees
are the most common sites of involvement (1).
The introduction of biological agents has
revolutionized the management of moderate-to-severe psoriasis, and
these drugs are considered as second-line therapy following
administration of first-line systemic medications to control the
disease. Since the introduction of the first drug belonging to this
family [an anti-tumour necrosis factor-α (TNF-α) indicated for
psoriasis in adults], several other agents have become available
(2).
Adalimumab is one of the most extensively
investigated biological agents. This drug is indicated for the
treatment of adults with moderate-to-severe disease manifesting as
plaques that are refractory to treatment, or is used in patients
with contraindications or intolerance to other systemic therapies,
including cyclosporin, methotrexate or psoralen and ultraviolet A
therapy (3).
Adalimumab is administered as a biweekly
subcutaneous injection. Its use is associated with a high risk of
infection, particularly tuberculosis (as is observed with the use
of other TNF-α inhibitors) (3). The
evaluation of the risk of malignancy in patients with psoriasis
receiving treatment with adalimumab and other TNF-α inhibitors may
be challenging due to the following reasons: i) Possible
dysregulation of the immune system is known to occur in these
patients; ii) the patients may receive other immunomodulatory
drugs; iii) the duration of exposure to these drugs varies between
patients; and iv) only few reports in the literature have described
TNF-α inhibitor-induced tumours (4).
Case report
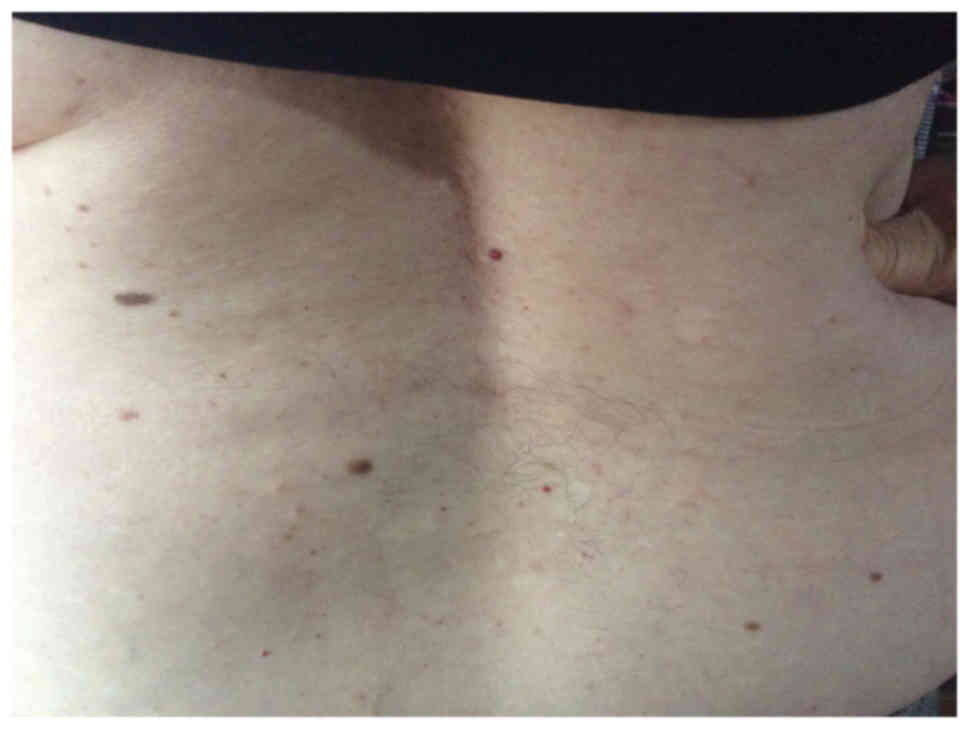
A 47-year-old woman was diagnosed with psoriasis 20
years prior to presentation. The patient was treated with
methotrexate, phototherapy and other topical treatments, and had
been receiving adalimumab since 2009. Following remission,
psoriasis has been completely controlled from 2016 onwards. In the
beginning of 2017, a lesion compatible with an intramuscular myxoma
was detected in the patient's right gluteus maximus on magnetic
resonance imaging examination (Fig.
1). At that time, adalimumab administration was discontinued.
Subsequently, an echocardiography-guided percutaneous biopsy was
performed, and a low-grade myxoid tumour was initially diagnosed.
In May 2017, surgery was performed under spinal anaesthesia, with
wide resection of the tumour. The resected tissue specimen measured
7×7×9 cm; a longitudinal section revealed a whitish, shiny and
gelatinous pseudotrabeculated tumour sized 4×3.5×3.7 cm. The tumour
was well-delineated and encapsulated with a tendency for
spontaneous enucleation. The tumour was surrounded by muscle and
adipose tissue. The patient was diagnosed with low-grade (grade 1)
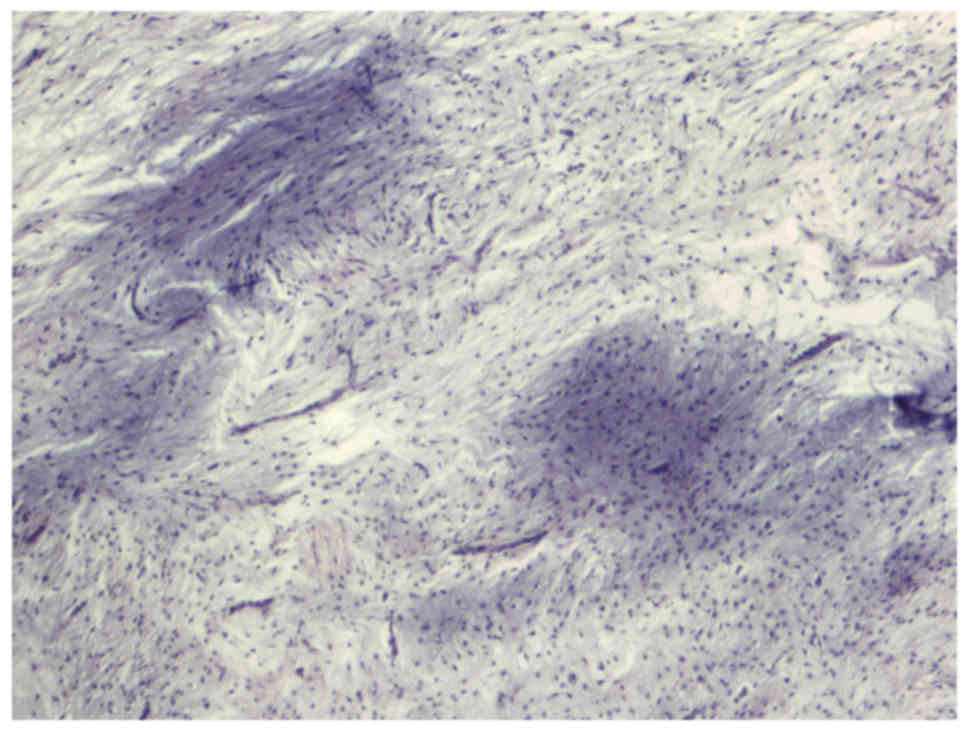
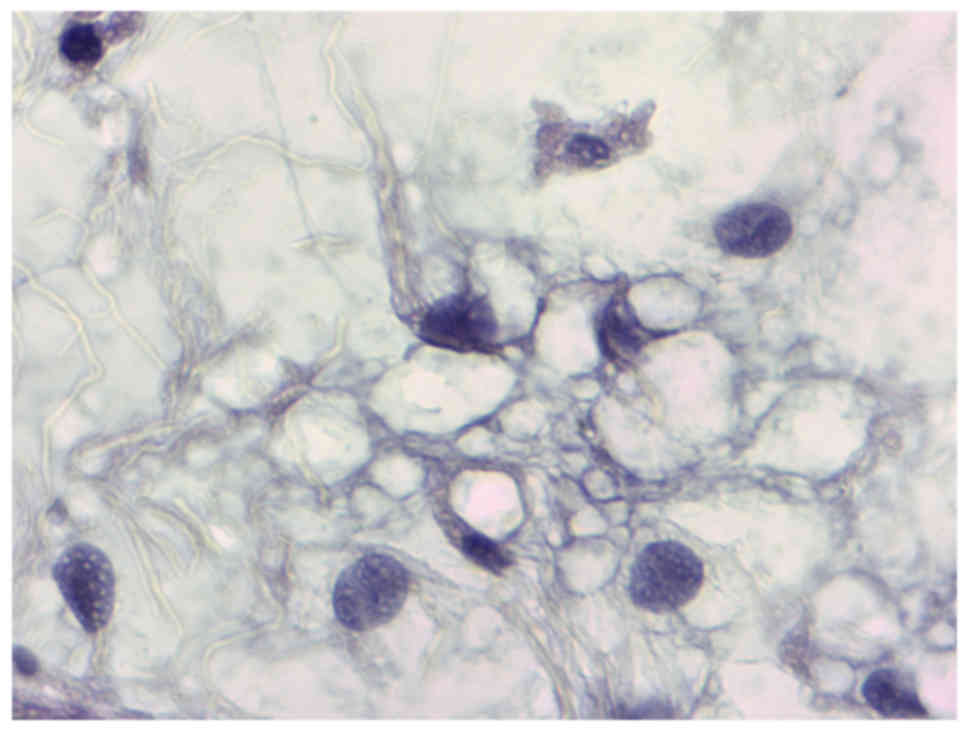
myxoid liposarcoma. Histopathological examination of the tumour
revealed a myxoid mesenchymal neoplasm with variable cell density
between mild and moderate and fusiform cells showing a non-specific
distribution pattern (Figs. 2 and
3). Immunohistochemistry
demonstrated that the Ki67 proliferative index was 1%, and p53
expression was not observed. The patient received radiotherapy
between July and September 2017. After time activity curve
simulation, three-dimensional planning was performed to administer
a 60-Gy dose into the surgical bed with wide margins, in 2 phases.
During the first phase, an additional 46 Gy was administered into
the surgical bed with wide margins. During the second phase, an
additional 14 Mv was administered into the surgical bed with the
lower margins. The patient tolerated the treatment well with no
significant toxicity. Regarding the follow-up, the patient is still
periodically monitored by the Dermatology and Radiotherapy Service
and there has been no recurrence data in the last monitored
follow-up.
Discussion
Psoriasis is an autoimmune chronic inflammatory
disease of the skin that manifests as oedematous and inflamed
cutaneous lesions, with a variable clinical presentation and
aetiology (5), which is associated
with a high risk of skin cancer development (6).
Treatment for psoriasis ranges from topical
preparations, such as corticosteroid-based ointments, to systemic
therapy, including methotrexate, retinoids, cyclosporin A or
phototherapy, which may be used as combination therapy or
monotherapy. However, these aforementioned therapeutic options have
limited efficacy and are associated with adverse effects (7). Thus, in recent years, the use of
biological agents, such as TNF-α inhibitors (e.g., adalimumab) is
becoming increasingly popular.
Recently, a class of drugs referred to as biological
agents (or biologics) has been developed to treat several diseases,
including cancer, multiple sclerosis, diabetes, rheumatoid
arthritis, and/or psoriasis (6). The
active ingredient in these biological agents is usually a protein
(e.g., anti-TNF-α) that exerts a therapeutic effect. Thus, these
agents mimic the actions of proteins and biological processes in
the host, suppress the symptoms of the disease and prevent or delay
its evolution. However, the use of these immunomodulatory agents is
associated with a high risk of serious infections and
neoplasms.
Despite evidence-based studies supporting its
efficacy, adalimumab is known to be associated with the development
of skin tumours in a proportion of the patients.
A previously published report described a case of
adalimumab-induced epithelioid myxofibrosarcoma in a patient with
psoriatic synovitis. The 65-year-old patient described in that
report was treated for psoriasis with methotrexate and etanercept
over 10 years. The disease was poorly controlled with this regimen,
and adalimumab therapy was initiated. Following 20 months of
adalimumab therapy, the patient developed a myxofibrosarcoma at the
site of the adalimumab injections (8). Another report described the development
of a Merkel cell carcinoma in a patient with rheumatoid arthritis
who received adalimumab, methotrexate and prednisone (9). A similar case report described a
61-year-old patient with rheumatoid arthritis who was treated with
leflunomide and methylprednisolone between July 2005 and February
2008. Following initiation of adalimumab therapy, a lesion
corresponding to Kaposi's sarcoma was detected in November 2008.
Adalimumab was withdrawn in November 2009, and the patient was
switched to low-dose corticosteroid treatment. By June 2010,
complete resolution of the papulonodular lesions was observed
(10). Another 67-year-old patient
with rheumatoid arthritis developed Kaposi's sarcoma following
treatment with adalimumab. The patient was initially treated with
methotrexate and etanercept and achieved a 2-year remission.
Following a psoriatic flare-up, etanercept was discontinued and
adalimumab was added to the methotrexate regimen. Two months later,
the patient developed skin lesions that were compatible with
Kaposi's sarcoma (11).
The present case may be of interest to clinicians
as, to the best of our knowledge, this is the first report of
myxoid liposarcoma associated with adalimumab treatment. As
mentioned above, several clinical reports have described some type
of tumour associated with the use of biological agents; however,
there is currently lack of evidence to support a possible
association between the administration of anti-TNF-α agents and the
risk of cancer (12).
Large-scale studies are warranted in the future to
conclusively establish a direct (if any) association between tumour
development and the administration of adalimumab.
Acknowledgements
The authors would like to thank Jose Carlos Medraño
(Internal Medicine Department, Meixoeiro Hospital) for his
assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CRC, VMG and MCG followed up the patient, wrote the
manuscript and designed the study. Moreover, they contributed to
addressing all questions related to the accuracy and integrity of
this study. JINV and CFV provided the histopathological results.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The authors declare that the procedures followed
were in accordance with the ethical standards of the responsible
committee on human experimentation (institutional and national) and
with the Helsinki Declaration of 1975, as revised in 2000 (5).
Patient consent for publication
Written informed consent was obtained from the
patient regarding the publication of the case details and any
accompanying images.
Competing interests
The authors declare no potential competing interests
with respect to the research, authorship and/or publication of this
article.
References
|
1
|
Augustin M, Langenbruch A, Gutknecht M,
Reich K, Körber A, Maaßen D, Mrowietz U, Thaçi D, von Kiedrowski R
and Radtke MA: Definition of psoriasis severity in routine clinical
care: Current guidelines fail to capture the complexity of
long-term psoriasis management. Br J Dermatol. 179:1385–1391. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carrera CG, Dapavo P, Malagoli P, Naldi L,
Arancio L, Gaiani F, Egan CG, Di Mercurio M and Cattaneo A: PACE
study: Real-life Psoriasis Area and Severity Index (PASI) 100
response with biological agents in moderate-severe psoriasis. J
Dermatolog Treat. 29:481–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Billi AC and Gudjonsson JE: Adalimumab in
Psoriasis: How much is enough? J Invest Dermatol. 139:19–22. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esposito M, Prignano F, Rongioletti F,
Hansel K, Bianchi L, Pescitelli L, Lazzeri L, Ricceri F, Mugheddu
C, Bavetta M, et al: Efficacy and safety of adalimumab after
failure of other anti-TNFα agents for plaque-type psoriasis:
Clinician behavior in real life clinical practice. J Dermatolog
Treat. 6:1–5. 2018. View Article : Google Scholar
|
|
5
|
Ruiz V, Velásquez M and Barrera LF:
Immunogenetic aspects of psoriasis with emphasis on micro-RNA.
Inmunol. 33:137–146. 2014.
|
|
6
|
Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A
and Gelfand JM: The risk of cancer in patients with psoriasis: A
population-based cohort study in the health improvement network.
JAMA Dermatol. 152:282–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rønholt K and Iversen L: Old and new
biological therapies for psoriasis. Int J Mol Sci. 18(pii):
E22972017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farshid G, Prowse P and True B:
Epithelioid myxofibrosarcoma developing at the injection site of
Adalimumab therapy for psoriatic synovitis. Eur J Rheumatol.
5:131–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krishna SM and Kim CN: Merkel cell
carcinoma in a patient treated with adalimumab: Case report. Cutis.
87:81–84. 2011.PubMed/NCBI
|
|
10
|
Amadu V, Satta R, Montesu MA and Cottoni
F: Kaposi's sarcoma associated with treatment with adalimumab.
Dermatol Ther (Heidelb). 25:619–620. 2012. View Article : Google Scholar
|
|
11
|
Bret J, Hernandez J, Aquilina C,
Zabraniecki L and Fournie B: Kaposi's disease in a patient on
adalimumab for rheumatoid arthritis. Joint Bone Spine. 76:721–722.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonovas S, Minozzi S, Lytras T,
González-Lorenzo M, Pecoraro V, Colombo S, Polloni I, Moja L,
Cinquini M, Marino V, et al: Risk of malignancies using anti-TNF
agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing
spondylitis: A systematic review and meta-analysis. Expert Opin
Drug Saf. 15 (Suppl 1):35–54. 2016. View Article : Google Scholar : PubMed/NCBI
|