Introduction
Mixed carcinoma of the pancreas is defined as the
coexistence of exocrine and endocrine carcinomatous components
within the same pancreatic neoplasm. Broadly stated, mixed
carcinoma of the pancreas involves the coexistence of ductal and
acinar cell carcinoma (1). Acinar
cell carcinoma accounts for no more than 1–2% of all adult
pancreatic tumors (2). The World
Health Organization (WHO) classification categorizes mixed
ductal-acinar cell carcinoma as a sub-class of acinar cell
neoplasms (3), and its diagnosis is
established when 25% of the tumor displays acinar and ductal
elements based on the pathological findings.
Mixed ductal-acinar cell carcinoma is extremely
rare, with only 21 cases reported in the English and Japanese
literature to date (4–10), and its clinicopathological
characteristics have not been clearly determined thus far. The
current study herein describes our experience with a case of mixed
ductal-acinar cell carcinoma in a 74-year-old male patient and
discuss the relevant literature.
Case report
A 74-year-old man presented with an abrupt
deterioration of blood glucose control, while undergoing treatment
for diabetes and hypertension by a local physician in February
2016. Oral medication for both diseases had been prescribed at the
age of 71 years. The patient had a history of pharyngeal cancer,
for which he had received surgical treatment at the age of 69
years. The family history was unremarkable. Ultrasonography and
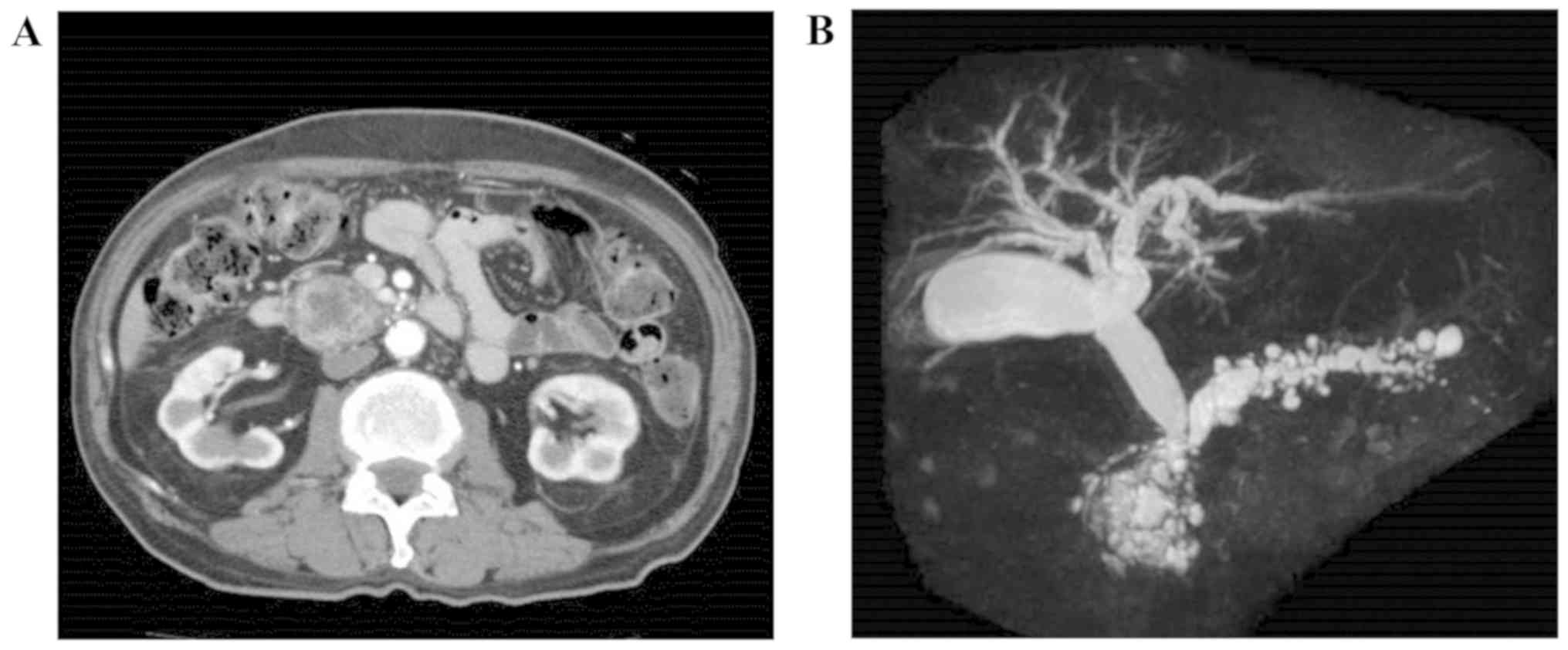
computed tomography (CT) examination revealed a tumor measuring 30
mm in the head of the pancreas, as well as dilation of the main
pancreatic duct. The patient was then referred to Tagawa Hospital
for extensive evaluation and treatment. On physical examination,
the patient's height and body weight were 167.0 cm and 52.5 kg,
respectively. No anemia was detected in the palpebral conjunctiva,
and the patient did not have jaundice, abdominal tenderness, or
back pain. No other abnormal findings were observed. The results of
the blood tests revealed that tumor marker levels were high.
Carcinoembryonic antigen, 6.2 ng/ml (normal range, <5.0 ng/ml);
carbohydrate antigen 19-9, 1131.4 U/ml (normal range, <37.0
U/ml); DUPAN-2, 240 U/ml (normal range, <150 U/ml) and SPan-1,
140.1 U/ml (normal range, <30.0 U/ml). Abnormal blood glucose
levels were also observed [hemoglobin A1c, 9.0% (normal range,
4.3–5.8%); fasting blood sugar, 381 mg/dl (normal range, 80–109
mg/dl)].
An abdominal ultrasound examination was performed,
which revealed a well-differentiated solid tumor, measuring ~35×30
mm, with cystic components. In addition, the main pancreatic duct
was dilated peripheral to the tumor. An abdominal contrast-enhanced
CT scan revealed a mixed cystic and solid tumor measuring ~30 mm in
the head of the pancreas, and dilation of the main pancreatic duct
in the body and tail of the pancreas. Microcysts were visible in
the dorsal pancreas. There was no invasion into the portal vein or
the superior mesenteric vein; however, the lower common bile duct
and intrapancreatic bile duct were compressed by the tumor. The
part of the bile duct cranial to the lower common bile duct and
intrapancreatic bile duct was dilated towards the head of the
pancreas (Fig. 1A). The surrounding
lymph nodes were not enlarged, and there were no findings
indicative of distant metastasis or peritoneal dissemination.
Magnetic resonance cholangiopancreatography identified a
multilocular cystic lesion measuring 40 mm, presenting as an
aggregation of microcysts in the head of the pancreas with a solid
component. Dilation of the pancreatic duct was observed towards the
tail of the pancreas (Fig. 1B).
Endoscopic retrograde cholangiopancreatography
revealed stenosis of the lower bile duct 10 mm peripheral to the
papilla of Vater. On pancreatography, almost none of the main
pancreatic duct in the head of the pancreas was visualized, and
dilation of the pancreatic duct dorsal to the head and body of the
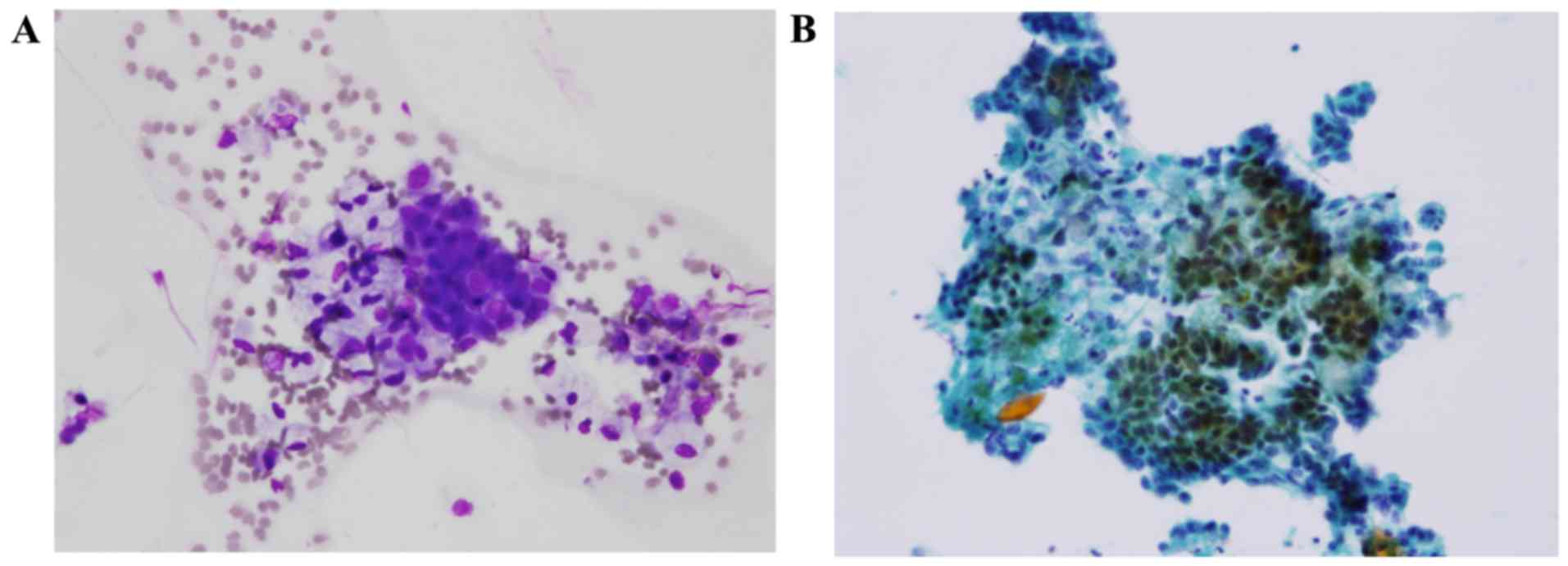
pancreas was observed. Concurrently, the pancreatic juice cytology
revealed adenocarcinoma (Fig.
2).
The abovementioned findings indicated an intraductal
proliferative neoplasm with mixed solid and cystic components.
Primary pancreatic cancer, pancreatic cancer with intraductal
papillary mucinous neoplasm (IPMN), and pancreatic cancer with
intraductal tubulopapillary neoplasm (ITPN) were considered in the
differential diagnosis, and subtotal stomach-preserving
pancreaticoduodenectomy was performed. During surgery, a tumor was
identified in the head of the pancreas, which was firm and elastic
in texture, measuring 40 mm in greatest diameter. The tumor was
adjacent to the portal vein and the superior mesenteric vein;
however, there was no gross invasion, and the tumor could be easily
separated from these structures. Neither liver metastasis nor
peritoneal dissemination were observed, and lymph node metastasis
was not readily identified intraoperatively. As scheduled, subtotal
stomach-preserving pancreaticoduodenectomy was performed, along
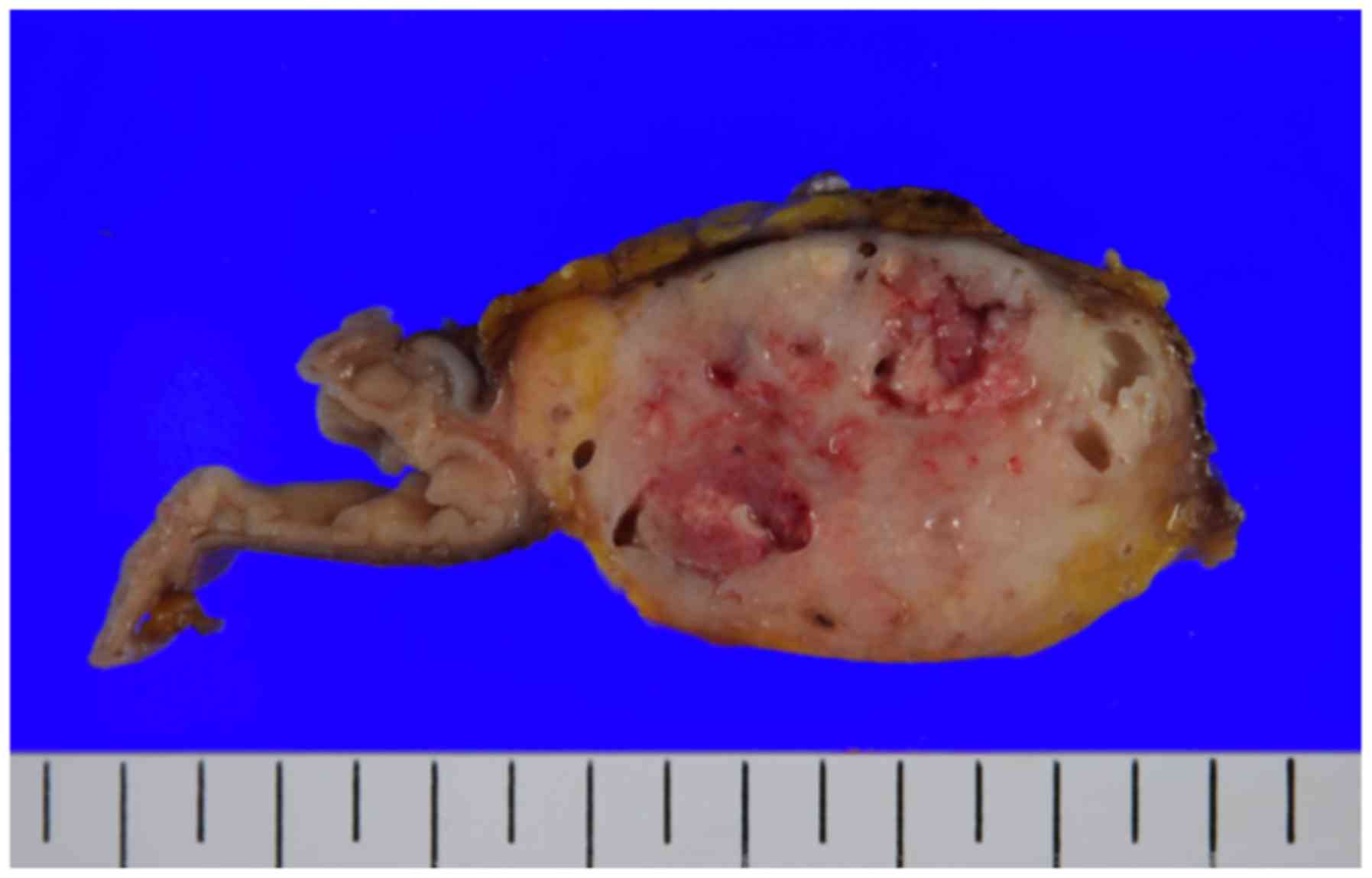
with reconstruction per Child's method. The surgical specimen
included a poorly differentiated tumor with mixed cystic and solid
components (Fig. 3). On histological
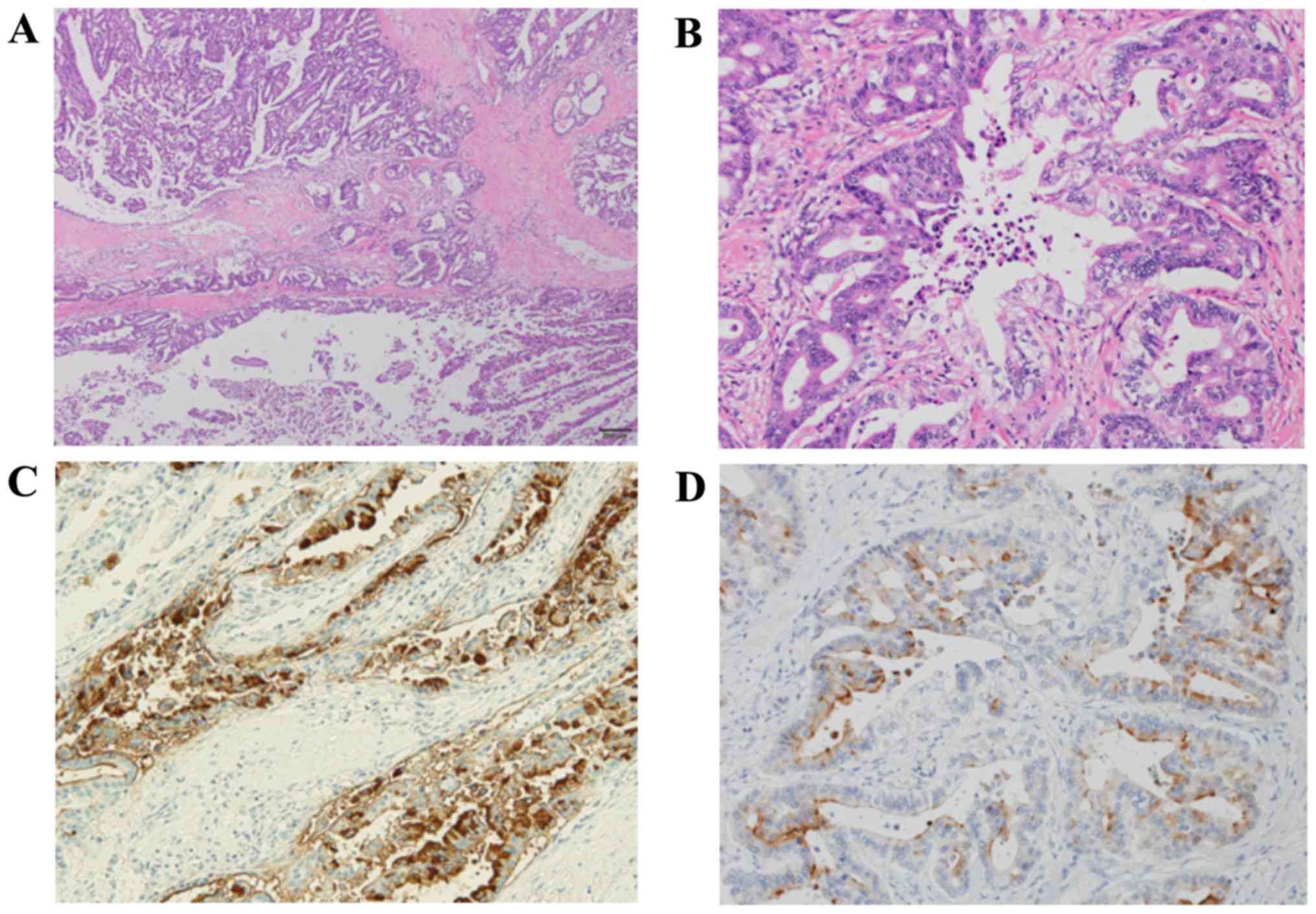
examination, the lesion was primarily located in the main
pancreatic duct; clear boundaries and portal invasion were observed
(Fig. 4A). The lesion comprised two
components (Fig. 4B): One component
formed irregularly sized tubular structures, which upon
immunohistological examination were positive for epithelial tumor
markers and carbohydrate antigen 19-9, therefore suggesting ductal
carcinoma (Fig. 4C). The other
component exhibited proliferation of tumor cells with oval-shaped
nuclei and eosinophilic cytoplasm, forming acinar structures. Upon
immunostaining, this component was diffusely positive for trypsin,
suggestive of acinar cell carcinoma (Fig. 4D). Due to the concurrent existence of
both components in the same tumor, the patient was diagnosed with
mixed ductal-acinar carcinoma of the pancreas.
The patient's postoperative progress was uneventful,
and no complications were reported. On day 27, the patient was
discharged from the hospital and was prescribed titanium silicate
(TS)-1 as postoperative adjuvant chemotherapy; however, due to
marked general malaise, TS-1 was discontinued 2 months later. The
patient succumbed to recurrence of tumor in the residual pancreas
12 months after the surgery.
Discussion
Although several cases of mixed acinar-endocrine
carcinoma of the pancreas, composed of both acinar and endocrine
tumor cells, have been reported, there have only been a few cases
of resected mixed ductal-acinar cell carcinomas (1). The World Health Organization (WHO)
classification categorizes mixed ductal-acinar cell carcinoma as a
sub-class of acinar cell neoplasms (3). The diagnosis of mixed ductal-acinar
cell carcinoma is established when 25% of the tumor displays acinar
and ductal elements, based on pathological findings.
Mixed ductal-acinar cell carcinoma is extremely rare
and, to the best of our knowledge, only 21 cases have been reported
in the English and Japanese literature to date (4–10)
(Table I). According to these
reports, the mean age of the patients was 69.8 years, the
male:female ratio was 15:6, and the mean diameter of the tumors was
42.8 mm. In six cases the tumors were present in the tail of the
pancreas, while in the remaining cases, the tumors were located in
the head of the pancreas.
 | Table I.The 21 previously reported cases of
mixed duct-acinar carcinoma in the English and Japanese
literature. |
Table I.
The 21 previously reported cases of
mixed duct-acinar carcinoma in the English and Japanese
literature.
| Author | Sex | Age (years) | Chief complaint | Site | Tumor diameter | Treatment | Follow-up | Prognosis | Refs. |
|---|
| Stelow et
al | M | 74 | Painless
jaundice | Head | 31 | PD | 20 | Alive | (5) |
| Stelow et
al | M | 75 | Weight loss,
diarrhea | Head | 25 | PD | 39 | Deceased | (5) |
| Stelow et
al | M | 73 | Not available | Tail | 20 |
| 52 | Deceased | (5) |
| Stelow et
al | M | 74 | Weight loss,
diarrhea | Head | 40 | PD | 51 | Deceased | (5) |
| Stelow et
al | M | 70 | Pain | Head | 40 | PD | 38 | Deceased | (5) |
| Stelow et
al | F | 77 | Weight loss | Head | 30 | PD | 9 | Deceased | (5) |
| Stelow et
al | M | 77 | Weight loss,
pain | Head | 37 | Rdx | 0.5 | Deceased | (5) |
| Stelow et
al | M | 52 | Pain | Head | 55 | PD | 12 | Deceased | (5) |
| Stelow et
al | M | 76 | Painless
jaundice | Head | 35 | PD | 8 | Deceased | (5) |
| Stelow et
al | M | 79 | Painless
jaundice | Head | 34 | PD | 11 | Alive | (5) |
| Stelow et
al | M | 69 | Painless
jaundice | Head | 54 | PD | 36 | Alive | (5) |
| Webb | M | 71 | Not mentioned | Head | 30 | – | 12 | Deceased | (6) |
| Webb | F | 51 | Not mentioned | Tail | – | – | 3 | Deceased | (6) |
| Webb | F | 51 | Not mentioned | Tail | 30 | – | 4 | Deceased | (6) |
| Webb | M | 85 | Not mentioned | Tail | – | – | 6 | Deceased | (6) |
| Sakata et
al | F | 67 | Abdominal pain | Body-tail | 110 | DP | 7 | Deceased | (7) |
| Inaba et
al | M | 63 | Abdominal pain | Head | 65 | PD+HR | 39 | Alive | (8) |
| Goto et
al | F | 75 | Weight loss | Head-body | 43 | TP | 6 | Alive | (9) |
| Sakai et
al | M | 63 | Worsening of
diabetes | Head | 35 | PD | 8 | Deceased | (10) |
| Shonaka et
al | F | 71 | Epigastric
discomfort | Head/tail | 35/72 | TP | 36 | Alive | (4) |
| Present case | M | 74 | Hyperglycemia | Head | 35 | PD | 12 | Deceased |
|
Preoperative diagnostic imaging of mixed tumors
often reflects imaging findings for pancreatic ductal carcinoma and
acinar cell carcinoma. On contrast-enhanced CT, pancreatic ductal
carcinoma appears as a solid tumor with poor vascularity, whereas
acinar cell carcinoma often has relatively rich vascularity and, in
case of necrosis, cystic components are often observed. In the
present case, we observed a mixed cystic/solid tumor in the head of
the pancreas with an acinar cell carcinomatous component in
addition to typical pancreatic ductal carcinoma. Histologically, no
necrosis was observed, but there was stenosis of the pancreatic
duct due to the protrusion of the tumor into the duct lumen;
therefore, dilation of the main pancreatic duct distal to the head
of the pancreas was observed.
Moreover, the present case exhibited pathological
characteristics of both pancreatic ductal carcinoma and acinar cell
carcinoma. The acinar cell carcinoma consisted of granular,
eosinophilic cells forming acinar structures, while the pancreatic
ductal carcinoma consisted of tubular formations. The differential
diagnosis of tumors consisting primarily of cystic lesions
originating from the pancreatic duct include IPMN and ITPN. There
were no papillae observed in the present case, but rather
conspicuous tubular structures were observed, which differentiated
it from IPMN. The presence of tubular structures resembles ITPN;
however, the mucous production in the present case ruled out ITPN.
On immunohistochemistry, the acinar cell carcinoma component was
diffusely positive for trypsin, while the ductal carcinoma
component was positive for MUC1, MUC5AC and MUC6. Synaptophysin and
chromogranin A were both negative, ruling out a neuroendocrine
origin. Based on the aforementioned findings, the patient was
diagnosed with mixed ductal-acinar cell carcinoma. Adenocarcinoma
cells (of ductal origin) were confirmed on preoperative pancreatic
juice cytology. In retrospect, however, small oval-shaped cells
with a high N/C ratio were also present; the difference between
these cells and normal columnar epithelial cells suggested an
acinar cell origin. The ability to distinguish the disease
characteristics in the present case, i.e., the ability to conduct
special staining methods using cytology specimens, may have
facilitated the preoperative diagnosis of this mixed neoplasm.
The mechanism underlying the onset of this mixed
neoplasm remains largely elusive; however, a previous study
revealed that all pancreatic cells differentiate from
developmentally common progenitors (11), namely the pancreatic duodenal
homeobox gene-1-positive progenitor cells; however, the ductal
lineage diverges at an early stage from p48-positive exocrine and
endocrine progenitor cells (12,13).
Pancreatic endocrine cells originate from embryonic
Ngn-3-expressing cells (14). Also,
under certain conditions, acinar cells can transdifferentiate into
endocrine cells (15,16). This may be one reason that ductal
differentiation is rarer, despite the fact that acinar cell
neoplasms often differentiate into pancreatic endocrine cells.
Approximately 30% of all ACC cases exhibit chromogranin and
synaptophysin neuroendocrine markers and neuroendocrine tumors may
also focally express acinar markers (17,18).
In terms of molecular pathology, pancreatic ductal
carcinoma progresses through a multistage process from a
premalignant lesion to invasive carcinoma due to the accumulation
of mutations in KRAS and other genes; however, acinar cell
carcinoma almost never harbors KRAS mutations or mutations
of tumor suppresor genes such as P16, DPC4/Smad4, or
P53 (19–21). As described above, the mechanism
underlying the mixture of biologically different neoplasms is
unclear and requires further investigation.
Mixed neoplasms of the pancreas are extremely rare,
with only a few reports published in the literature to date. The
developmental and clinical characteristics of these tumors have not
been fully elucidated. Owing to the small number of reports on
mixed pancreatic carcinoma, there is no consistent trend regarding
outcome. We hope that further accumulation of cases will lead to
the elucidation of the mechanism of onset and the establishment of
optimal multimodal treatment in the future.
Acknowledgements
The authors would like to thank Sugako Kajiwara and
Jun Uchida for their technical assistance.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TS designed the current study and wrote the
manuscript. TH and HK analyzed and interpreted the data and wrote
the manuscript. YN, YA, FF, RM, TO, IS, AH and TT collected and
interpreted the data and critically revised the manuscript. All
authors approved the final version of the manuscript, and agree to
be accountable for all aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests to disclose.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
IPMN
|
intraductal papillary mucinous
neoplasm
|
|
ITPN
|
intraductal tubulopapillary
neoplasm
|
References
|
1
|
Ohike N, Jürgensen A, Pipeleers-Marichal M
and Klöppel G: Mixed ductal-endocrine carcinomas of the pancreas
and ductal adenocarcinomas with scattered endocrine cells:
Characterization of the endocrine cells. Virchows Arch.
442:258–265. 2003.PubMed/NCBI
|
|
2
|
Hruban RH, Pitman MB and Klimstra DS:
Tumors of the Pancreas, AFIP Atlas of Tumor Pathology. Fourth
series, Fascicle. 6:American Registry of Pathology. (Washington,
DC). 191–218. 2007.
|
|
3
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
9th. IARC/WHO; Lyon: pp. 279–337. 2010
|
|
4
|
Shonaka T, Inagaki M, Akabane H, Yanagida
N, Shomura H, Yanagawa N, Oikawa K and Nakano S: Total
pancreatectomy for metachronous mixed acinar-ductal carcinoma in a
remnant pancreas. World J Gastroenterol. 20:11904–11909. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stelow EB, Shaco-Levy R, Bao F, Garcia J
and Klimstra DS: Pancreatic acinar cell carcinomas with prominent
ductal differentiation: Mixed acinar ductal carcinoma and mixed
acinar endocrine ductal carcinoma. Am J Surg Pathol. 34:510–518.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Webb JN: Acinar cell neoplasms of the
exocrine pancreas. J Clin Pathol. 30:103–112. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakata T, Mimura H, Takakura N, Hamazaki
K, Kin H, Kimura T, Hosoba T, Orita K, Mizobuchi K, Taguchi K, et
al: A case of mixed duct and acinar cell carcinoma the pancreas
showing peculiar modes of spread. J Biliary Tract Pancreas.
8:1349–1353. 1987.(In Japanese).
|
|
8
|
Inaba N, Kasahara K, Kashii A, Kanazawa K,
Yamaguchi T, Saito K and Kamisawa T: Mixed ductal and acinar cell
cancer of the pancreas head; report of a case. Nihon Geka Gakkai
Zasshi. 88:773–778. 1987.(In Japanese). PubMed/NCBI
|
|
9
|
Goto H, Yamazaki Y, Takeuchi T, Kokehara
N, Ota M, Ohashi N, Kusakawa M and Soga T: Total pancreatectomy for
combined carcinoma of the pancreas-report of a case. J Biliary
Tract Pancreas. 9:463–467. 1988.(In Japanese).
|
|
10
|
Sakai M, Takeda S, Ishikawa T, Kanazumi N,
Inoue S, Kaneko T, Nakao A and Nagasaka T: Mixed duct-acinar cell
carcinoma of the pancreas: Report of a case. Jpn J Gastroenterol
Surg. 38:1821–1827. 2005. View Article : Google Scholar
|
|
11
|
Fishman MP and Melton DA: Pancreatic
lineage analysis using a retroviral vector in embryonic mice
demonstrates a common progenitor for endocrine and exocrine cells.
Int J Dev Biol. 46:201–207. 2002.PubMed/NCBI
|
|
12
|
Edlund H: Pancreas: How to get there from
the gut? Curr Opin Cell Biol. 11:663–668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krapp A, Knöfler M, Ledermann B, Bürki K,
Berney C, Zoerkler N, Hagenbüchle O and Wellauer PK: The bHLH
protein PTF1-p48 is essential for the formation of the exocrine and
the correct spatial organization of the endocrine pancreas. Genes
Dev. 12:3752–3763. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Offield MF, Jetton TL, Labosky PA, Ray M,
Stein RW, Magnuson MA, Hogan BL and Wright CV: PDX-1 is required
for pancreatic outgrowth and differentiation of the rostral
duodenum. Development. 122:983–995. 1996.PubMed/NCBI
|
|
15
|
Song KH, Ko SH, Ahn YB, Yoo SJ, Chin HM,
Kaneto H, Yoon KH, Cha BY, Lee KW and Son HY: In vitro
transdifferentiation of adult pancreatic acinar cells into
insulin-expressing cells. Biochem Biophys Res Commun.
316:1094–1100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minami K, Okuno M, Miyawaki K, Okumachi A,
Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T and Seino
S: Lineage tracing and characterization of insulin-secreting cells
generated from adult pancreatic acinar cells. Proc Natl Acad Sci
USA. 102:15116–15121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohike N and Morohoshi T: Pathological
assessment of pancreatic endocrine tumors for metastatic potential
and clinical prognosis. Endocr Pathol. 16:33–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohike N, Kosmahl M and Klöppel G: Mixed
acinar-endocrine carcinoma of the pancreas. A clinicopathological
study and comparison with acinar-cell carcinoma. Virchows Arch.
445:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoorens A, Lemoine NR, McLellan E,
Morohoshi T, Kamisawa T, Heitz PU, Stamm B, Rüschoff J, Wiedenmann
B and Klöppel G: Pancreatic acinar cell carcinoma. An analysis of
cell lineage markers, p53 expression, and Ki-ras mutation. Am J
Pathol. 143:685–698. 1993.PubMed/NCBI
|
|
20
|
Moore PS, Orlandini S, Zamboni G, Capelli
P, Rigaud G, Falconi M, Bassi C, Lemoine NR and Scarpa A:
Pancreatic tumours: Molecular pathways implicated in ductal cancer
are involved in ampullary but not in exocrine nonductal or
endocrine tumorigenesis. Br J Cancer. 84:253–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Terhune PG, Heffess CS and Longnecker DS:
Only wild-type c-Ki-ras codons 12, 13, and 61 in human pancreatic
acinar cell carcinomas. Mol Carcinog. 10:110–114. 1994. View Article : Google Scholar : PubMed/NCBI
|