Introduction
Mucosal melanoma (MM) represents a highly aggressive
variant of malignant melanoma that arises within the resident
melanocytes of mucous linings. Comprising barely one-hundredth
fraction of all melanomas, it is an entity that is notorious for
the infinitesimal 5-year survival rate (<25%) (1). Although MM is often understood as a
blanket term for any extracutaneous melanoma, it nevertheless comes
with somewhat hazy disease definition; some authors regard uveal or
conjunctival melanomas as bona fide MM, while others are less
inclined to label the ocular tumours as such. The head and neck
(H&N) is cited as the region most heavily represented (~50%),
followed by the ano-rectum, and the female genital tract (FGT)
(2). The insidious nature of the
disease compounds accurate diagnosis, depriving the affected of any
remaining chance for an early detection. Failure to intervene early
often boomerangs with the amplified lethality, which is the
hallmark of the mucosal disease.
Given the miniscule incidence and patient survival
rate, randomised clinical trials (RCT) have been understandably
difficult to come by. The resulting paucity of evidence have long
clouded our understanding of tumour behaviour. Field clinicians
facing therapeutic decisions inevitably suffer from general lack of
consensus over virtually all aspects of the disease, from staging
to management. While it is tempting to extrapolate from CM-derived
data, the notion, that MM is fundamentally a distinctive entity, is
now considered canonical (3). Such
discrepancies include female preponderance, limited role of UV
(ultraviolet) light, and mutation status (4). The different makeup of mutation
landscape is thought to be the impetus that drives the divergence
between the two (5–7).
In the present meta-analysis and systematic review,
the authors present a comprehensive assessment of available
evidence to elaborate crucial factors that determine clinical
outcome in MM.
Materials and methods
Data collection and inclusion
criteria
Literature search was conducted using multiple
engines, most notably but not limited to, PubMed, EMBASE, Cochrane,
MEDLINE, and Google Scholar, up to March of 2018. The query
employed various keywords, such as ‘mucosal malignant melanom’,
‘anorectal melanoma’, ‘sino-nasal melanoma’ and ‘survival’; the
search was intended to include any abstract proceedings or graduate
theses [www.thesis.de], so as not to discount
‘grey’ literature from the study. No restriction was applied in
terms of the language of publication. The following criteria were
considered for selection: i) primary mucosal melanomas, ii)
reporting of Kaplan-Meier survival analysis results, or iii) Cox
regression analysis with time-to-event information. Where HR were
not explicitly given, they were imputed using the method described
by Tierney et al (8).
Excluded were studies i) on leptomeningeal melanomatosis, ii) based
on cell lines iii) performed on canine, murine or other non-human
subjects. The present study was conducted in accordance to the
Meta-analysis of Observational Studies in Epidemiology guidelines
for the reporting of meta-analyses of observational studies (MOOSE)
(9).
Statistical analysis
The principal parameter of effect size (ES)
reporting used in the study was hazard ratio (HR), in terms of
melanoma-specific survival (=disease-specific survival, DSS) and
overall survival (=all-cause survival, OS). The main surrogate for
detecting between-study heterogeneity was the I2
statistic. The assumption of homogeneity was considered valid if
I2 was <50%, in which cases the fixed effect model
was used; for all other cases, the random effect model was used.
Before incorporating a study into analysis, sensitivity testing was
performed to decide if there was a pulling effect by single studies
with substantial weight. Publication bias was assessed with funnel
plots and Egger test. Statistical analyses were carried out with
Comprehensive Meta-Analysis Software (v3.0; Biostat, Englewood, NJ,
USA). P<0.05 were considered to indicate a statistically
significant difference.
Results
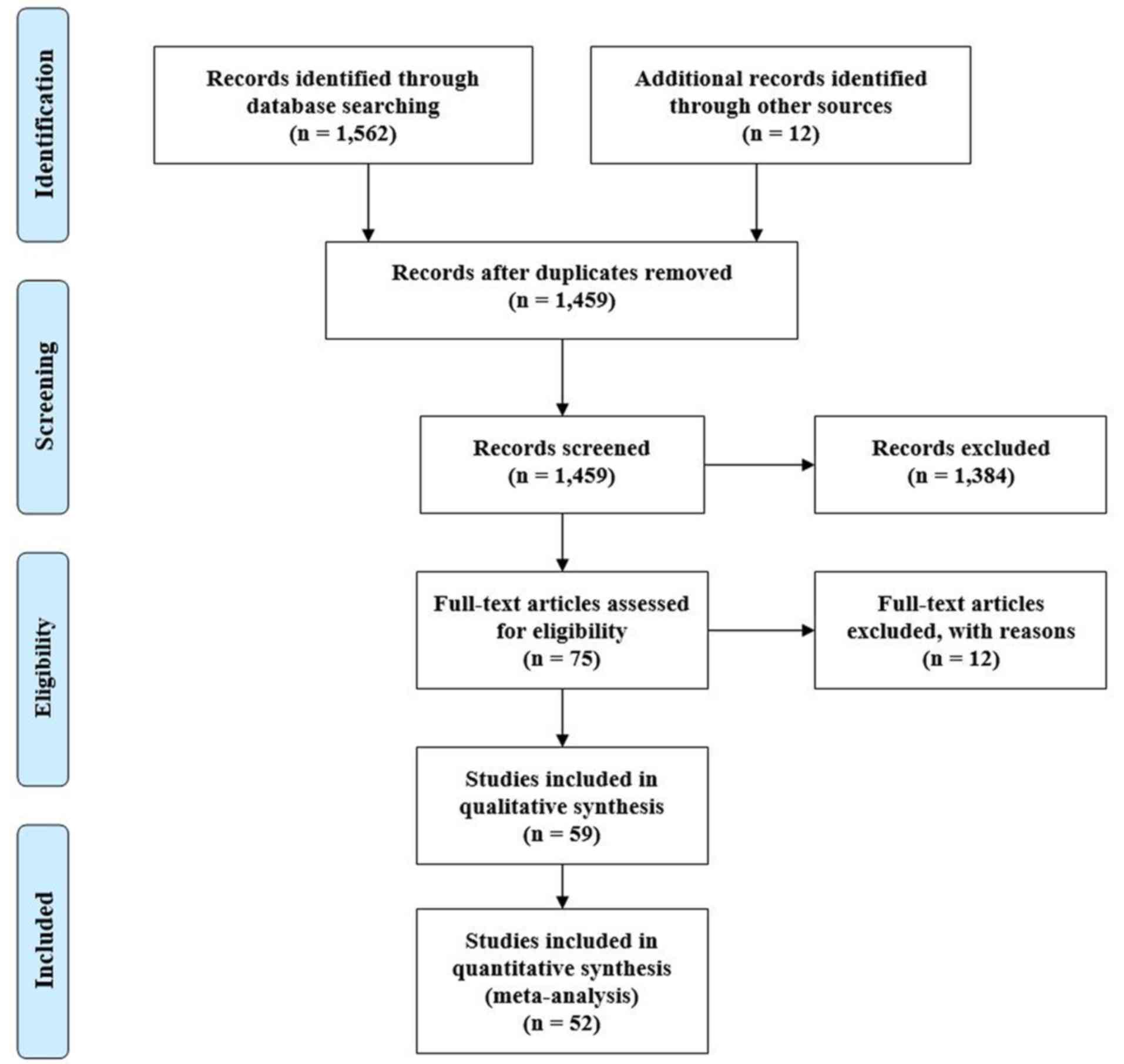
PRISMA (Preferred Reporting Items for Systematic
Reviews and Meta-Analyses) (10)
flow diagram of the search strategy, and characteristics of the
included studies are given in Fig. 1
and Table I, respectively. Search
query using the aforementioned keywords initially returned 1,459
articles from 8 different databases, of which 52 were deemed to
suit our agenda. All the studies originated from three continent
regions: North/Central America (18, 34.6%), Asia/Indian
subcontinent/Oceania (21, 40.4%), and the European Union (13,
25.0%). Topographically, 27 studies (51.9%) were on head and neck
region (MMHN), 4 (7.7%) on gastrointestinal tract, 3 (5.8%) on
urinary/female genital tract, and 18 (34.6%) on all mucosal sites.
Potential survival variables were arbitrarily categorised into
three groups: i) host factors, which is demographic characteristics
of the affected individual, ii) tumour factors, relating to various
aspects of tumour histology, behaviour, and staging, and iii)
treatment factors, which are parameters that assess the impact of
differing treatment modalities on survival.
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
| Author, year |
Countryb | Location | No. of
patients | Follow-up | Ref. |
|---|
| Abugideiri et
al, 2016 | USA | H&N | 39 (SRT=27;
S=12) | Median 8.1
years | 17 |
| Ahn et al,
2010 | Korea | H&N | 32 (SRT=16;
S=16) | Median 25.3
months | 18 |
| Aiempanakit et
al, 2018 | Thailand | All mucosal | 17 (S=14,
UN=3) | Median 18.2
months | 19 |
| Ajmani et
al, 2017 | USA | SN | 704 (SRT=399;
S=305) | NR | 20 |
| Amit et al,
2018 | USA | SN | 198 (SRT=81; S=79;
SCRT=24; C or CRT=14) | Median 26
months | 21 |
| D'Angelo et
al, 2016 | USA | All mucosal | 889 (ipilimumab and
nivolumab) | 6.2 months | 22 |
| Benlyazid et
al, 2010 | France | H&N | 160 (SRT=78;
S=82) | Median 65.2
months | 23 |
| Bishop and
Olszewski 2014 | USA | All, including
CMa | 229,976 (NR) | NR | 24 |
| Chiu and Weinstock,
1996 | USA | OC | 40,320 (NR) | NR | 25 |
| Ciarrocchi et
al, 2017 | Italy | Anorectum | 208 (SRT=32;
S=167) | Median 14
months | 26 |
| Ercelep et
al, 2016 | Turkey | All mucosal | 229,976 (NR) | Median 27
months | 27 |
| Harada et
al, 2016 | Japan | Oesophagus | 10 (S=10) | NR | 28 |
| Hasebe et
al, 2017 | Japan | H&N | 85 (RT=85) | Median 42.5
months | 29 |
| Heinzelmann- | Australia | Vulva | 33 (NR) | NR | 30 |
| Schwarz et
al, 2014 |
| Heppt et al,
2017 | Germany | All mucosal | 444 (NR) | NR | 31 |
| Hughes et
al, 2013 | Australia | All, including
CMa | 485
(Lymphadenectomy) | Median 17.4
months | 32 |
| Jang et al,
2014 | Korea | All, including
CMa | 206 (S=197; C=46;
RT=31) | NR | 33 |
| Kang et al,
2018 | China | All mucosal | 60 (NR) | Median 36
months | 34 |
| Khan et al,
2014 | USA | SN | 567 (NR) | NR | 35 |
| Kirchoff et
al, 2016 | USA | All mucosal | 227 (S=53; S +
other=174) | NR | 36 |
| Kirschner et
al, 2013 | USA | Vagina | 201 (SRT=53; S=87;
RT=30) | Median 14
months | 37 |
| Kong et al,
2016 | China | All, including
CMa | 412 (NR) | Median 31
months | 38 |
| Konuthula et
al, 2017 | USA | SN | 695 (SRT=271;
S=206; SC=29; SCRT=49; C=21; RT=42) | NR | 39 |
| Koto et al,
2017 | Japan | H&N | 260 (RT=105;
CRT=155) | Median 22
months | 40 |
| Kuk et al,
2016 | Korea | OC | 39 (S=22; S + C or
RT=17) | NR | 41 |
| Lansu et al,
2018 | Netherlands | SN | 63 (SRT=63) | Median 23
months | 42 |
| Lawaetz et
al, 2016 | Denmark | H&N | 98 (SRT=26; S=49;
SC=2; SCRT=2; RT=8; None=8) | Median 24.5
months | 43 |
| Lee et al,
2017 | Korea | H&N | 31 (SRT=13; S=9;
SC=7; SCRT=2) | Mean 9 months | 44 |
| Lee et al,
2017 | USA | OC | 232 (NR) | NR | 45 |
| Lombardi et
al, 2016 | Italy | SN | 58 (SRT=13; S=42;
SCRT=3) | Median 30
months | 46 |
| Mücke et al,
2009 | Germany | OC | 10 (NR) | NR | 47 |
| Nakamura et
al, 2018 | Japan | All mucosal | 45 (checkpoint
inhibitors) | NR | 48 |
| Oba et al,
2011 | Japan | All, including
CMa | 78 (NR) | Median 40
months | 49 |
| Pandey et
al, 2002 | India | H&N | 60 (SRT=6; S=17;
SC=3; SCRT=1; C=8; RT=7) | NR | 50 |
| Pfeil et al,
2011 | Germany | All mucosal | 172 (NR) | Median 24
months | 51 |
| Plavc et al,
2016 | Slovenia | H&N | 61 (SRT=14; S=17;
C=1; RT=15) | Median 16.5
months | 52 |
| Roh et al,
2016 | Korea | All mucosal | 392 (NR) | Mean 55.4
months | 53 |
| Samstein et
al, 2016 | USA | SN | 78 (SRT=64;
S=14) | Median 21
months | 54 |
| Sanchez et
al, 2016 | USA | Genitourinary
tract | 1,586 (NR) | NR | 55 |
| Schaefer et
al, 2017 | Germany | All mucosal | 75 (checkpoint
inhibitors) | NR | 56 |
| Schmidt et
al, 2017 | USA | H&N | 1,368 (SRT=704;
S=566; RT=98) | Median 55.2
months | 57 |
| Shoushtari et
al, 2017 | USA | All mucosal | 81 (NR) | NR | 58 |
| Shuman et
al, 2011 | USA | H&N | 52 (SRT=15; S=13;
SC=18; NR=6) | Median 97
months | 59 |
| Song et al,
2016 | China | OC | 62 (NR) | Median 32.5
months | 60 |
| Sun et al,
2014 | China | SN | 65 (SRT=13; S=18;
SC=9; C=6; RT=4; CRT= 2) | NR | 61 |
| Tchelebi et
al, 2016 | USA | Rectum | 63 (SRT=18;
S=45) | Median 17
months | 62 |
| Thariat et
al, 2011 | France | SN | 155 (NR) | Median 37
months | 63 |
| Wang et al,
2013 | China | OC | 81 (NR) | NR | 64 |
| Wen et al,
2017 | China | All mucosal | 52 (checkpoint and
PD-1 inhibitors) | NR | 65 |
| Won et al,
2015 | Korea | SN | 155 (NR) | NR | 66 |
| Yeh et al,
2006 | USA | Anorectum | 46 (S=23;
C=23) | Median 29
months | 67 |
| Yi et al,
2011 | Korea | All, including
CMa | 95 (NR) | Median 41
months | 68 |
Host factors
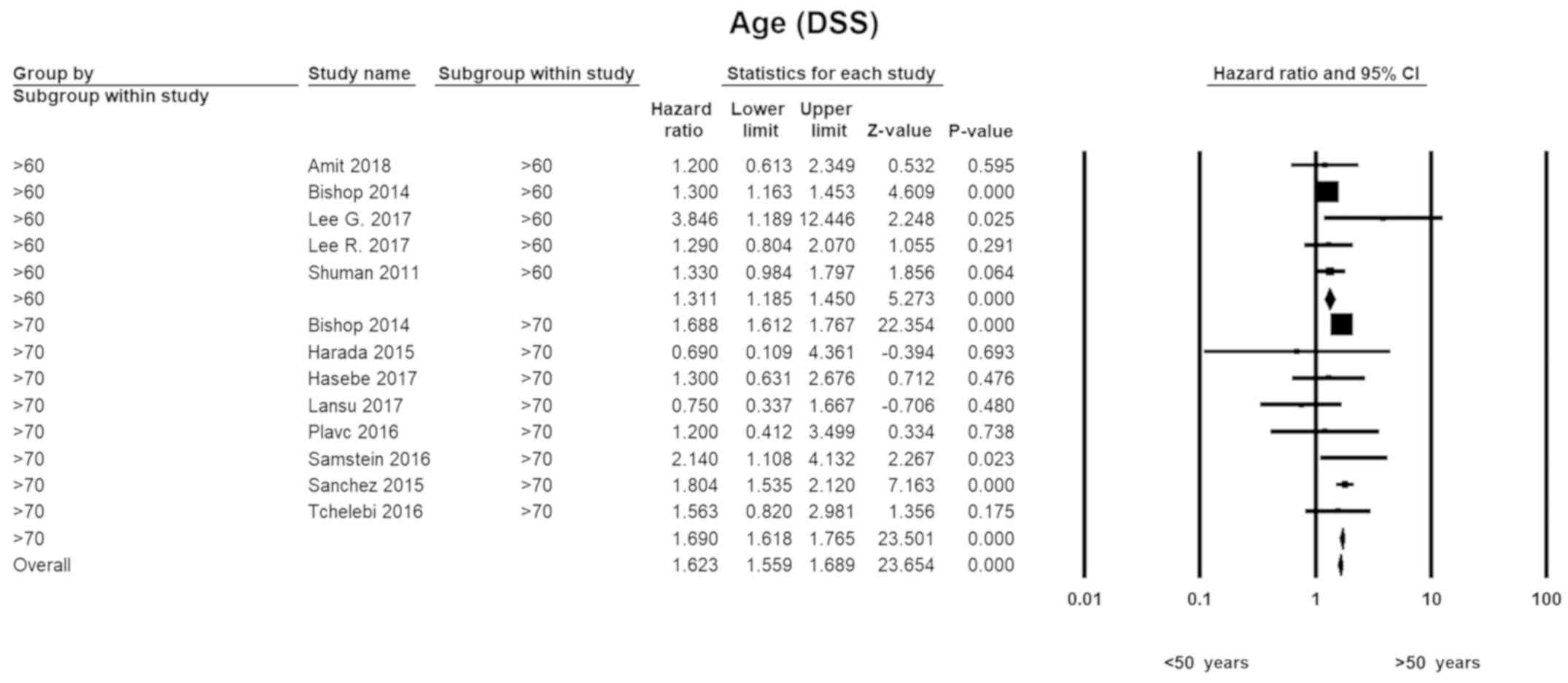
Age
With respect to younger individuals (<50 years),
the HR for those in the seventh decade of life was 1.3 (HR=1.31;
95% CI, 1.19–1.45; P=0.00). The disease-specific hazards for
patients in their 70's were 1.7 (HR=1.69; 95% CI, 1.62–1.77;
P=0.00). A similar pattern was seen with overall survival. There
was no evidence of heterogeneity in any of the subgroups (Fig. 2).
Sex
The HR for males was calculated to be 1.1 (HR=1.11;
95% CI, 0.93–1.31; P=0.26). The value was similar for OS (HR=1.12;
95% CI, 1.03–1.23; P=0.01). No statistical heterogeneity was found
(I2=32.14).
Ethnicity
Pooled HR, with non-Hispanic white Caucasians as
reference, was computed for patients with African, Asian/Pacific
Island, and other (including white Hispanic, Native American and
Mestizos) ancestries. Compared to Caucasian individuals, the hazard
to overall survival for non-Caucasians as a whole was ~1.4
(HR=1.39, 95% CI, 1.06–1.82; P=0.02). Apart from the overall death
risk, ethnicity of the affected per se did not have seem to be a
major influence on survival (Table
II).
 | Table II.Hazard ratios for non-Caucasian
ethnicities. |
Table II.
Hazard ratios for non-Caucasian
ethnicities.
| Ethnicity
comparison | Survival | No. of studies | Pooled HR | 95% CI | Z-value | P-value | I2 |
|---|
| Non-Caucasian vs.
Caucasian | DSS | 5 | 1.12 | 1.05–1.20 | 3.354 | 0.001 | 0.0001 |
| Non-Caucasian vs.
Caucasian | OS | 3 | 1.39 | 1.06–1.82 | 2.358 | 0.018 | 0.0001 |
| Afro-American vs.
Caucasian | DSS | 6 | 1.13 | 0.95–1.34 | 1.421 | 0.155 | 4.451 |
| API vs.
Caucasian | DSS | 2 | 1.09 | 0.80–1.49 | 0.563 | 0.574 | 91.47 |
Comorbidities and ‘High-risk’
lifestyle
Having one or more major comorbidities showed a weak
correlation to increased risk in all-cause mortality (HR=1.43, 95%
CI, 1.01–2.04; P=0.04). On the other hand, the mode of life
traditionally considered ‘high-risk’-e.g., sedentariness, obesity,
smoking-was found to be a significant threat to neither
disease-specific (HR=1.41, 95% CI, 0.98–2.03; P=0.07) nor overall
(HR=1.24, 95% CI, 0.98–1.56; P=0.14) survival.
Tumour factors
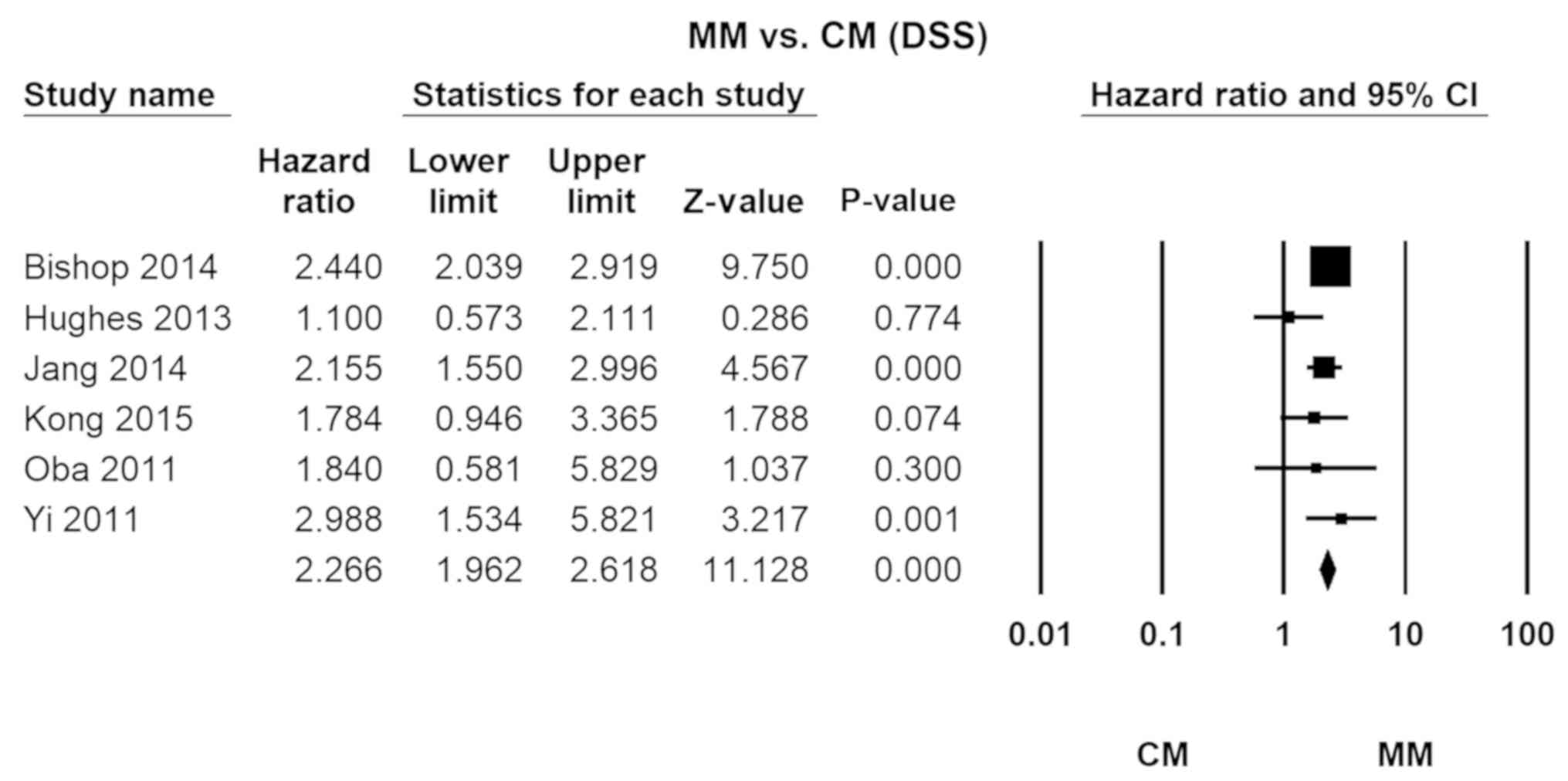
Cutaneous melanoma
The relative lethality of MM vs. CM was 2.25
(HR=2.27, 95% CI, 1.96–2.62; P=0.00). No significant heterogeneity
was detected across the studies (I2=26.41; Fig. 3).
Location
A primary lesion originating within the sino-nasal
(SN) cavity was found to be 1.4 times more deadly compared to other
locations (HR=1.44; 95% CI, 1.28–1.63; P=0.00). The HR for OS was
nearly 2.0 (HR=1.93; 95% CI, 1.59–2.33; P=0.00). Head and neck
lesions (MMHN) as a whole showed an HR of 1.4 (HR=1.35; 95% CI,
1.02–1.79; P=0.00) for overall survival.
Multifocal disease
MM is a devastating cancer partly because of its
tendency to arise from multiple foci. The associated
disease-specific death risk was nearly 3.0 (HR=2.95; 95% CI,
2.72–3.19; P=0.00).
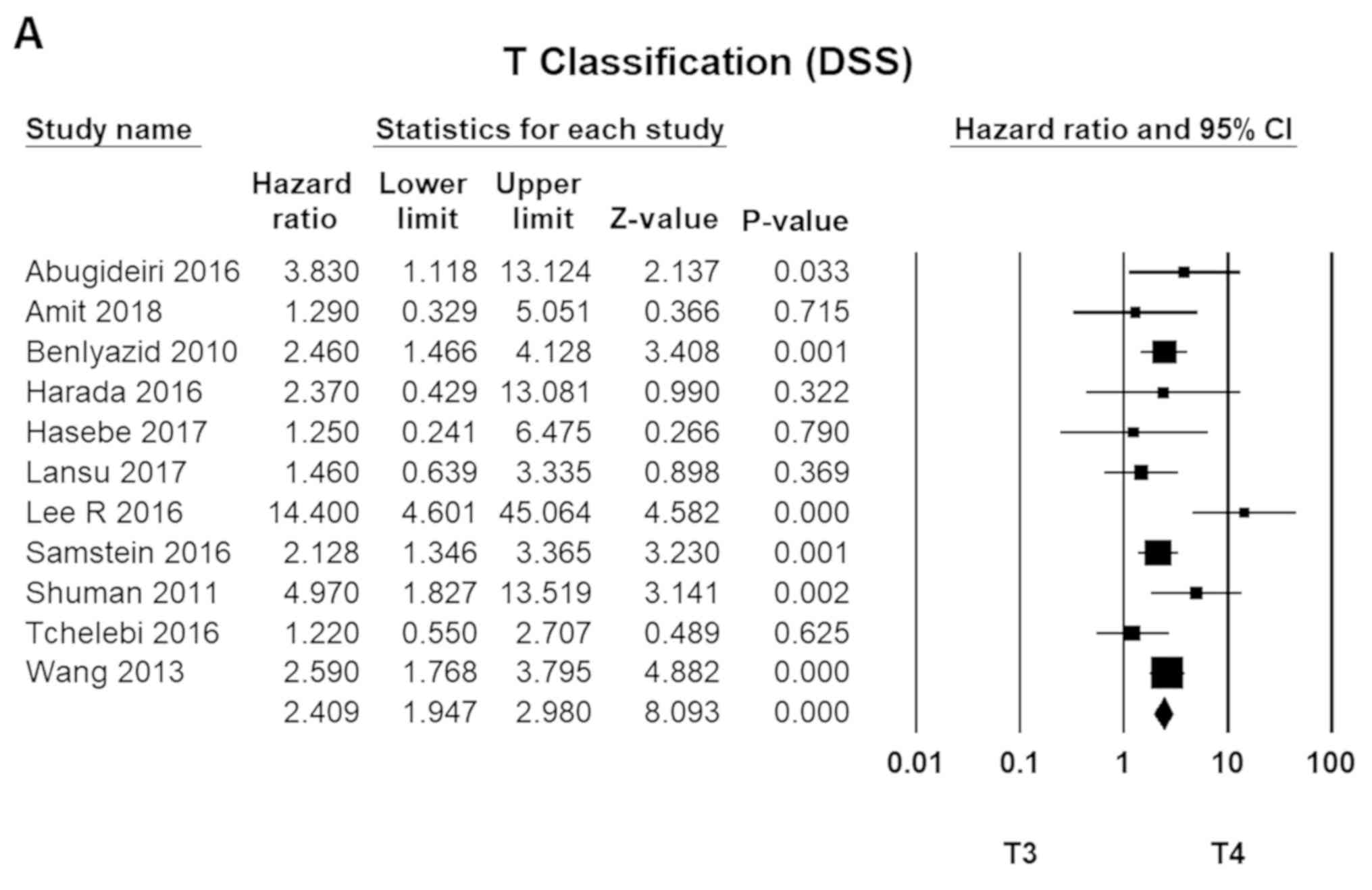
Clinical staging (MMHN)
The TNM staging system, developed by the American
Joint Committee on Cancer (AJCC), is one of the most widely
accepted standards for MMHN staging and conventionally the most
accurate predictor of survival. T4 disease (T4a and T4b) was 2.4
times more fatal than T3 tumours (95% CI, 1.75–2.98; P=0.00).
Meanwhile, N1 disease had an HR of 2.0 compared to N0 (HR=1.90; 95%
CI, 1.62–2.23; P=0.00). For metastatic diseases (M1), the HR was
3.2 (HR=3.17; 95% CI, 2.72–3.70; P=0.00; Fig. 4).
Clinical
features/Macro-morphology
Elevated lactate dehydrogenase (LDH) level was
associated with the greatest HR for disease-specific survival
(HR=2.06; 95% CI, 1.56–2.72; P=0.00). Higher performance score (PS)
was correlated with increased risk for OS (HR=1.71; 95% CI,
1.32–2.21; P=0.00). Ulceration of primary lesions was also linked
to unfavourable OS. The verdict on pigmentation (HR=0.87; 95% CI,
0.66–1.15; P=0.34), necrosis, and nodularity of primary tumours was
inconclusive (Table III).
 | Table III.Hazard ratios for
clinical/macro-morphological features. |
Table III.
Hazard ratios for
clinical/macro-morphological features.
| Feature
comparison | Survival | No. of studies | Pooled HR | 95% CI | Z-value | P-value | I2 |
|---|
| Elevated LDH vs.
WNL | DSS | 4 | 2.06 | 1.56–2.72 | 5.104 | 0.001 | 0.001 |
| PS>1 vs.
PS<0 | OS | 4 | 1.71 | 1.32–2.21 | 4.112 | 0.001 | 0.001 |
| Ulceration vs. no
ulceration | DSS | 3 | 1.32 | 0.91–1.90 | 1.465 | 0.143 | 6.401 |
| Ulceration vs. no
ulceration | OS | 4 | 1.44 | 1.04–2.01 | 2.191 | 0.215 | 32.95 |
| Pigmentation vs. no
pigmentation | OS | 3 | 0.93 | 0.70–1.25 | 0.464 | 0.642 | 0.001 |
| Necrosis vs. no
necrosis | DSS | 2 | 1.29 | 0.96–1.73 | 1.708 | 0.088 | 0.001 |
| Necrosis vs. no
necrosis | OS | 2 | 0.96 | 0.55–1.68 | 0.013 | 0.989 | 72.12 |
Microscopic features
Margin status was the most important
micro-morphological determinant of survival. The HR attributed to
margin positivity was nearly 2.0 (HR=1.85; 95% CI, 1.34–2.54;
P=0.00). The effect of perineural invasion (PNI) and
lympho-vascular invasion (LVI) was not statistically significant.
Meanwhile, Breslow thickness, depth of invasion, and mitotic count
did not seem to play a significant role in either terms of survival
(Table IV).
 | Table IV.Hazard ratios for microscopic
features. |
Table IV.
Hazard ratios for microscopic
features.
| Feature
comparison | Survival | No. of studies | Pooled HR | 95% CI | Z-value | P-value | I2 |
|---|
| (+) Margin vs. (−)
margin | DSS | 10 | 1.85 | 1.34–2.54 | 3.759 | 0.001 | 23.84 |
| (+) Margin vs. (−)
margin | OS | 10 | 1.59 | 1.21–2.08 | 3.365 | 0.001 | 44.22 |
| Breslow >1 mm
vs. Breslow <1 mm | DSS | 6 | 1.07 | 0.99–1.19 | 1.755 | 0.079 | 29.63 |
| Breslow >1 mm
vs. Breslow <1 mm | OS | 3 | 1.07 | 0.99–1.17 | 1.621 | 0.105 | 11.23 |
| Invasion >2 mm
vs. invasion <2 mm | DSS | 3 | 2.02 | 0.68–6.03 | 1.259 | 0.208 | 81.02 |
| Invasion >2 mm
vs. invasion <2 mm | OS | 4 | 2.02 | 1.26–0.23 | 2.913 | 0.004 | 0.001 |
| Mitosis (+) vs.
mitosis (−) | DSS | 4 | 1.09 | 1.03–1.15 | 2.875 | 0.004 | 0.001 |
| Mitosis (+) vs.
mitosis (−) | OS | 4 | 1.06 | 1.01–1.12 | 2.405 | 0.016 | 0.001 |
| PNI vs. PNI
(−) | DSS | 2 | 2.08 | 0.97–4.4 | 1.884 | 0.06 | 42.65 |
| Lymphovascular
invasion vs. no invasion | DSS | 3 | 1.24 | 0.94–1.64 | 1.537 | 0.124 | 0.001 |
| Epithelioid type
vs. non-epithelioid | DSS | 3 | 1.29 | 0.94–1.78 | 1.561 | 0.118 | 0.001 |
Treatment factors
Extent of treatment
Radical operation was found to amplify overall death
risk by 2.5 (HR=2.61; 95% CI, 2.04–3.34; P=0.00); When surgery was
the sole modality of treatment, it was associated with a
significant risk elevation in both terms of survival (HR of 1.72
and 2.21, respectively). Conversely, when any modality but surgery
was used, similar increase in mortality was observed. For
therapeutic regimen consisted entirely of chemotherapy, the
attributed risk in mortality was around 1.5. Meanwhile,
radiotherapy (RT) apparently carried the least detriment to patient
survival as monotherapy.
The value of lymphadenectomy for primary tumours in
the cephalo-cervical subsite was dubious (HR=0.86; 95% CI,
0.73–1.02; P=0.07). Likewise, endoscopic resection showed neither
inferior nor superior results compared to the more traditional
approach in terms of survival benefit (P=0.83 and 0.68,
respectively; Table V).
 | Table V.Hazard ratios for different
modalities of treatment. |
Table V.
Hazard ratios for different
modalities of treatment.
| Modality
comparison | Survival | No. of studies | Pooled HR | 95% CI | Z-value | P-value | I2 |
|---|
| Radical op. vs.
Conservative Tx | OS | 5 | 2.61 | 2.04–3.34 | 15.079 | 0.001 | 55.35 |
| Op. alone vs.
SC/SRT | DSS | 11 | 1.78 | 1.55–2.05 | 8.192 | 0.001 | 30.85 |
| RT alone vs.
SRT | DSS | 5 | 1.29 | 1.08–1.54 | 2.831 | 0.005 | 19.37 |
| RT alone vs.
SRT | OS | 4 | 1.52 | 1.35–1.70 | 7.087 | 0.001 | 26.97 |
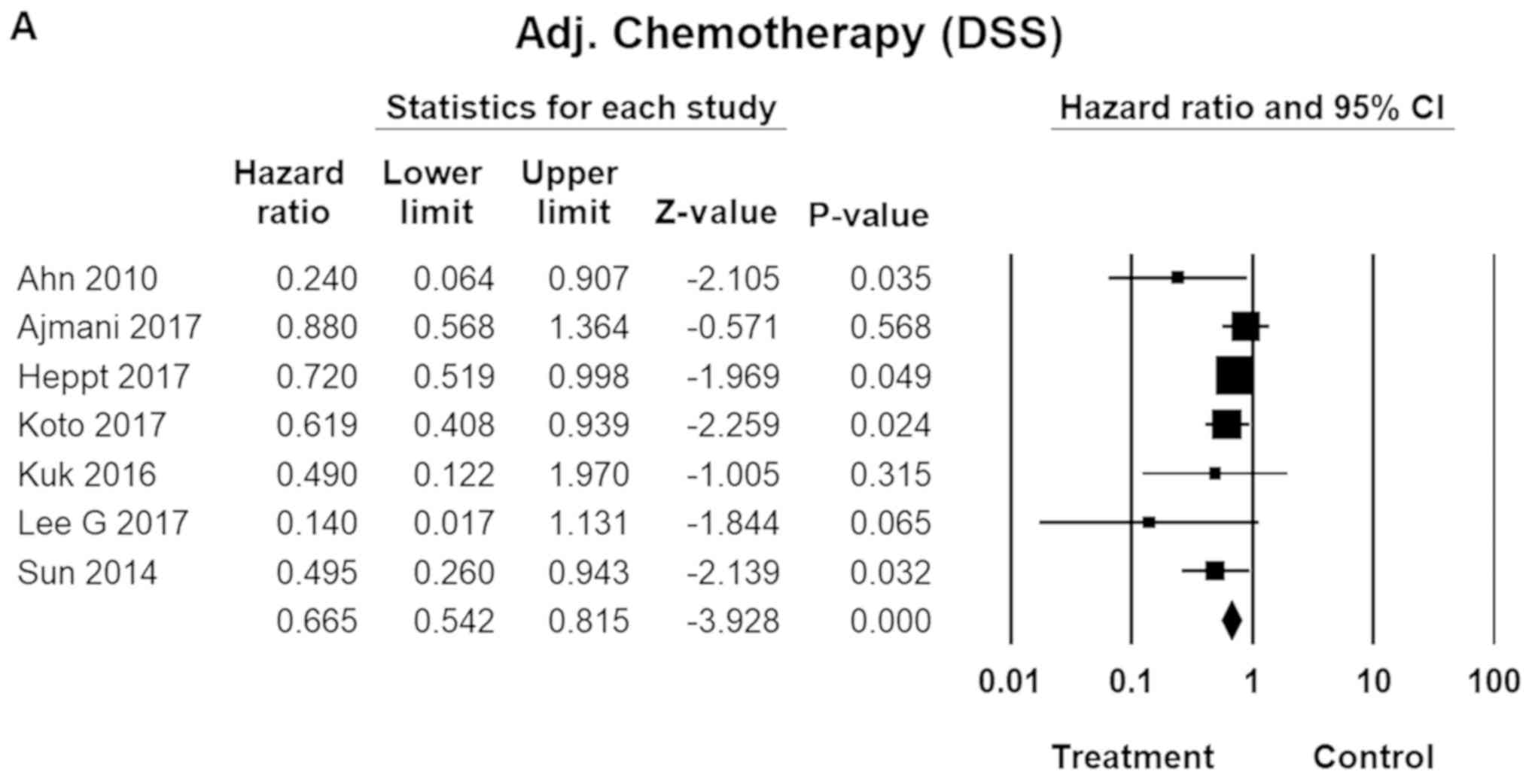
Adjuvant therapy
Adjuvant chemotherapy was found to reduce both
disease-specific and overall death by some 30 percent. The
therapeutic regimen included cisplatin/tamoxifen, dacarbazine
(DTIC), and interferon-γ (INF-γ). RT, while also significantly
effective, tended to be somewhat less efficacious (HR=0.84; 95% CI,
0.82–0.86; P=0.01; Fig. 5).
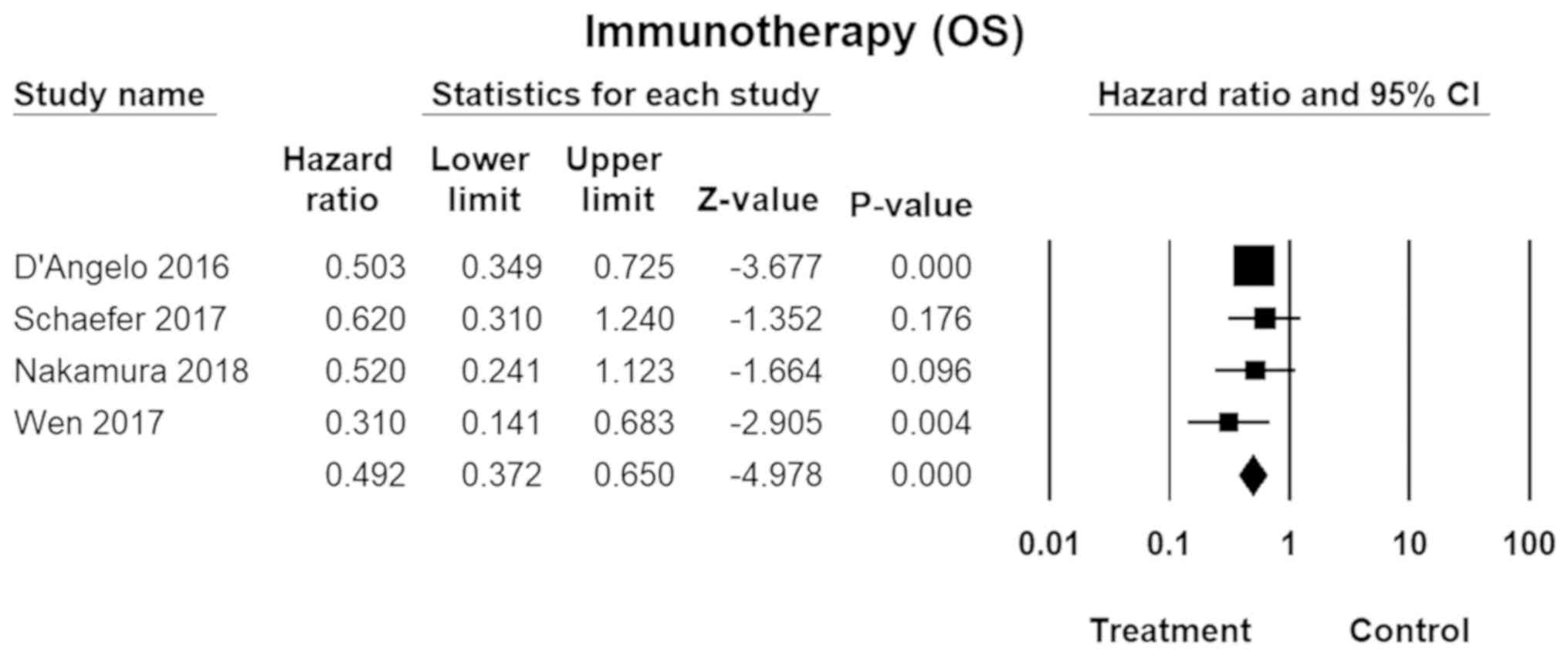
Immunotherapy
Immunotherapy, usually involving PD-1 (programmed
death protein-1), immune checkpoint inhibitors (e.g., CTLA-4), or a
combination of the two, was shown to more effective for MM than CM.
The pooled HR was 0.49 (95% CI, 0.37–0.65; P=0.00; Fig. 6) for overall survival. No inter-study
heterogeneity was found across the studies
(I2=0.00).
Discussion
The present meta-analysis had aimed to provide an
updated review on various aspects of MM, with data from the most
recent studies. The genetic and molecular underpinning behind the
distinctive biologic behaviour is believed to stem from
amplification of c-Kit (11),
a receptor tyrosine kinase (RTK). In contrast, b-Raf and
n-Ras mutations are infrequent in MM. This oncogenic
mutation profile is reminiscent of the acral lentiginous subtype of
CM (ALM). Quite fittingly, ALM shares several characteristics with
MM in common, namely i) infrequency (1–2% of all CM), ii) delayed
detection and hence worse prognosis, and iii) relative
preponderance in non-Caucasian ethnic groups.
Although what is known about MM pales in comparison
to the cutaneous disease, a few generalities can be drawn from our
analysis: in the authors' estimation, MM was two-and-a-quarter
times more life-threatening than CM. As a whole, the influence of
the ‘host factors’ was not imposing; one pattern that stood out was
advanced age. The median age of onset for MM is higher than CM, at
67 years (vs. 55 years for CM). The death risk in this age group
was more than 1.5, compared to the younger cohort (<50 years),
which might partially account for the higher mortality. While the
incidence tends to be higher and the prognosis grimmer for male
melanoma patients in general, MM is an exception; it is reasonably
well established that MM shows predilection for females (12). Moreover, there seemed to be no
respect of sexes with MM when it comes to mortality, although male
individuals may be at a slight disadvantage as far as overall
survival is concerned. MM is also peculiar from ethnic perspectives
because the higher proportion of non-Caucasian patients (especially
African and Asian races) (13) is
higher. This point is underlined by the fact that 40% of the
referenced studies came from regions where the indigenous
population is not of white Caucasian ancestry. Nevertheless, racial
disparities did not appear to be a major deciding factor in
MM-specific mortality. The higher all-cause mortality for non-white
cohorts may point to either supposedly superior overall quality of
care in Western facilities, or a legitimate, ethno-genetic
differences in the ability of the body system to cope with the
cancer or mount anti-tumour immune defence against. The fact that
undesirable health-related behaviours played negligible role in
survival may be one indication that the intrinsic cancer behaviour
wields an overriding influence above other variables.
Mucosal melanoma of the head and neck (MMHN), cited
as the most common location of MM occurrence overall, also carried
the worst prognosis. Tumours in the paranasal sinuses
(PNS)-maxillary and ethmoid, etc.-predisposed the individuals to
significantly higher disease-specific and overall mortality, with
the latter perhaps reflecting the inaccessibility of the subsite,
rendering it all the more unfeasible to carry out effective
surgical manoeuvres. Tumour thickness would normally be one of
objective prognosticators for solid organ cancers. That said, the
usefulness of the AJCC clinical stageing system in CM cannot be
readily engrafted into mucosal patients, the reason for which is
questionable validity of tumour thickness as a prognostic index
(14). This notion has been backed
by the authors' findings, that neither thickness nor depth of
invasion is a significant determinant of survival (Table IV).
Although surgery constitutes the backbone of
management strategy in many cases, radical excision seems to be a
poor choice of treatment for the considerable morbidity and added
mortality associated. Any mono-modality therapy was shown to
increase death risk by at least 1.5. For inoperable cases,
immunotherapeutic regimen, usually consisting of combination of
CTLA-4 and PD-1 inhibitors (e.g., nivolumab and ipilimumab), may be
the most rational option. Also, both chemotherapy and radiotherapy
were found to be survival-benefitting adjuvant modalities. However,
as of now, there is no clearly established formula for specific
combination of for chemotherapeutic agents and anti-tumour
biologics (‘cocktails’).
The current study was hampered by a few limitations.
The validity of disease-specific survival (DSS), the primary
measure of effect sizes, is grounded on the premise of the reported
cause of death being accurate. This inherent risk can potentially
be a limiting factor with cancers such as MM, in which the high
lethality can often obscure the true cause of death. In addition,
all but two of the included studies came out after the year 2010.
This is mainly due to the rarity of the disease, with many studies
taking several decades to complete.
In summing up, mucosal melanoma is a highly
malignant entity that is difficult to detect, treat, and even
study. It is accentuated by an oncogenic profile that is at odds
with the more prevalent cutaneous disease. Microscopic frequency,
coupled with air of pessimism surrounding the gross ineffectuality
of conventional arsenal, may have pushed it into relative obscurity
and disinterest. Nonetheless, a body of recent evidence indicates
its incidence is on the rise (15,16), and
may well be on its way to becoming a force to be reckoned with.
Further studies, elaborating on the oncogenic pathways and driver
mutations, are needed to improve the overall outlook of this
fearsome cancer, especially now that the era of three
P's-precision, personalized, and preventive oncology-is looming
over the horizon.
Acknowledgements
Not applicable.
Funding
This work was supported by Konyang University
Myunggok Research Fund of 16.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HMH and KGL designed and conducted the study. HJH
and KGL produced the manuscript. WC, KGL, HJH, HMH, SHC and KBM
performed the statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
ALM
|
acral lentiginous melanoma
|
|
CI
|
confidence interval
|
|
CM
|
cutaneous melanoma
|
|
DSS
|
disease-specific survival
|
|
ES
|
effect sizes
|
|
H&N
|
head and neck
|
|
HR
|
hazard ratio
|
|
I2
|
degree of inconsistency
|
|
LDH
|
lactate dehydrogenase
|
|
LVI
|
lympho-vascular invasion
|
|
LRC
|
loco-regional control
|
|
MM
|
mucosal melanoma
|
|
MMHN
|
mucosal melanoma of head and neck
|
|
MSS
|
melanoma-specific survival
|
|
NR
|
not reported
|
|
OC
|
oral cavity
|
|
OS
|
overall survival
|
|
PNI
|
perineural invasion
|
|
PNS
|
paranasal sinuses
|
|
PS
|
performance score
|
|
RCT
|
randomised controlled trials
|
|
RT
|
radiotherapy
|
|
RTK
|
receptor tyrosine kinase
|
|
SN
|
sino-nasal
|
|
UV
|
ultraviolet
|
References
|
1
|
Patrick RJ, Fenske NA and Messina JL:
Primary mucosal melanoma. J Am Acad Dermatol. 56:828–834. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tacastacas JD, Bray J, Cohen YK, Arbesman
J, Kim J, Koon HB, Honda K, Cooper KD and Gerstenblith MR: Update
on primary mucosal melanoma. J Am Acad Dermatol. 71:366–375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gru AA, Becker N, Dehner LP and Pfeifer
JD: Mucosal melanoma: Correlation of clinicopathologic, prognostic,
and molecular features. Melanoma Res. 24:360–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thierauf J, Veit JA, Affolter A, Bergmann
C, Grünow J, Laban S, Lennerz JK, Grünmüller L, Mauch C, Plinkert
PK, et al: Identification and clinical relevance of PD-L1
expression in primary mucosal malignant melanoma of the head and
neck. Melanoma Res. 25:503–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ragnarsson-Olding BK, Karsberg S, Platz A
and Ringborg UK: Mutations in the TP53 gene in human malignant
melanomas derived from sun-exposed skin and unexposed mucosal
membranes. Melanoma Res. 12:453–463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu W, Li S, Zou B, Liu H and Wang S:
Expressions and clinical significance of HER4 and CD44 in sinonasal
mucosal malignant melanoma. Melanoma Res. 28:105–110. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SY, Kim SN, Hahn HJ, Lee YW, Choe YB
and Ahn KJ: Metaanalysis of BRAF mutations and clinicopathologic
characteristics in primary melanoma. J Am Acad Dermatol.
72:1036–46.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohashi A, Funasaka Y, Ueda M and Ichihashi
M: c-KIT receptor expression in cutaneous malignant melanoma and
benign melanotic naevi. Melanoma Res. 6:25–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Postow MA, Hamid O and Carvajal RD:
Mucosal melanoma: Pathogenesis, clinical behavior, and management.
Curr Oncol Rep. 14:441–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Altieri L, Wong MK, Peng DH and Cockburn
M: Mucosal melanomas in the racially diverse population of
California. J Am Acad Dermatol. 76:250–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luna-Ortiz K, Aguilar-Romero M,
Villavicencio-Valencia V, Zepeda-Castilla E, Vidrio-Morgado H,
Peteuil N and Mosqueda-Taylor A: Comparative study between two
different staging systems for mucosal melanomas of the Head and
Neck. Med Oral Patol Oral Cir Bucal. 21:e425–e430. 2016.PubMed/NCBI
|
|
15
|
Youssef D, Vasani S, Marquess J and Cervin
A: Rising incidence of head and neck mucosal melanoma in Australia.
J Laryngol Otol. 131:S25–S28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marcus DM, Marcus RP, Prabhu RS, Owonikoko
TK, Lawson DH, Switchenko J and Beitler JJ: Rising incidence of
mucosal melanoma of the head and neck in the United States. J Skin
Cancer. 2012:2316932012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abugideiri M, Patel K, Switchenko JM,
Magliocca K, Buchwald ZS, Delgaudio J, Beitler JJ and Khan MK:
Adjuvant radiation therapy improved local control for primary
mucosal melanoma of the head and neck. Int J Radiation Oncol.
96:E384–E385. 2016. View Article : Google Scholar
|
|
18
|
Ahn HJ, Na II, Park YH, Cho SY, Lee BC,
Lee GH, Koh JS, Lee YS, Shim YS, Kim YK, et al: Role of adjuvant
chemotherapy in malignant mucosal melanoma of the head and neck.
Oral Oncol. 46:607–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aiempanakit K, Chiratikarnwong K,
Auepemkiate S and Sriplung H: Clinicopathologic characteristics and
survival outcomes of primary mucosal melanomas: A 10-year
retrospective analysis from a single tertiary medical center in
Thailand. Zhonghua Pifuke Yixue Zazhi xxx. 1–3. 2018.
|
|
20
|
Ajmani GS, Liederbach E, Kyrillos A, Wang
CH, Pinto JM and Bhayani MK: Adjuvant radiation and survival
following surgical resection of sinonasal melanoma. Am J
Otolaryngol. 38:663–667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amit M, Tam S, Abdelmeguid AS, Roberts DB,
Raza SM, Su SY, Kupferman ME, DeMonte F and Hanna EY: Approaches to
regional lymph node metastasis in patients with head and neck
mucosal melanoma. Cancer. 124:514–520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D'Angelo SP, Larkin J, Sosman JA, Lebbé C,
Brady B, Neyns B, Schmidt H, Hassel JC, Hodi FS, Lorigan P, et al:
Efficacy and safety of nivolumab alone or in combination with
ipilimumab in patients with mucosal melanoma: A pooled analysis. J
Clin Oncol. 35:226–235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benlyazid A, Thariat J, Temam S, Malard O,
Florescu C, Choussy O, Makeieff M, Poissonnet G, Penel N, Righini
C, et al: Postoperative radiotherapy in head and neck mucosal
melanoma: A GETTEC study. Arch Otolaryngol Head Neck Surg.
136:1219–1225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bishop KD and Olszewski AJ: Epidemiology
and survival outcomes of ocular and mucosal melanomas: A
population-based analysis. Int J Cancer. 134:2961–2971. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiu NT and Weinstock MA: Melanoma of
oronasal mucosa. Population-based analysis of occurrence and
mortality. Arch Otolaryngol Head Neck Surg. 122:985–988. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ciarrocchi A, Pietroletti R, Carlei F and
Amicucci G: Extensive surgery and lymphadenectomy do not improve
survival in primary melanoma of the anorectum: Results from
analysis of a large database (SEER). Colorectal Dis. 19:158–164.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ercelep O, Topcu TO, Bayoglu IV, Ekinci
AS, Koca S, Kavgaci H, Ozcelik M, Alacacioglu A, Uzunoglu S,
Bozkurt O, et al: Retrospective multicenter evaluation of patients
diagnosed with mucosal melanoma: A study of Anatolian Society of
Medical Oncology. Tumour Biol. 37:12033–12038. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harada K, Mine S, Yamada K, Shigaki H, Oya
S, Baba H and Watanabe M: Long-term outcome of esophagectomy for
primary malignant melanoma of the esophagus: A single-institute
retrospective analysis. Dis Esophagus. 29:314–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hasebe M, Yoshikawa K, Nishii R, Kawaguchi
K, Kamada T and Hamada Y: Usefulness of 11C-methionine-PET for
predicting the efficacy of carbon ion radiation therapy for head
and neck mucosal malignant melanoma. Int J Oral Maxillofac Surg.
46:1220–1228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heinzelmann-Schwarz VA, Nixdorf S, Valadan
M, Diczbalis M, Olivier J, Otton G, Fedier A, Hacker NF and Scurry
JP: A clinicopathological review of 33 patients with vulvar
melanoma identifies c-KIT as a prognostic marker. Int J Mol Med.
33:784–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heppt MV, Roesch A, Weide B, Gutzmer R,
Meier F, Loquai C, Kähler KC, Gesierich A, Meissner M, von Bubnoff
D, et al: Prognostic factors and treatment outcomes in 444 patients
with mucosal melanoma. Eur J Cancer. 81:36–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hughes MC, Wright A, Barbour A, Thomas J,
Smithers BM, Green AC and Khosrotehrani K: Patients undergoing
lymphadenectomy for stage III melanomas of known or unknown primary
site do not differ in outcome. Int J Cancer. 133:3000–3007.
2013.PubMed/NCBI
|
|
33
|
Jang HS, Kim JH, Park KH, Lee JS, Bae JM,
Oh BH, Rha SY, Roh MR and Chung KY: Comparison of melanoma subtypes
among Korean patients by morphologic features and ultraviolet
exposure. Ann Dermatol. 26:485–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang X, Zeng Y, Liang J, Li J, Ren D, Chai
L, Sun Z, Yu S, Wu X, Han W, et al: Aberrations and clinical
significance of BRAF in malignant melanoma: A series of 60 cases in
Chinese Uyghur. Medicine (Baltimore). 97:e95092018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khan MN, Kanumuri VV, Raikundalia MD,
Vazquez A, Govindaraj S, Baredes S and Eloy JA: Sinonasal melanoma:
Survival and prognostic implications based on site of involvement.
Int Forum Allergy Rhinol. 4:151–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kirchoff DD, Deutsch GB, Foshag LJ, Lee
JH, Sim MS and Faries MB: Evolving Therapeutic Strategies in
Mucosal Melanoma Have Not Improved Survival Over Five Decades. Am
Surg. 82:1–5. 2016.PubMed/NCBI
|
|
37
|
Kirschner AN, Kidd EA, Dewees T and
Perkins SM: Treatment approach and outcomes of vaginal melanoma.
Int J Gynecol Cancer. 23:1484–1489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kong Y, Si L, Li Y, Wu X, Xu X, Dai J,
Tang H, Ma M, Chi Z, Sheng X, et al: Analysis of mTOR Gene
Aberrations in Melanoma Patients and Evaluation of Their
Sensitivity to PI3K-AKT-mTOR Pathway Inhibitors. Clin Cancer Res.
22:1018–1027. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Konuthula N, Khan MN, Parasher A, Del
Signore A, Genden EM, Govindaraj S and Iloreta AM: The presentation
and outcomes of mucosal melanoma in 695 patients. Int Forum Allergy
Rhinol. 7:99–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koto M, Demizu Y, Saitoh JI, Suefuji H,
Tsuji H, Okimoto T, Ohno T, Shioyama Y, Takagi R, Nemoto K, et al
Japan Carbon-Ion Radiation Oncology Study Group, : Multicenter
study of carbon-ion radiation therapy for mucosal melanoma of the
head and neck: Subanalysis of the Japan Carbon-Ion Radiation
Oncology Study Group (J-CROS) Study (1402 HN). Int J Radiat Oncol
Biol Phys. 97:1054–1060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuk SK, Won CH, Lee WJ, Shin WJ, Yoon HJ,
Hong SD, Hong SP and Lee J: Prognostic significance of nestin in
primary malignant melanoma of the oral cavity. Melanoma Res.
26:457–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lansu J, Klop WM, Heemsbergen W, Navran A,
Al-Mamgani A, Langendijk JA, Kaanders JH, Terhaard C, Karakullukcu
B and Hamming-Vrieze O: Local control in sinonasal malignant
melanoma: Comparing conventional to hypofractionated radiotherapy.
Head Neck. 40:86–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lawaetz M, Birch-Johansen F, Friis S,
Eriksen JG, Kiss K, Gade S, Møller-Madsen M, Pourbordbari N and von
Buchwald C: Primary mucosal melanoma of the head and neck in
Denmark, 1982–2012: Demographic and clinical aspects. A
retrospective DAHANCA study. Acta Oncol. 55:1001–1008. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee G, Baek CH, Choi NY and Chung MK: The
prognostic role of the surgical approach and adjuvant therapy in
operable mucosal melanoma of the head and neck. Clin Exp
Otorhinolaryngol. 10:97–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee RJ, Lee SA, Lin T, Lee KK and
Christensen RE: Determining the epidemiologic, outcome, and
prognostic factors of oral malignant melanoma by using the
Surveillance, Epidemiology, and End Results database. J Am Dent
Assoc. 148:288–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lombardi D, Bottazzoli M, Turri-Zanoni M,
Raffetti E, Villaret AB, Morassi ML, Ungari M, Vermi W, Battaglia
P, Castelnuovo P, et al: Sinonasal mucosal melanoma: A 12-year
experience of 58 cases. Head Neck. 38 (Suppl 1):E1737–E1745. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mücke T, Hölzle F, Kesting MR, Loeffelbein
DJ, Robitzky LK, Hohlweg-Majert B, Tannapfel A and Wolff KD: Tumor
size and depth in primary malignant melanoma in the oral cavity
influences survival. J Oral Maxillofac Surg. 67:1409–1415. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nakamura Y, Fujisawa Y, Tanaka R, Maruyama
H, Ishitsuka Y, Okiyama N, Watanabe R and Fujimoto M: Use of immune
checkpoint inhibitors prolonged overall survival in a Japanese
population of advanced malignant melanoma patients: Retrospective
single institutional study. J Dermatol. 45:1337–1339. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Oba J, Nakahara T, Abe T, Hagihara A,
Moroi Y and Furue M: Expression of c-Kit, p-ERK and cyclin D1 in
malignant melanoma: An immunohistochemical study and analysis of
prognostic value. J Dermatol Sci. 62:116–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pandey M, Mathew A, Iype EM, Sebastian P,
Abraham EK and Nair KM: Primary malignant mucosal melanoma of the
head and neck region: Pooled analysis of 60 published cases from
India and review of literature. Eur J Cancer Prev. 11:3–10. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pfeil AF, Leiter U, Buettner PG, Eigentler
TK, Weide B, Meier F and Garbe C: Melanoma of unknown primary is
correctly classified by the AJCC melanoma classification from 2009.
Melanoma Res. 21:228–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Plavc G, But-Hadžić J, Aničin A, Lanišnik
B, Didanović V and Strojan P: Mucosal melanoma of the head and
neck: A population-based study from Slovenia, 1985–2013. Radiat
Oncol. 11:1372016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roh MR, Gupta S, Park KH, Chung KY, Lauss
M, Flaherty KT, Jönsson G, Rha SY and Tsao H: Promoter Methylation
of PTEN Is a Significant Prognostic Factor in Melanoma Survival. J
Invest Dermatol. 136:1002–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Samstein RM, Carvajal RD, Postow MA,
Callahan MK, Shoushtari AN, Patel SG, Lee NY and Barker CA:
Localized sinonasal mucosal melanoma: Outcomes and associations
with stage, radiotherapy, and positron emission tomography
response. Head Neck. 38:1310–1317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sanchez A, Rodríguez D, Allard CB, Bechis
SK, Sullivan RJ, Boeke CE, Kuppermann D, Cheng JS, Barrisford GW,
Preston MA, et al: Primary genitourinary melanoma: Epidemiology and
disease-specific survival in a large population-based cohort. Urol
Oncol. 34:166.e7–166.e14. 2016. View Article : Google Scholar
|
|
56
|
Schaefer T, Satzger I and Gutzmer R:
Clinics, prognosis and new therapeutic options in patients with
mucosal melanoma: A retrospective analysis of 75 patients. Medicine
(Baltimore). 96:e57532017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schmidt MQ, David J, Yoshida EJ, Scher K,
Mita A, Shiao SL, Ho AS and Zumsteg ZS: Predictors of survival in
head and neck mucosal melanoma. Oral Oncol. 73:36–42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shoushtari AN, Bluth MJ, Goldman DA, Bitas
C, Lefkowitz RA, Postow MA, Munhoz RR, Buchar G, Hester RH, Romero
JA, et al: Clinical features and response to systemic therapy in a
historical cohort of advanced or unresectable mucosal melanoma.
Melanoma Res. 27:57–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shuman AG, Light E, Olsen SH, Pynnonen MA,
Taylor JM, Johnson TM and Bradford CR: Mucosal melanoma of the head
and neck: Predictors of prognosis. Arch Otolaryngol Head Neck Surg.
137:331–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Song H, Jing G, Wang L, Guo W and Ren G:
Periodic acid-Schiff-positive loops and networks as a prognostic
factor in oral mucosal melanoma. Melanoma Res. 26:145–152. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sun CZ, Li QL, Hu ZD, Jiang YE, Song M and
Yang AK: Treatment and prognosis in sinonasal mucosal melanoma: A
retrospective analysis of 65 patients from a single cancer center.
Head Neck. 36:675–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tchelebi L, Guirguis A and Ashamalla H:
Rectal melanoma: Epidemiology, prognosis, and role of adjuvant
radiation therapy. J Cancer Res Clin Oncol. 142:2569–2575. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Thariat J, Poissonnet G, Marcy PY, Lattes
L, Butori C, Guevara N, Dassonville O, Santini J, Bensadoun RJ and
Castillo L: Effect of surgical modality and hypofractionated
split-course radiotherapy on local control and survival from
sinonasal mucosal melanoma. Clin Oncol (R Coll Radiol). 23:579–586.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang X, Wen W, Wu H, Chen Y, Ren G and Guo
W: Heparanase expression correlates with poor survival in oral
mucosal melanoma. Med Oncol. 30:6332013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wen X, Ding Y, Li J, Zhao J, Peng R, Li D,
Zhu B, Wang Y and Zhang X and Zhang X: The experience of immune
checkpoint inhibitors in Chinese patients with metastatic melanoma:
A retrospective case series. Cancer Immunol Immunother.
66:1153–1162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Won TB, Choi KY, Rhee CS, Jin HR, Yi JS,
Dhong HJ, Kim SW, Choi JH, Kim JK, Chung YJ, et al: Treatment
outcomes of sinonasal malignant melanoma: A Korean multicenter
study. Int Forum Allergy Rhinol. 5:950–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yeh JJ, Shia J, Hwu WJ, Busam KJ, Paty PB,
Guillem JG, Coit DG, Wong WD and Weiser MR: The role of
abdominoperineal resection as surgical therapy for anorectal
melanoma. Ann Surg. 244:1012–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yi JH, Yi SY, Lee HR, Lee SI, Lim DH, Kim
JH, Park KW and Lee J: Dacarbazine-based chemotherapy as first-line
treatment in noncutaneous metastatic melanoma: Multicenter,
retrospective analysis in Asia. Melanoma Res. 21:223–227. 2011.
View Article : Google Scholar : PubMed/NCBI
|