Introduction
Appendiceal carcinomas occur in adults with a mean
age at onset of 55–65 years for primary tumors and 38 years for
malignant tumors (1,2). First described by Gagne et al
(2,4)
in 1969, goblet cell carcinoids (GCCs) exhibit mixed neuroendocrine
differentiation and intestinal-type goblet cell morphology; for
this reason, they are described as an entity separate from
carcinoids and mucinous adenocarcinomas. The incidence of GCC is
~1.2 cases for every million individuals per year among Caucasian
women and it is less common among children (2–5).
Metastasis has been documented in 8–20% of the cases, with 5-year
survival rates ranging from 55 to 80% (6,7).
The diagnosis of GCC is confirmed by pathological
examination based on consensus guidelines. Currently, a variety of
different classification systems for the nomenclature, grading and
staging of neuroendocrine tumors (NETs) are available in an attempt
to segregate groups by prognostic value, management and survival
(8–13). The 2010 World Health Organization
(WHO) tumor classification (8)
considered GCCs as a subgroup of mixed adenoneuroendocrine
carcinomas (MANECs). The tumor-node-metastasis (TNM) classification
of malignant tumors by the Union for International Cancer Control,
the American Joint Committee on Cancer and the European
Neuroendocrine Tumor Society (ENETS), consider GCCs to be
adenocarcinomas (5,9). However, their complexity is such that
GCCs were not included in the 2016 ENETS consensus guidelines for
Neuroendocrine Neoplasms of the Appendix. Another diagnostic
classification for GCC was proposed by Tang et al (10), based on the TNM classification for
appendiceal adenocarcinomas, and has been proven useful for
predicting clinical behavior and prognosis. In the Tang
classification, tumors are subclassified into group A (typical
GCC), group B (adenocarcinoma ex-GCC) and group C (adenocarcinoma
ex-GCC; poorly differentiated). Additionally, several pathological
markers and clinical findings are used to determine prognosis and
the course of action for NETs, including origin, stage, grade,
tumor size (<2 or >2 cm), histological differentiation (well-
or poorly differentiated), invasion of muscularis propria,
histopathological examination (hematoxylin and eosin, chromogranin
A, synaptophysin and CD56), assessment of mitotic index (mitoses
per high-power field), Ki-67 index (<2, >2 and >30%),
biological behavior (benign, low-grade and high-grade),
lymphovascular invasion and metastasis (10,14). In
parallel, a general classification has been established for midgut,
hindgut and foregut NETs based on Ki-67 index, including grades 1
(≤2%), 2 (3–20%) and 3 (>20%), as described by Rindi et
al (15). In addition, in a
recent study by Yozu et al (12), a new grading system was proposed,
based on the classification of GCCs as adenocarcinomas, similar to
colorectal adenocarcinoma. This complex grading system represents a
challenge for the pathologist in routine practice.
Upon diagnosis, surgical management by right
hemicolectomy is recommended as the standard surgical approach by
the North American Neuroendocrine Tumor Society (NANETS) consensus
guidelines if the tumor invasion is at the base of the appendix,
for tumors sized >2 cm, and/or if there is evidence of
mesoappendiceal or lymphovascular infiltration with lymph node
involvement and for intermediate or high-grade tumors (13). Postoperatively, adjuvant chemotherapy
includes regimens with or without debulking followed by
chemotherapy similar to that for the treatment of adenocarcinoma of
the colon (7,10,13). We
herein report the cases of two GCC patients with varied clinical
presentation who underwent right hemicolectomy, and provide a
literature review of similar clinical cases.
Case report
Case 1
A 45-year-old female patient presented to the
emergency department of Barzilai Medical Hospital with lower
abdominal pain and nausea that started 1 day prior to admission.
The findings on physical examination and blood tests were
unremarkable, except for abdominal tenderness in the right lower
quadrant. There were no associated comorbidities. Abdominal
contrast-enhanced computed tomography confirmed the diagnosis of
acute appendicitis and an appendectomy was performed. Additionally,
the patient had previously undergone hysterectomy due to leiomyoma.
Macroscopically, the appendix appeared inflamed and dilated; on
palpation, a solid, moderately hard, elastic mass with an estimated
size of 1.5×0.5 cm was identified. Histopathological examination of
the appendix revealed a well-preserved appendiceal epithelium, with
no evidence of neoplastic changes. However, circumferential
involvement of the appendiceal wall by a poorly differentiated
adenocarcinoma with longitudinal extension along the length of the
appendix was observed. The main morphological characteristics
included i) the presence of mucin-containing goblet-shaped
epithelial cells arranged in small round or oval clusters; ii)
disorganized arrangement of the tumor cells, with predominant
signet ring cells with focal moderate/severe cellular atypia and
irregular hyperchromatic nuclei; iii) cells exhibiting a
single-cell infiltrating pattern with areas of confluent growth and
iv) desmoplastic response within the appendiceal submucosal wall,
and muscle bundles of the muscularis propria divided by tumor cell
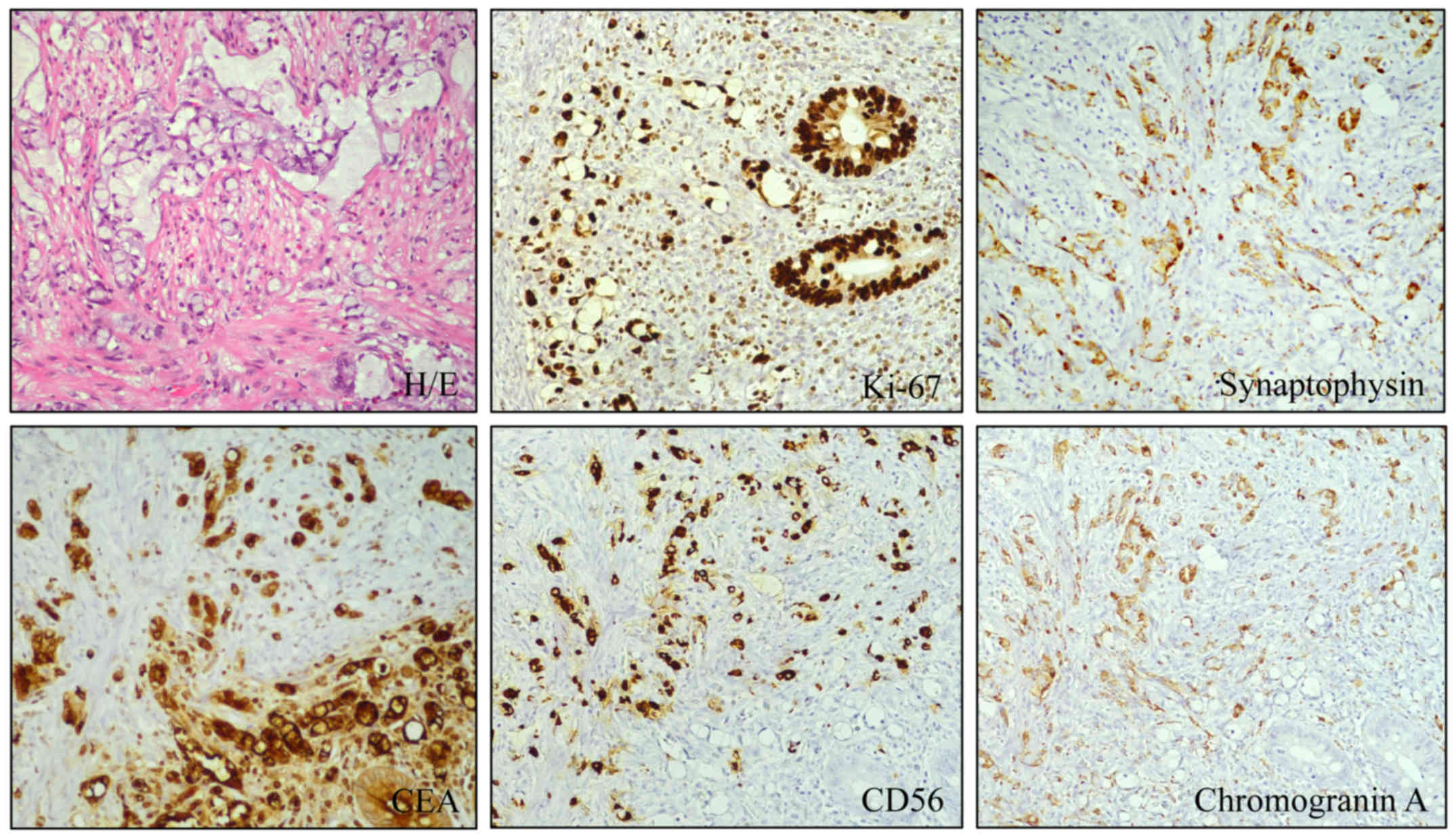
clusters (Fig. 1). The tumor cells
were positive for MNF-116, chromogranin A, synaptophysin,
cytokeratin-20, CDX-2, CD56, carcinoembryonic antigen (CEA) (signet
ring cell and goblet cell type); however, they were negative for
Wilms' tumor-1 (WT-1) and cytokeratin-7. Ki-67 was positive in ~25%
of the tumor cells (grade 3). Signet ring cells were positive for
mucicarmine, a natural gastrointestinal tumor type mucin, and PAS.
The tumor invaded through the muscularis propria into the subserosa
(Table I). Elective right
hemicolectomy and bilateral oophorectomy were performed, according
to the current guidelines (13),
followed by adjuvant chemotherapy with capecitabine and oxaliplatin
(XeLox) for eight cycles. Macroscopic examination revealed no
changes in the large intestine, with preserved rugal folds and
ileocecal valve. The analysis of 24 regional lymph nodes revealed
no metastatic changes. Since then, the patient has been on regular
follow-up and no signs of disease recurrence have been detected
within 2 years.
 | Table I.Comparison of clinicopathological
characteristics of GCC between the two cases. |
Table I.
Comparison of clinicopathological
characteristics of GCC between the two cases.
| Clinical
characteristics | Case 1 | Case 2 |
|---|
| Age, years | 45 | 60 |
| Carcinoid
syndrome | No | No |
| Primary symptoms | Abdominal tenderness
in the right lower quadrant | Abdominal pain,
fever, nausea and decreased appetite |
| Gross appearance | <2 cm,
well-defined mass | <2 cm, ill-defined
mass |
| Microscopic
appearance |
|
|
|
Morphology | Clusters of goblet
cells or signet ring cells | Cords of goblet
cells |
|
Atypia | Minimal | Minimal |
|
Mitoses | Absent | Present |
| Vascular
and perineural invasion | Absent | Absent |
|
Infiltrative margins | Absent | Absent |
| Staining |
|
|
|
Mucicarmine/PAS | Positive in goblet
cells | Positive in goblet
cells |
| IHC |
|
|
|
MNF-116 | Positive | Positive |
|
Chromogranin A | Positive | Positive |
|
Synaptophysin | Positive | Positive |
|
Cytokeratin-20 | Positive | Positive |
|
CDX-2 | Positive | Positive |
| CD56 | Positive | Positive |
| CEA | Positive | Positive |
| WT-1 | Negative | Negative |
|
Cytokeratin-7 | Negative | Negative |
|
Ki-67 | 25% | 15% |
Case 2
A 60-year-old male patient presented to the
emergency department with abdominal pain, fever, nausea and
decreased appetite over the previous 2 days. The patient displayed
no signs of acute abdomen suggestive of acute appendicitis. There
were no associated comorbidities. Abdominal ultrasound revealed
appendiceal inflammation, with a transverse appendiceal diameter of
8 cm. The patient was operated for acute appendicitis.
Macroscopically, the specimen was intact, with neoplastic
proliferation in the distal portion of the appendix (1×1.2 cm).
Additionally, a superimposed perforated diverticular structure with
exudate over the serosal surface was identified. The microscopic
appearance indicated a tumor cell nest pattern composed of large
goblet cells mimicking lumen-devoid crypts. Additionally, cords of
single enlarged cuboidal-shaped goblet cells with macronucleoli and
some mitotic figures were observed, which were absent in Case 1.
The specimen exhibited no lymphovascular space invasion. The
immunohistochemical profile of the tumor was identical to that in
Case 1. Ki-67 staining was positive in ~15% of the tumor cells
(Grade 2). Signet cells were mucicarmine- and PAS-positive
(Table I). Elective right
hemicolectomy was performed (13).
Macroscopic examination revealed no macroscopic changes in the
large intestine, and regional analysis of 21 lymph nodes revealed
no metastatic changes. The patient declined adjuvant chemotherapy.
Over a clinical follow-up period of 10 years, no tumor recurrence
has been observed, and the 5-HIAA levels have remained normal.
Discussion
Appendiceal carcinomas are found incidentally during
surgery in cases of acute appendicitis, representing 1% of
appendectomies (2). Appendiceal
cancer presents with significant morphological diversity and is
further classified into carcinoid (NET), mucinous
cystadenocarcinoma, adenocarcinoma, GCC and signet ring cell tumors
(2,3). Due to the fact that GCCs are discovered
incidentally during routine appendectomy, there is a lack of a
standardized classification system and discrepancies regarding
specific reliable markers, such as Ki-67; this may lead to
misdiagnosis and suboptimal treatment and surgical approaches
(i.e., hemicolectomy or multivisceral resection). A literature
review of GCCs revealed a constant steady increase in the number of
GCC cases over the past decade, evidenced by an increase in case
series reports, possibly due to improved detection methods and
clinicians' awareness (Table II).
The aim of the present case report was to emphasize the lack of a
standardized classification system and reliable markers for
adequate prognosis, management and/or treatment.
 | Table II.Characteristics and outcomes of GCC
patients. |
Table II.
Characteristics and outcomes of GCC
patients.
| Author | All patients | Median age (range),
years | Sex | Ki-67 | R/H (%) | (Refs.) |
|---|
| Clift et
al | 21 | 55 (32–77) | 9 M, 12 F | <2%: 3/18 3–20%:
6/18 | 15/21 (71) | (6) |
|
|
|
|
| <20%: 9/18 |
|
|
| Tsang et
al | 86 | 54 (25–91) | 42 M, 44 F | <2%: 1/86 3–20%:
12/86 | 51/67 (76) | (16) |
|
|
|
|
| <20%: 6/86
Unknown: 67/86 |
|
|
| Madsen et
al | 48 | 52 (32–75) | 18 M, 30 F | N/A | 16/21 (76) | (17) |
| Nonaka et
al | 105 | 54 (25–79) | 54 M, 51 F | N/A | 45/105 (43) | (18) |
| Yu et
al | 15 | 52 (36–74) | 9 M, 6 F |
31.9±6.3%a | N/A | (19) |
| Lamarca et
al | 74 | 56 (26–83) | 34 M, 40 F | N/A | 42/74 (57) | (21) |
For clinicians, it is a challenging task to develop
an evidence-based treatment plan. In addition, there remains the
question of whether a right hemicolectomy should have been
performed in Case 2. Although surgery in this case is recommended
by both NANETS and ENETS (5,13), the extent of surgical resection with
appendectomy versus right hemicolectomy is debated. Recent evidence
suggests limited or no benefit of right hemicolectomy, primarily in
patients with low-grade and/or limited disease burden (20). In another study, Lamarca et al
(21) assessed the effects of right
hemicolectomy on disease-free survival. The results suggested a
higher risk of relapse in patients who underwent right
hemicolectomy vs. those receiving appendectomy alone. Despite these
results, the authors concluded that appendectomy alone is only
justifiable in patients with Tang class A, stage I/II tumours that
are unable to undergo surgery due to comorbidities. A meta-analysis
by Varisco et al (22)
including 100 patients with GCC also failed to identify a
significant benefit of hemicolectomy relative to appendectomy.
The marker Ki-67, used to measure cell
proliferation, is a widely used marker for NET grading and staging
(23,24). Additionally, Ki-67 has exhibited a
positive correlation with known prognostic factors (tumor size and
metastatic status) and has been extensively investigated in
pancreatic and gastrointestinal NETs (25,26);
however, no studies have yet provided sufficient evidence for GCCs.
Currently, prognosis based on the pathological gradient is mostly
dependent on the Ki-67 proliferative index, despite its dynamic
change over time (21). A recent
study by Liu et al (27)
examined the role of Ki-67 as a prognostic factor for GCC. That
study, which included 12 patients with GCC, revealed no prognostic
significance for GCC. The fact that NETs comprise a heterogeneous
group of tumors renders the interpretation of the Ki-67 index for
GCC unreliable without an adequate researched cut-off value, which
is currently set between 20 and 30% for digestive tract NETs
(10,14). In the present case report, Case 1 had
a Ki-67 index of 25%, whereas Case 2 had a Ki-67 index of 15%. Both
patients underwent right hemicolectomy based on the guidelines;
however, surgical intervention should be based on tumor size,
invasiveness and careful evaluation of the morphological
characteristics of GCC in addition to the Ki-67 index. An important
morphological characteristic in GCC reflecting prognosis and
survival is the adenocarcinoma component, which may be classified
into signet ring-cell and non-signet ring-cell types (28,29).
Based on the results reported by Taggart et al (28), the amount of the carcinomatous
component should be included in the diagnosis of GCC, since it is
associated with the clinical characteristics and stage.
Consequently, a consensus guideline assessing the value of the new
staging classification is of paramount importance, since there is a
40% risk of morbidity with right hemicolectomy in elderly patients
with respiratory and cardiovascular complications (cardiac arrest,
pneumonia, pulmonary embolism) (7).
Finally, an update of the current 2008 guidelines (7) should consider the following
recommendations on the section for GCC of the appendix: i) Tumor
marker use (MNF-116, chromogranin A, synaptophysin, keratin-20,
CDX-2, CD56, CEA and Ki-67) along with the current staging system
(7,20); ii) addition of a Ki-67 cut-off point
of >25% in cases treated with right hemicolectomy; and iii) in
the early stages, when the tumor is confined to the mucosa and
defined as carcinoma in situ, appendectomy alone is
adequate, regardless of the Ki-67 value (Tang class A, stage I/II
tumors). However, in more advanced stages, when submucosa
involvement and possibly lymphatic spread have occurred, prognosis
should be revised along with other markers (i.e., Ki-67) to
determine whether right hemicolectomy should be performed. To
conclude, the overall survival for patients with GCCs varies
according to the different references, classifications or staging
criteria used. Hence, there is a need for standardization of the
classification system to ensure optimal clinical management and
outcome predictions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NA and TH were involved in diagnosis, treatment and
data acquisition. EGC was involved in analysis and interpretation
of the data and drafting of the manuscript. AL performed the
pathological examination of the specimens. MS was involved in
diagnosis and critical revision of the manuscript. AD, EGC and MS
contributed to the critical revision and final approval of the
manuscript. All authors have read and approved the final version of
this manuscript for publication.
Ethics approval and consent to
participate
Institutional Ethics Board approval was obtained and
both patients signed an informed consent form. The analysis of the
data was conducted according to the principles outlined in the
Declaration of Helsinki.
Patient consent for publication
Written consent was obtained from the patients
regarding surgical treatment, pathological examination and
publication, including associated images.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Connor SJ, Hanna GB and Frizelle FA:
Appendiceal tumors: Retrospective clinicopathologic analysis of
appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum.
41:75–80. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCusker ME, Coté TR, Clegg LX and Sobin
LH: Primary malignant neoplasms of the appendix: A population-based
study from the surveillance, epidemiology and end-results program,
1973–1998. Cancer. 94:3307–3312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vukovic J, Vrebalov Cindro P, Tomic S and
Tonkic A: Signet ring carcinoma of the appendix presenting as
Crohn's disease in a young male. Case Rep Gastroenterol.
12:277–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gagné F, Fortin P, Dufour V and Delage C:
Tumors of the appendix associating histologic features of carcinoid
and adenocarcinoma. Ann Anat Pathol (Paris). 14:393–406. 1969.(In
French). PubMed/NCBI
|
|
5
|
Pape UF, Perren A, Niederle B, Gross D,
Gress T, Costa F, Arnold R, Denecke T, Plöckinger U, Salazar R, et
al: ENETS consensus guidelines for the management of patients with
neuroendocrine neoplasms from the jejuno-ileum and the appendix
including goblet cell carcinomas. Neuroendocrinology. 95:135–156.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clift AK, Kornasiewicz O, Drymousis P,
Faiz O, Wasan HS, Kinross JM, Cecil T and Frilling A: Goblet cell
carcinomas of the appendix: Rare but aggressive neoplasms with
challenging management. Endocr Connect. 7:268–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plöckinger U, Couvelard A, Falconi M,
Sundin A, Salazar R, Christ E, de Herder WW, Gross D, Knapp WH,
Knigge UP, et al: Consensus guidelines for the management of
patients with digestive neuroendocrine tumours: Well-differentiated
tumour/carcinoma of the appendix and goblet cell carcinoma.
Neuroendocrinology. 87:20–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestive
system4th. Intrenation agency for research on cancer; Lyon:
2010
|
|
9
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumoursJohn Wiley & Sons;
2011
|
|
10
|
Tang LH, Shia J, Soslow RA, Dhall D, Wong
WD, O'Reilly E, Qin J, Paty P, Weiser MR, Guillem J, et al:
Pathologic classification and clinical behavior of the spectrum of
goblet cell carcinoid tumors of the appendix. Am J Surg Pathol.
32:1429–1443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee LH, McConnell YJ, Tsang E, Zerhouni S,
Speers C, Kennecke H and Schaeffer DF: Simplified 2-tier histologic
grading system accurately predicts outcomes in goblet cell
carcinoid of the appendix. Hum Pathol. 46:1881–1889. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yozu M, Johncilla ME, Srivastava A, Ryan
DP, Cusack JC, Doyle L, Setia N, Yang M, Lauwers GY, Odze RD and
Misdraji J: Histologic and outcome study supports reclassifying
appendiceal goblet cell carcinoids as goblet cell adenocarcinomas,
and grading and staging similarly to colonic adenocarcinomas. Am J
Surg Pathol. 42:898–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boudreaux JP, Klimstra DS, Hassan MM,
Woltering EA, Jensen RT, Goldsmith SJ, Nutting C, Bushnell DL,
Caplin ME and Yao JC; North American Neuroendocrine Tumor Society
(NANETS), : The NANETS consensus guideline for the diagnosis and
management of neuroendocrine tumors: Well-differentiated
neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum.
Pancreas. 39:753–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oberg K, Modlin IM, De Herder W, Pavel M,
Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg
J, et al: Consensus on biomarkers for neuroendocrine tumour
disease. Lancet Oncol. 16:e435–e446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rindi G: The ENETS guidelines: The new TNM
classification system. Tumori J. 96:806–809. 2010. View Article : Google Scholar
|
|
16
|
Tsang ES, McConnell YJ, Schaeffer DF, Lee
L, Yin Y, Zerhouni S, Schaff K, Speers C and Kennecke HF: Outcomes
of surgical and chemotherapeutic treatments of goblet cell
carcinoid tumors of the appendix. Ann Surg Oncol. 25:2391–2399.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Madsen AH, Ladekarl M, Villadsen GE,
Grønbæk H, Sørensen MM, Stribolt K, Verwaal VJ and Iversen LH:
Effects of cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy (HIPEC) in the treatment of goblet cell carcinoma: A
prospective cohort study. Ann Surg Oncol. 25:422–430. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nonaka D, Papaxoinis G, Lamarca A, Fulford
P, Valle J and Chakrabarty B: A study of appendiceal crypt cell
adenocarcinoma (so-called goblet cell carcinoid and its related
adenocarcinoma). Hum Pathol. 72:18–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu HH, Yonemura Y, Hsieh MC, Mizumoto A,
Wakama S and Lu CY: Cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy for appendiceal goblet cell carcinomas
with peritoneal carcinomatosis: Results from a single specialized
center. Cancer Manag Res. 9:513–523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gilmore G, Jensen K, Saligram S, Sachdev
TP and Arekapudi SR: Goblet cell carcinoid of the
appendix-diagnostic challenges and treatment updates: A case report
and review of the literature. J Med Case Rep. 12:2752018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamarca A, Nonaka D, Lopez Escola C,
Hubner RA, O'Dwyer S, Chakrabarty B, Fulford P and Valle JW:
Appendiceal goblet cell carcinoids: Management considerations from
a reference peritoneal tumour service centre and ENETS centre of
excellence. Neuroendocrinology. 103:500–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varisco B, McAlvin B, Dias J and Franga D:
Adenocarcinoid of the appendix: Is right hemicolectomy necessary? A
meta-analysis of retrospective chart reviews. Am Surg. 70:593–599.
2004.PubMed/NCBI
|
|
23
|
Nadler A, Cukier M, Rowsell C, Kamali S,
Feinberg Y, Singh S and Law CH: Ki-67 is a reliable pathological
grading marker for neuroendocrine tumors. Virchows Arch.
462:501–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jamali M and Chetty R: Predicting
prognosis in gastroentero-pancreatic neuroendocrine tumors: An
overview and the value of Ki-67 immunostaining. Endocr Pathol.
19:282–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vilar E, Salazar R, Pérez-García J,
Cortes J, Öberg K and Tabernero J: Chemotherapy and role of the
proliferation marker Ki-67 in digestive neuroendocrine tumors.
Endocr Relat Cancer. 14:221–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rorstad O: Prognostic indicators for
carcinoid neuroendocrine tumors of the gastrointestinal tract. J
Surg Oncol. 89:151–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu E, Telem DA, Warner RR, Dikman A and
Divino CM: The role of Ki-67 in predicting biological behavior of
goblet cell carcinoid tumor in appendix. Am J Surg. 202:400–403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taggart MW, Abraham SC, Overman MJ,
Mansfield PF and Rashid A: Goblet cell carcinoid tumor, mixed
goblet cell Carcinoid-Adenocarcinoma, and adenocarcinoma of the
appendix: Comparison of clinicopathologic features and prognosis.
Arch Pathol Lab Med. 139:782–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burke AP, Sobin LH, Federspiel BH,
Shekitka KM and Helwig EB: Goblet cell carcinoids and related
tumors of the vermiform appendix. Am J Clin Pathol. 94:27–35. 1990.
View Article : Google Scholar : PubMed/NCBI
|