Introduction
Many trials to reduce local recurrence in locally
advanced lower rectal cancer have been reported worldwide. In
Japan, the standard therapy for locally advanced rectal cancer is
total mesorectal excision (TME) and lateral lymph node dissection
(LLND), whereas neoadjuvant chemoradiotherapy (NACRT) combined with
TME is commonly used in many Western countries. NACRT has been
reported to reduce the local recurrence rate, but the prognosis is
not improved (1). Furthermore, NACRT
may induce anal dysfunction, postoperative complications, and late
effects related to radiation (2–4).
Therefore, intensive neoadjuvant chemotherapy (NAC) has recently
been tried in Japan, since NAC is likely to reduce postoperative
complications, maintain better anal function, and achieve early
systemic control by using a strong agent, such as oxaliplatin. An
advantage of NACRT is improvement of local control. Therefore, if
local control of NAC is equivalent to NACRT, NAC may be useful as
preoperative therapy. In this retrospective study, we compared
histopathological changes and evaluated therapeutic effects in
patients treated with NAC or NACRT.
Patients and methods
This retrospective study was approved by the Human
Research Ethics Committee of the Hirosaki University Graduate
School of Medicine (Aomori, Japan; reference no. 2017-1009).
Histopathological evaluation was performed retrospectively with
opt-out consent reusing previously obtained specimens for routine
pathological diagnosis.
The subjects were 26 patients with locally advanced
lower rectal cancer who were treated with NAC (S-1 and oxaliplatin,
3 courses; n=16) or NACRT (5-FU base chemotherapy and 40–45 Gy
radiation; n=10) before surgery in our department between June 2002
and June 2016. NAC was introduced for patients participated in the
clinical trial of TME and LLND after NAC [phase II study of S-1 and
L-OHP neoadjuvant chemotherapy with total mesorectal excision and
lateral lymph node dissection for resectable rectal cancer
(ACCS-01, UMIN 000019606)] from October 2015 to June 2016. NACRT
was introduced for patients with locally advanced low RC from June
2002 to January 2012. Patients of NACRT group were collected
retrospectively. TME was performed in all cases and LLND was
performed in 21 patients (15 in the NAC group and 6 in the NACRT
group).
The stage was classified using the Japanese
Classification of Colorectal Carcinoma 8th Edition (5). Resected specimens and lymph nodes were
fixed in 10% formalin and embedded in paraffin, prior to staining
with hematoxylin-eosin. In the primary tumor, we selected a slice
for analysis containing the deepest residual tumor in each case.
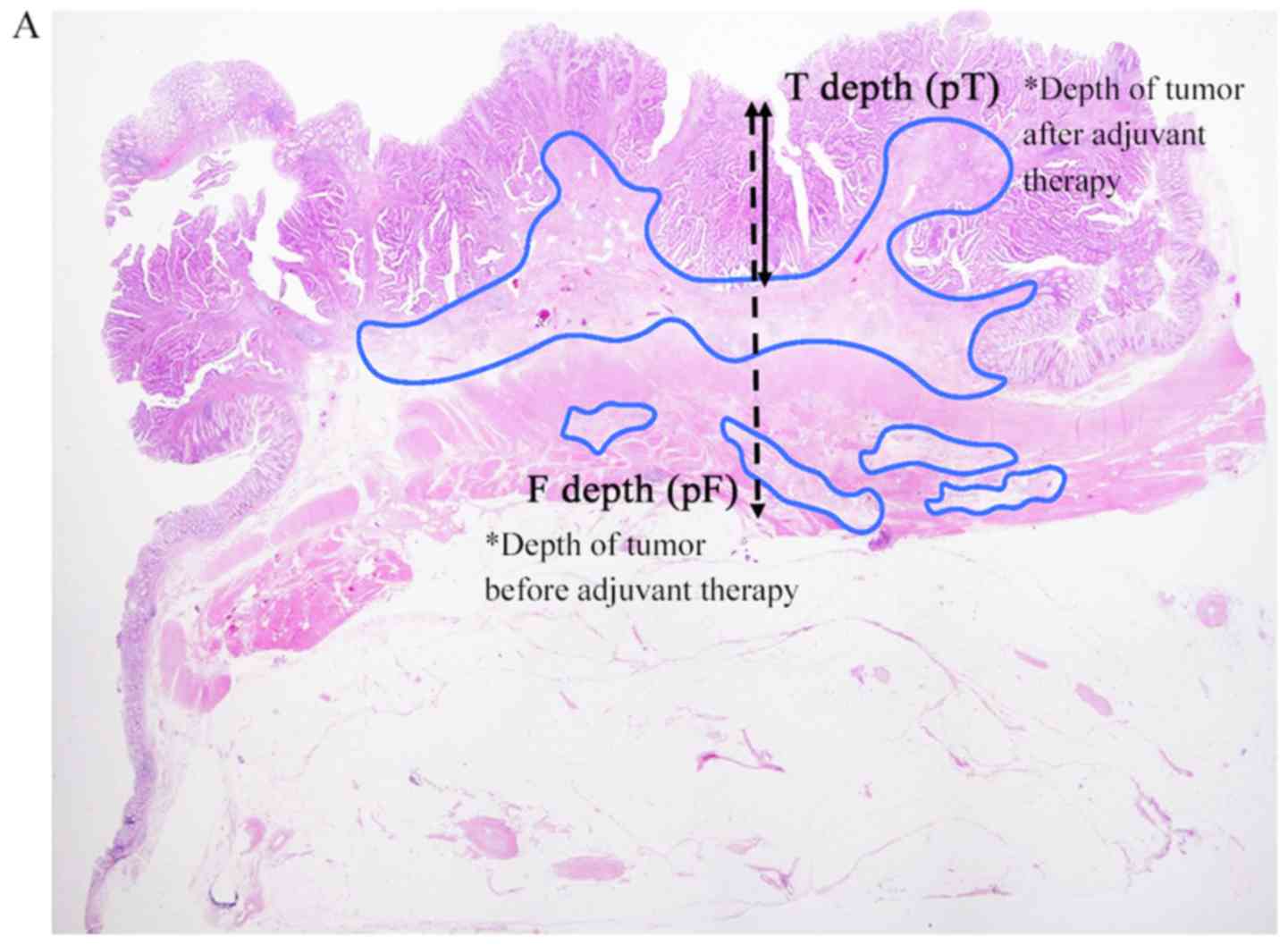
Parameters for therapeutic effects were defined based on fibrosis
(Fig. 1). The invasion depth of the
tumor before preoperative therapy was defined as the depth of
fibrosis [distance from mucosa: F depth (mm); invasion depth:
Pathological F (pF)]; and the invasion depth after preoperative
therapy was defined as the depth of residual tumor [distance from
mucosa: T depth (mm); invasion depth: Pathological T (pT)].
Measurements of these depths were performed and evaluated by two
surgeons (K.S. and T.M.) and one pathologist (S.M.).
Histopathological therapeutic effects in the NAC and
NACRT groups were compared using the following parameters: T
downgrade rate: Improvement of invasion depth after preoperative
therapy compared with clinical T (cT) and pT (example, in a case of
cT3→pT2, T downgrade was achieved); T depth/F depth ratio: The
smaller this ratio, the greater the therapeutic effect; pT
downgrade rate: Improvement of invasion depth after preoperative
therapy compared with pT and pF (example, in a case with pF of A
and pT of MP, pT downgrade was achieved); pathological complete
response (pCR) rate: Comparison of pCR rate of primary legion and
lymph nodes between the NAC and NACRT groups [an example of pCR for
lymph nodes is shown in Fig. 1B; in
lymph nodes, cases that were pN negative and without fibrosis were
excluded (NAC: n=6, NACRT: n=8)]; and N negative conversion rate:
Conversion rate from cN positive (NAC: n=10, NACRT: n=8) to
pathological N (pN) negative. Statistical analyses were performed
by Mann-Whitney U test and χ2 test, with P<0.05 considered to
indicate a significant difference. These analyses were performed
using EZR on R commander version 1.36, programed by Kanda (6).
Results
Clinical characteristics
The clinical characteristics of the patients are
shown in Table I. In the NAC group,
the median distance of the tumor from the anal verge was
significantly longer (5 vs. 2.5 mm, P=0.01) than in the NACRT
group. Our department introduced laparoscopic surgery from 2014.
All patients in the NACRT group were performed operation before
2014 and all patients in the NAC group were performed operation
after 2014, so all operations in the NACRT group were performed by
laparotomy and all operations in the NAC group were performed
laparoscopically. The median operation time was significantly
longer (286.5 vs. 194.5 min, P<0.01) and median blood loss was
significantly lower (55 vs. 546.5 ml, P<0.01) in the NAC group.
In all cases in both groups, histological types in biopsy before
preoperative therapy were well or moderately differentiated
adenocarcinoma. LLND was performed in 15 patients (93.8%) in the
NAC group and in 6 (60%) in the NACRT group. Patients of NAC group
participated in the clinical trial of LLND after NAC (ACCS-01, UMIN
000019606, described in Patients and methods), so most patients in
the NAC group were performed LLND. One patient of NAC group didn't
be performed lateral lymph node dissection because the lower margin
of tumor didn't present in lower rectum by the results of
reexaminations. In the NACRT group, LNND was performed only for
patients suspected lateral pelvic lymph node (LPLN) metastasis from
preoperative findings. cT grades and cN-positive rates did not
differ significantly between the groups.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | NAC (n=16) | NACRT (n=10) | P-value |
|---|
| Median age (range),
years old | 67.5 (43–77) | 66 (53–71) | 0.44 |
| Male, n (%) | 14 (87.5) | 5 (50) | 0.07 |
| Median BMI, kg/m2
(range) | 21.6 (17.9–29.0) | 22.6 (17.8–25.7) | 0.90 |
| Diabetes, n (%) | 2 (12.5) | 1 (10) | 1 |
| Cardiovascular
disease, n (%) | 2 (12.5) | 1 (10) | 1 |
| Median distance of
tumor from AV, cm (range) | 5 (2–7) | 2.5 (0–8) | 0.01 |
| Anal preservation, n
(%) | 10 (62.5) | 4 (40) | 0.42 |
| pap, tub, n (%) | 16 (100) | 10 (100) | – |
| cT4, n (%) | 8 (50) | 5 (50) | 1 |
| cN positive, n
(%) | 10 (62.5) | 8 (80) | 0.42 |
| Laparoscopic surgery,
n (%) | 16 (100) | 0 (0) | <0.01 |
| Median operation
time, min (range) | 286.5 (249–376) | 194.5 (149–300) | <0.01 |
| Median blood loss, ml
(range) | 55 (3–170) | 546.5 (105–1485) | <0.01 |
| LLND, n (%) | 15 (93.8) | 6 (60) | 0.05 |
Pathological characteristics
The pathological characteristics of resected
specimens and lymph nodes are shown in Table II. The median T depth (6.0 vs. 6.8
mm, P=0.75) and F depth (10.0 vs. 10.5 mm, P=0.34) were not
significantly different between the groups. pF grade (invasion
depth before preoperative therapy) and pT grades (invasion depth
after preoperative therapy) were not significantly different
between the groups (pF, P=0.05, pT, P=0.05). Positive rates of
pathological metastasis to pararectal lymph nodes (PRLNs) were
18.8% in the NAC group and 40% in the NACRT group (P=0.37).
Positive pathological LPLN metastasis was seen in only one case in
the NACRT group.
 | Table II.Histological features. |
Table II.
Histological features.
| Parameter | NAC (n=16) | NACRT (n=10) | P-value |
|---|
| Median T depth, mm
(range) | 6.0 (0–19) | 6.8 (1–23) | 0.75 |
| Median F depth, mm
(range) | 10.0 (3–20) | 10.5 (5–30) | 0.34 |
| pF, n (%) |
|
| 0.05 |
| 1
(M-SM) | 1 (6.3) | 0 (0) |
|
| 2
(MP) | 7 (43.8) | 1 (10) |
|
| 3
(A) | 7 (43.8) | 9 (90) |
|
| 4
(AI) | 1 (6.3) | 0 (0) |
|
| pT, n (%) |
|
| 0.05 |
| 0
(pCR) | 2 (12.5) | 0 (0) |
|
| 1
(M-SM) | 0 (0) | 3 (30) |
|
| 2
(MP) | 7 (43.8) | 2 (20) |
|
| 3
(A) | 7 (43.8) | 4 (40) |
|
| 4
(AI) | 0 (0) | 1 (10) |
|
| pN, n (%) |
|
|
|
|
Pararectal (+) | 3 (18.8) | 4 (40) | 0.37 |
| Lateral
(+)a | 0 (0) | 1 (16.7) | 0.29 |
Pathological effects
The pathological effects evaluated using the five
parameters described in the methods are shown in Table III. None of these parameters showed
a significant difference between the NAC and NACRT groups: The T
downgrade rates were good in both groups (87.5 vs. 80%, P=0.63),
and the median T depth/F depth ratios (0.61 vs. 0.73, P=0.75) and
pT downgrade rates (25 vs. 40%, P=0.66) were similar. For the pCR
rates, pCR for a primary lesion was achieved only in 2 cases in the
NAC group (12.5 vs. 0%, P=0.51), and pCR rates for lymph nodes were
similar in the two groups (33 vs. 37.5%, P=1). The N-negative
conversion rate: Was slightly but not significantly higher in the
NAC group (80 vs. 37.5%, P=0.15).
 | Table III.Pathological therapeutic effects of
NAC and NACRT. |
Table III.
Pathological therapeutic effects of
NAC and NACRT.
| Parameter | NAC (n=16) | NACRT (n=10) | P-value |
|---|
| T downgrade, n
(%) | 14 (87.5) | 8 (80) | 0.63 |
| T depth/F depth,
median (range) | 0.61 (0–1.58) | 0.73 (0.1–1) | 0.75 |
| pT downgrade, n
(%) | 4 (25) | 4 (40) | 0.66 |
| pCR rate for primary
lesion, n (%) | 2
(12.5) | 0 (0) | 0.51 |
| pCR rate for lymph
nodes, n (%)a | 2
(33.3) | 3
(37.5) | 1 |
| N negative
conversion, n (%)b | 8 (80) | 3
(37.5) | 0.15 |
Discussion
Many studies of multimodal therapies for locally
advanced lower rectal cancer have been reported to improve
outcomes. In western countries, preoperative radiotherapy and NACRT
have been used for many years, and several randomized controlled
trials have shown that preoperative radiotherapy and NACRT improve
the local recurrence rate (7–9).
Therefore, preoperative radiotherapy and NACRT combined with TME
are now standard therapies for local advanced lower rectal cancer,
but this approach does not improve overall survival (OS) (10). In contrast, in Japan, TME and LLND
are recommended, and preoperative therapy is not included in
standard therapy. However, in cases with lymph node metastasis,
prognosis is poor even after LLND (11) and this has led to recent introduction
of NAC in Japan. The advantages of NAC include possible improved OS
by introducing intensive chemotherapy (such as FOLFOX and XELOX,
including oxaliplatin) at an early stage; reduced postoperative
anal dysfunction, postoperative complications, and late effects due
to radiation; and a high completion rate of chemotherapy compared
to postoperative adjuvant chemotherapy.
A summary of reports (10,12–19) of
pCR rates using NAC and NACRT is shown in Table IV. In recent reports on the effects
of NAC on local control, Schrag et al (14) found a pCR rate for the primary lesion
of 25% after 6 courses of FOLFOX and bevacizumab, and Kamiya et
al (15) reported a pCR rate of
12.2% after 4 courses of XELOX and a rate of 31.7% for a tumor
regression grade (TRG) ≥3. The pCR rates in these reports and our
result of 12.5% for NAC are comparable with results for 5-FU-based
NACRT. In Japan, SOX is sometimes used for unresectable lower RC as
one of the first-line chemotherapy with Bevacizumab. On the other
hands, SOX is not used as the adjuvant chemotherapy for colorectal
cancer, so therapeutic effects of SOX for NAC and adjuvant
chemotherapy is controversial. However, SOX have some good points
to introduce for NAC; needless to indwell central venous catheter
and port system; easy to introduce in outpatient clinic; does not
induce hand foot syndrome by Capecitabine, used in XELOX regimen.
Even though this study was small size, retrospective study, our
result may support the effectiveness of SOX for NAC in low RC.
 | Table IV.Studies of preoperative NAC and NACRT
for rectal cancer. |
Table IV.
Studies of preoperative NAC and NACRT
for rectal cancer.
| A, NAC |
|---|
|
|---|
| Author, year | n | Regimen of
chemotherapy | Courses | Radiation | pCR rate (%) | (Refs.) |
|---|
| Ishii et al,
2010 | 26 | 5-FU, LV,
Irinotecan | 2 | None | 3.8 | (12) |
| Hasegawa et
al, 2014 | 25 | XELOX,
Bevacizumab | 4 | None | 4 | (13) |
| Schrag et
al, 2014 | 32 | FOLFOX,
Bevacizumab | 6 | None | 25 | (14) |
| Kamiya et
al, 2016 | 41 | XELOX | 4 | None | 12.2 | (15) |
| Hasegawa et
al, 2017 | 60 | mFOLFOX6 | 6 | None | 16.7 | (16) |
|
| B,
NACRT |
|
| Author,
year | n | Regimen of
chemotherapy | Courses |
Radiation | pCR rate
(%) | (Refs.) |
|
| Rödel et al,
2003 | 32 | XELOX | 2 | 50.4 Gy | 19.0 | (17) |
| Bosset et
al, 2005 | 473 | 5-FU, LV | 5 | 45 Gy | 13.7 | (10) |
| Tulchinsky et
al, 2008 | 132 | 5-FU | 5 | 45-50.4 Gy | 28.0 | (18) |
| Roh et al,
2009 | 1,113 | 5-FU, LV | 7 | 45 Gy | 15.0 | (19) |
The pCR rate does not reveal detailed histological
changes because cases exclusive of pCR are not assessed. Therefore,
a more detailed pathological analysis is required in all cases.
Sakuyama et al (20)
evaluated histological differences between NAC and NACRT for rectal
cancer, using fibrosis as a parameter for therapeutic effects.
NACRT gave a smaller area of residual tumor and a shallower
residual tumor depth than NAC, and ypT downstaging was more
prominent with NACRT. However, NAC showed a higher N downgrade rate
than NACRT. In our results, both NAC and NACRT had good therapeutic
effects on primary lesions and lymph nodes, and the N-negative
conversion rate was higher with NAC (80%) than with NACRT (37.5%),
although the difference was not significant.
We used the median T depth/F depth ratio as a
parameter for therapeutic effects. This ratio became smaller when
the depth of fibrosis from the mucosal surface was deeper, with a
value of <1 indicating that the depth of fibrosis was deeper
than that of the residual tumor. In our cases, the median T depth/F
depth ratios were 0.61 with NAC and 0.73 with NACRT. Thus, the
values of <1 suggest that both NAC and NACRT had good
therapeutic effects that reached deep tissue. These results suggest
that NAC and NACRT can contribute to R0 resection. The T depth/F
depth ratio has not been used previously, but this parameter is
useful for quantitative assessment of therapeutic effects. Sakuyama
et al (20) found that NACRT
had greater effects than NAC beyond the muscular layer, but in our
cases the therapeutic effects of NAC and NACRT in deep regions, as
evaluated by the T depth/F depth ratio, did not differ
significantly.
This study has several limitations: The sample size
was small and cases were analyzed retrospectively; the chemotherapy
patients in the NAC group received (SOX) was not standard regimen
in the western countries; the patient characteristics in the NAC
and NACRT groups were markedly different, so selection bias may be
present; and the study only assessed histological effects, and did
not assess clinical outcomes, such as local recurrence rate and OS.
Therefore, a randomized controlled trial of clinical outcomes of
NAC and NACRT is required to compare actual clinical therapeutic
effects. Within these limitations, we conclude that both NAC and
NACRT are good preoperative therapy from a pathological
perspective. NAC gave local control equivalent to that of NACRT,
and therapeutic effects for lymph nodes might be better with NAC.
These findings suggest that NAC can serve as an alternative therapy
to NACRT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and analyzed in the present study
are included in this published article.
Authors' contributions
KS, TM, SM, YS, HM, TY and KH all contributed to the
study concept and design. KS, TM and SM carried out the
pathological analysis. YS, HM and TY performed the operations. KH
supervised the study. KS, TM, SM, YS, HM, TY and KH all
participated in the interpretation of the results and the writing
of the report, and all approved the final version. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Human Research Ethics Committee of the Hirosaki University Graduate
School of Medicine (Aomori, Japan; reference no. 2017-1009).
Histopathological evaluation was performed retrospectively with
opt-out consent reusing previously obtained specimens for routine
pathological diagnosis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NAC
|
neoadjuvant chemotherapy
|
|
NACRT
|
neoadjuvant chemoradiotherapy
|
|
TME
|
total mesorectal excision
|
|
LLND
|
lateral lymph node dissection
|
|
pCR
|
pathological complete response
|
|
PRLN
|
pararectal lymph node
|
|
LPLN
|
lateral pelvic lymph node
|
|
AV
|
anal verge
|
|
pap
|
papillary adenocarcinoma
|
|
tub
|
tubular adenocarcinoma
|
References
|
1
|
De Caluwé L, Van Nieuwenhove Y and Ceelen
WP: Preoperative chemoradiation versus radiation alone for stage II
and III resectable rectal cancer. Cochrane Database Syst Rev.
28:22013.
|
|
2
|
Marijnen CA, van de Velde CJ, Putter H,
van den Brink M, Maas CP, Martijn H, Rutten HJ, Wiggers T,
Kranenbark EK, Leer JW and Stiggelbout AM: Impact of short-term
preoperative radio-therapy on health-related quality of life and
sexual functioning in primary rectal cancer: Report of a
multicenter randomized trial. J Clin Oncol. 23:1847–1858. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camma C, Giunta M, Fiorica F, Pagliaro L,
Craxì A and Cottone M: Preoperative radiotherapy for resectable
rectal cancer: A meta-analysis. J Am Med Assoc. 284:1008–1015.
2000. View Article : Google Scholar
|
|
4
|
Holm T, Rutqvist LE, Johansson H and
Cedermark B: Postoperative mortality in rectal cancer treated with
or without preoperative radiotherapy: Causes and risk factors. Br J
Surg. 83:964–968. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Japanese Society for Cancer of the Colon
Rectum, . Japanese Classification of Colorectal Carcinoma (8th
edition). Kanehara Shuppan. Tokyo: 2013.
|
|
6
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplantat. 48:452–458. 2013. View Article : Google Scholar
|
|
7
|
Frykholm GJ, Glimelius B and Pahlman L:
Preoperative or postoperative irradiation in adenocarcinoma of the
rectum: Final treatment results of a randomized trial and an
evaluation of late secondary effects. Dis Colon Rectum. 36:864–872.
1993. View Article : Google Scholar
|
|
8
|
Peeters KC, Marijnen CA, Nagtegaal ID,
Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius
B, Leer JW, et al: The TME trial after a median follow-up of 6
years: Increased local control but no survival benefit in
irradiated patients with resectable rectal carcinoma. Ann Surg.
246:693–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swedish Rectal Cancer Trial, . Cedermark
B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE and Wilking N:
Improved survival with preoperative radiotherapy in resectable
rectal cancer. N Engl J Med. 336:980–987. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosset JF, Calais G, Mineur L, Maingon P,
Radosevic-Jelic L, Daban A, Bardet E, Beny A, Briffaux A and
Collette L: Enhanced tumorocidal effect of chemotherapy with
preoperative radiotherapy for rectal cancer: Preliminary
results-EORTC 22921. J Clin Oncol. 23:5620–5627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakafusa Y, Hirohashi Y, Tanaka T,
Kitajima Y, Sato S and Miyazaki K: Lateral lymph node dissection in
treatment for advanced lower rectal cancer. Jpn J Gastroenterol
Surg. 34:1512–1521. 2001. View Article : Google Scholar
|
|
12
|
Ishii Y, Hasegawa H, Endo T, Okabayashi K,
Ochiai H, Moritani K, Watanabe M and Kitagawa Y: Medium-term
results of neoadjuvant systemic chemotherapy using irinotecan,
5-fluorouracil, and leucovorin in patients with locally advanced
rectal cancer. Eur J Surg Oncol. 36:1061–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hasegawa J, Nishimura J, Mizushima T,
Miyake Y, Kim HM, Takemoto H, Tamagawa H, Noura S, Fujii M, Fujie
Y, et al: Neoadjuvant capecitabine and oxaliplatin (XELOX) combined
with bevacizumab for high-risk localized rectal cancer. Cancer
Chemother Pharmacol. 73:1079–1087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schrag D, Weiser MR, Goodman KA, Gonen M,
Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem
JG, et al: Neoadjuvant chemotherapy without routine use of
radiation therapy for patients with locally advanced rectal cancer:
A pilot trial. J Clin Oncol. 32:513–518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamiya T, Uehara K, Nakayama G, Ishigure
K, Kobayashi S, Hiramatsu K, Nakayama H, Yamashita K, Sakamoto E,
Tojima Y, et al: Early results of multicenter phase II trial of
perioperative oxaliplatin and capecitabine without radiotherapy for
high-risk rectal cancer: CORONA I study. Eur J Surg Oncol.
42:829–835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hasegawa S, Goto S, Matsumoto T, Hida K,
Kawada K, Matsusue R, Yamaguchi T, Nishitai R, Manaka D, Kato S, et
al: A multicenter phase 2 study on the feasibility and efficacy of
neoadjuvant chemotherapy without radiotherapy for locally advanced
rectal cancer. Ann Surg Oncol. 24:3587–3595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rödel C, Grabenbauer GG, Papadopoulos T,
Hohenberger W, Schmoll HJ and Sauer R: Phase I/II trial of
capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin
Oncol. 21:3098–3104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tulchinsky H, Shmueli E, Figer A, Klausner
JM and Rabau M: An interval >7 weeks between neoadjuvant therapy
and surgery improves pathologic complete response and disease-free
survival in patients with locally advanced rectal cancer. Ann Surg
Oncol. 15:2661–2667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roh MS, Colangelo LH, O'Connell MJ,
Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L,
Ursiny CS, Petrelli NJ and Wolmark N: Preoperative multimodality
therapy improves disease-free survival in patients with carcinoma
of the rectum: NSABP R-03. J Clin Oncol. 27:5124–5130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakuyama N, Kojima M, Kawano S, Akimoto T,
Saito N, Ito M and Ochiai A: Histological differences between
preoperative chemoradiotherapy and chemotherapy for rectal cancer:
A clinicopathological study. Pathol Int. 66:273–280. 2016.
View Article : Google Scholar : PubMed/NCBI
|