Introduction
Systemic therapy for metastatic renal cell carcinoma
(mRCC) has created a notable paradigm shift since the introduction
of novel molecular-targeted therapies (1). A standard agent for the treatment of
mRCC is sunitinib malate (Sutent; Pfizer Inc.), which is an orally
administered, small-molecule, multi-targeted inhibitor of tyrosine
kinases, including vascular endothelial growth factor (VEGF)
receptor, platelet-derived growth factor receptor, phosphorylation
of stem cell factor receptor, Fms-like tyrosine kinase-3,
colony-stimulating factor-1 receptor, and RET receptor tyrosine
kinases. In a randomized, multicenter, phase III trial that
enrolled 750 patients with previously untreated clear-cell mRCC to
receive either sunitinib or interferon (IFN)-α, sunitinib was
demonstrated to be superior to IFN-α regarding objective response
rate (47 vs. 12%, respectively), median progression-free survival
(PFS; 11.0 vs. 5.0 months, respectively), and median overall
survival (OS; 26.4 vs. 21.8 months, respectively) (2,3). Based
on these significant results, sunitinib was approved worldwide for
the first-line treatment of clear-cell mRCC.
The oncological outcomes of cytokine therapy for
Japanese patients with mRCC have been reported to be more favorable
compared with those of Western patients (4). Accordingly, selected patients with mRCC
still occasionally receive first-line cytokine therapy in Japan.
Furthermore, sorafenib tosylate (Nexavar; Bayer Pharmaceuticals
Corporation), which is another standard agent for mRCC, is
administered as second-line treatment after failure of cytokine
therapy due to its significant results demonstrated in a phase III
trial (5). Therefore, the use of
third-line sunitinib for selected patients with mRCC should be
evaluated, as should the therapeutic efficacy of third-line
sunitinib following failure of sequential therapy with cytokines
and sorafenib. The aim of the present study was to retrospectively
investigate the oncological and therapeutic outcomes of third-line
sunitinib for patients with clear-cell mRCC.
Patients and methods
Patients and treatments
The present study was conducted with the approval of
the Kitasato University Medical Ethics Organization (approval no.:
KMEO B16-156), which waived the requirement for informed consent
due to the retrospective nature of the analyses. Between December
2008 and February 2012, 14 consecutive patients with mRCC treated
with third-line sunitinib after sequential use of cytokine therapy
and sorafenib were enrolled in the present study. All patients had
histologically confirmed clear-cell mRCC. The group comprised 9 men
and 5 women with a median age of 62.5 years (range, 52–76 years) at
the time of sunitinib initiation. In general, 50 mg sunitinib was
administered orally once daily during a 6-week cycle consisting of
4 weeks of treatment followed by a 2-week break. Dose reductions
were permitted on the basis of individual tolerability. Comparisons
between third-line sunitinib and first- or second-line sunitinib
were performed and the results were assessed during the same study
period. The first-line sunitinib group comprised 20 consecutive
patients and the second-line sunitinib group comprised 14
consecutive patients with advanced renal cell carcinoma (a total of
48 consecutive patients). Tumor grading was performed according to
the Japanese classification (6).
Response and progression analysis
Response to treatment and disease progression were
assessed by the treating physician on the basis of the Response
Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (7), with computed tomography or magnetic
resonance imaging performed every 4–8 weeks. Adverse events were
evaluated by physical examination and laboratory assessments, such
as hematological and serum chemistry every 2–4 weeks during
treatment with sunitinib, and were graded according to the National
Cancer Institute Common Terminology Criteria for Adverse Events,
version 4.0 (8).
Disease control rate (DCR)
evaluation
Patient charts were retrospectively reviewed. DCR,
which was defined as complete response, partial response (PR) and
stable disease (SD), was evaluated. PFS, OS and relative dose
intensity (RDI) were analyzed. The RDI was calculated as follows:
RDI=cumulative dose ×100/1,400 mg.
Statistical analysis
Analysis of variance and post hoc Fisher's protected
least significant difference test were used to evaluate differences
of means between cohorts. The Chi-squared test was used to evaluate
differences in categorical variables. Non-parametric estimates of
survival were performed using Kaplan-Meier curves. Survival curves
were generated based on PFS and OS from the initiation of sunitinib
administration to the date of disease progression or death.
Log-rank tests were used for statistical comparisons. All analyses
were performed with StatView version 5.0 (SAS Institute); P<0.05
was considered to indicate statistically significant
differences.
Results
Patient characteristics
The clinical and pathological characteristics of
patients administered first-, second- and third-line sunitinib are
summarized in Table I. Prior to the
initiation of third-line sunitinib, all patients were sequentially
treated with first-line cytokine therapy [either IFN-α, or IFN-α
plus interleukin (IL)-2] and second-line sorafenib. The DCR for
first-line cytokine therapy and second-line sorafenib was 71.4 and
50.0%, respectively. The median PFS for first-line cytokine therapy
and second-line sorafenib was 10.0 and 5.0 months,
respectively.
 | Table I.Patient characteristics of the three
cohorts. |
Table I.
Patient characteristics of the three
cohorts.
|
| Sunitinib
treatment |
|
|---|
|
|
|
|
|---|
| Characteristics | First-line
(n=20) | Second-line
(n=14) | Third-line
(n=14) | P-value |
|---|
| Sex, n (%) |
|
|
| 0.6707 |
| Male | 15 (75.0) | 11 (78.6) | 9 (64.3) |
|
|
Female | 5 (25.0) | 3 (21.4) | 5 (35.7) |
|
| Age, years |
|
|
| 0.7408 |
|
Median | 65.5 | 66.5 | 62 |
|
|
Range | 36–75 | 46–80 | 52–76 |
|
| Mean ±
standard deviation | 63.3±9.3 | 65.6±9.3 | 63.9±7.8 |
|
| ECOG PS, n (%) |
|
|
| 0.6410 |
| 0 | 13 (65.0) | 11 (78.6) | 9 (64.3) |
|
| ≥1 | 7 (35.0) | 3 (21.4) | 5 (35.7) |
|
| MSKCC risk
classification, n (%) |
|
|
| 0.4252 |
|
Favorable | 3 (15.0) | 4 (28.6) | 2 (14.3) |
|
|
Intermediate | 9 (45.0) | 8 (57.1) | 9 (64.3) |
|
| Poor | 8 (40.0) | 2 (14.3) | 3 (21.4) |
|
| Pretreatment CRP, n
(%) |
|
|
| 0.0869 |
| Normal
(≤0.30 mg/dl) | 3 (15.0) | 7 (50.0) | 4 (28.6) |
|
| Elevated
(>0.30 mg/dl) | 17 (85.0) | 7 (50.0) | 10 (71.4) |
|
| Prior nephrectomy, n
(%) |
|
|
| 0.0004 |
| Yes | 11 (55.0) | 14 (100) | 14 (100) |
|
| No | 9 (45.0) | 0 (0) | 0 (0) |
|
| Histological
classification, n (%) |
|
|
| 0.2320 |
|
Clear-cell | 18 (90.0) | 14 (100) | 14 (100) |
|
|
Papillary | 2 (10.0) | 0 (0) | 0 (0) |
|
| T stage, n (%) |
|
|
| 0.5037 |
| T1,
T2 | 8 (40.0) | 8 (57.1) | 8 (57.1) |
|
| ≥T3 | 12 (60.0) | 6 (42.9) | 6 (42.9) |
|
| Grade, n (%) |
|
|
| 0.2288 |
| 1, 2 | 8 (40.0) | 11 (78.6) | 11 (78.6) |
|
| 3 | 7 (35.0) | 3 (21.4) | 3 (21.4) |
|
| Prior cytokine
therapy, n (%) |
|
|
| <0.0001 |
| Yes | 0 (0) | 11 (78.6) | 14 (100) |
|
| No | 0 (0) | 3 (21.4) | 0 (0) |
|
| Prior sorafenib, n
(%) |
|
|
| <0.0001 |
| Yes | 0 (0) | 3 (21.4) | 14 (100) |
|
| No | 0 (0) | 11 (78.6) | 0 (0) |
|
| No. of metastatic
sites, n (%) |
|
|
| 0.5530 |
| 1 | 6 (30.0) | 7 (50.0) | 6 (42.9) |
|
| ≥2 | 13 (65.0) | 7 (50.0) | 8 (57.1) |
|
DCR
Overall, 1 patient (7.1%) exhibited PR to treatment
and 5 patients (35.7%) had SD according to RECIST. The DCR was
42.9%. First- and second-line sunitinib achieved DCRs of 50.0 and
71.4%, respectively, and the differences were not statistically
significant (P=0.3429).
PFS and OS
Non-parametric estimates of PFS and OS were analyzed
by Kaplan-Meier curves for first-, second- and third-line
sunitinib. The median PFS for first-, second- and third-line
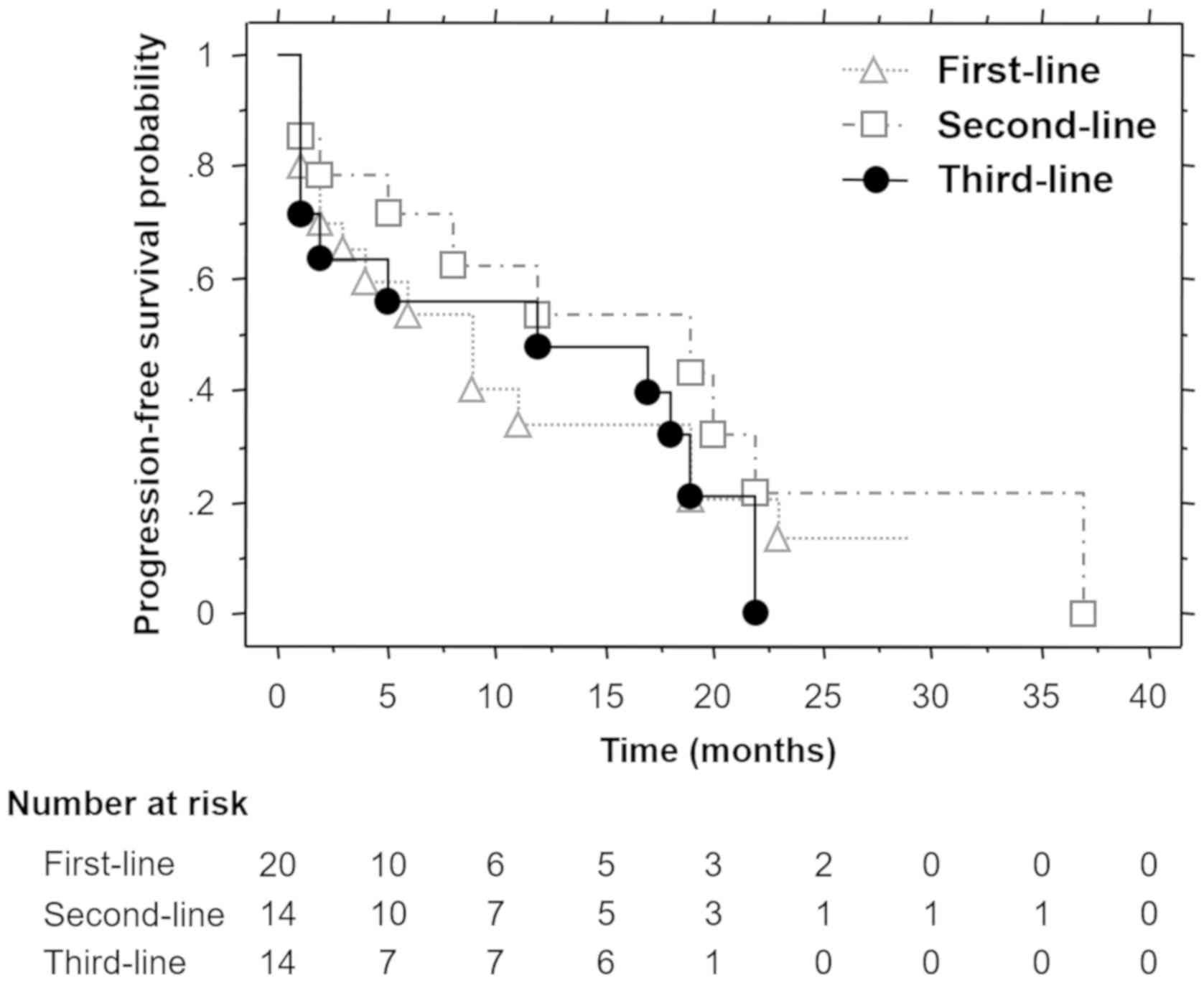
sunitinib was 9.0, 19.0 and 12.0 months, respectively (Fig. 1), and the differences were not
statistically significant (P=0.5326). The median OS for first-,
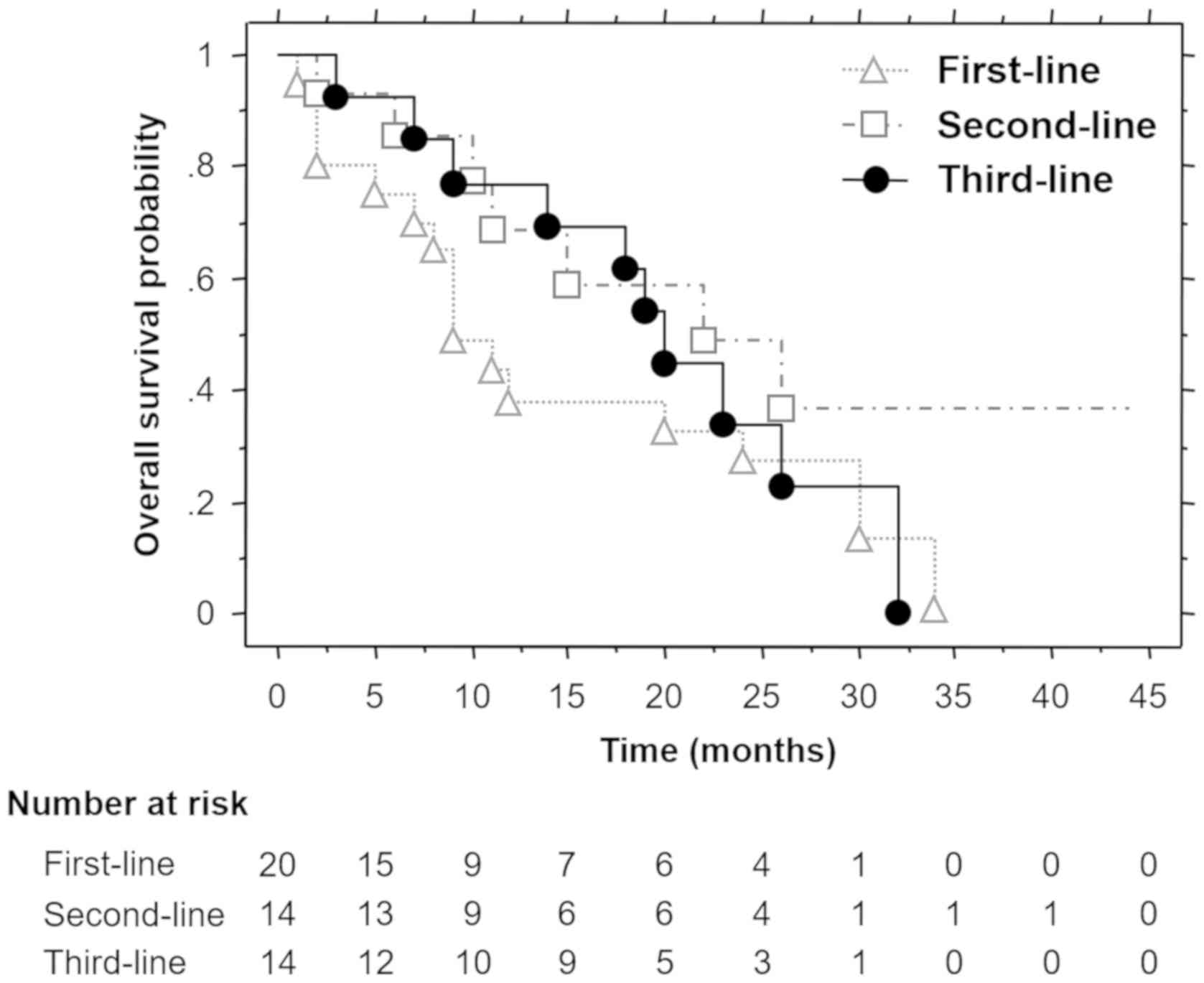
second- and third-line sunitinib was 9.0, 22.0 and 20.0 months,
respectively (Fig. 2), and the
differences were not statistically significant (P=0.2932).
Adverse events
Adverse events associated with third-line sunitinib
treatment are summarized in Table
II. The rates of treatment-related edema for first-, second-
and third-line sunitinib were 50.0, 21.4 and 7.1%, respectively.
Third-line sunitinib was associated with a significantly lower rate
of treatment-related edema compared with first- or second-line
sunitinib (P=0.0193). The adverse events of first-line sunitinib
were similar with those of second-line sunitinib treatment.
 | Table II.Treatment-related toxicity. |
Table II.
Treatment-related toxicity.
|
| NCI CTCAE
grade |
|---|
|
|
|
|---|
|
| Total | Grade 1 | Grade 2 | Grade 3 |
|---|
|
|
|
|
|
|
|---|
| Toxicity | n | % | n | % | n | % | n | % |
|---|
| Adverse events |
|
Hypertension | 6 | 42.9 | 2 | 14.3 | 4 | 28.6 | – | – |
|
Hand-foot syndrome | 4 | 28.6 | – | – | 1 | 7.1 | 3 | 21.5 |
|
Stomatitis | 5 | 35.7 | 3 | 21.5 | 2 | 14.3 | – | – |
|
Fatigue | 6 | 42.9 | 2 | 14.3 | 2 | 14.3 | 2 | 14.3 |
|
Diarrhea | 6 | 42.9 | 4 | 28.6 | 1 | 7.1 | 1 | 7.1 |
| Altered
taste | 6 | 42.9 | 4 | 28.6 | 2 | 14.3 | – | – |
|
Edema | 1 | 7.1 | – | – | – | – | 1 | 7.1 |
|
Nausea | 3 | 21.5 | – | – | 1 | 7.1 | 2 | 14.3 |
|
Fever | 3 | 21.5 | 3 | 21.5 | – | – | – | – |
|
Cholecystitis | 1 | 7.1 | – | – | – | – | 1 | 7.1 |
| Nasal
bleeding | 1 | 7.1 | – | – | – | – | 1 | 7.1 |
| Laboratory
abnormalities |
|
|
|
|
|
|
|
|
|
Leukopenia | 12 | 85.7 | 2 | 14.3 | 4 | 28.6 | 6 | 42.9 |
|
Anemia | 12 | 85.7 | 6 | 42.9 | 4 | 28.6 | 2 | 14.3 |
|
Thrombocytopenia | 13 | 92.9 | 4 | 28.6 | 5 | 35.7 | 4 | 28.6 |
|
Increased creatinine | 8 | 57.1 | 5 | 35.7 | 2 | 14.3 | 1 | 7.1 |
|
Increased alkaline
phosphatase | 1 | 7.1 | – | – | – | – | 1 | 7.1 |
|
Hypothyroidism | 10 | 71.4 | 1 | 7.1 | 9 | 64.3 | – | – |
|
Proteinuria | 8 | 57.1 | 4 | 28.6 | 2 | 14.3 | 2 | 14.3 |
RDI
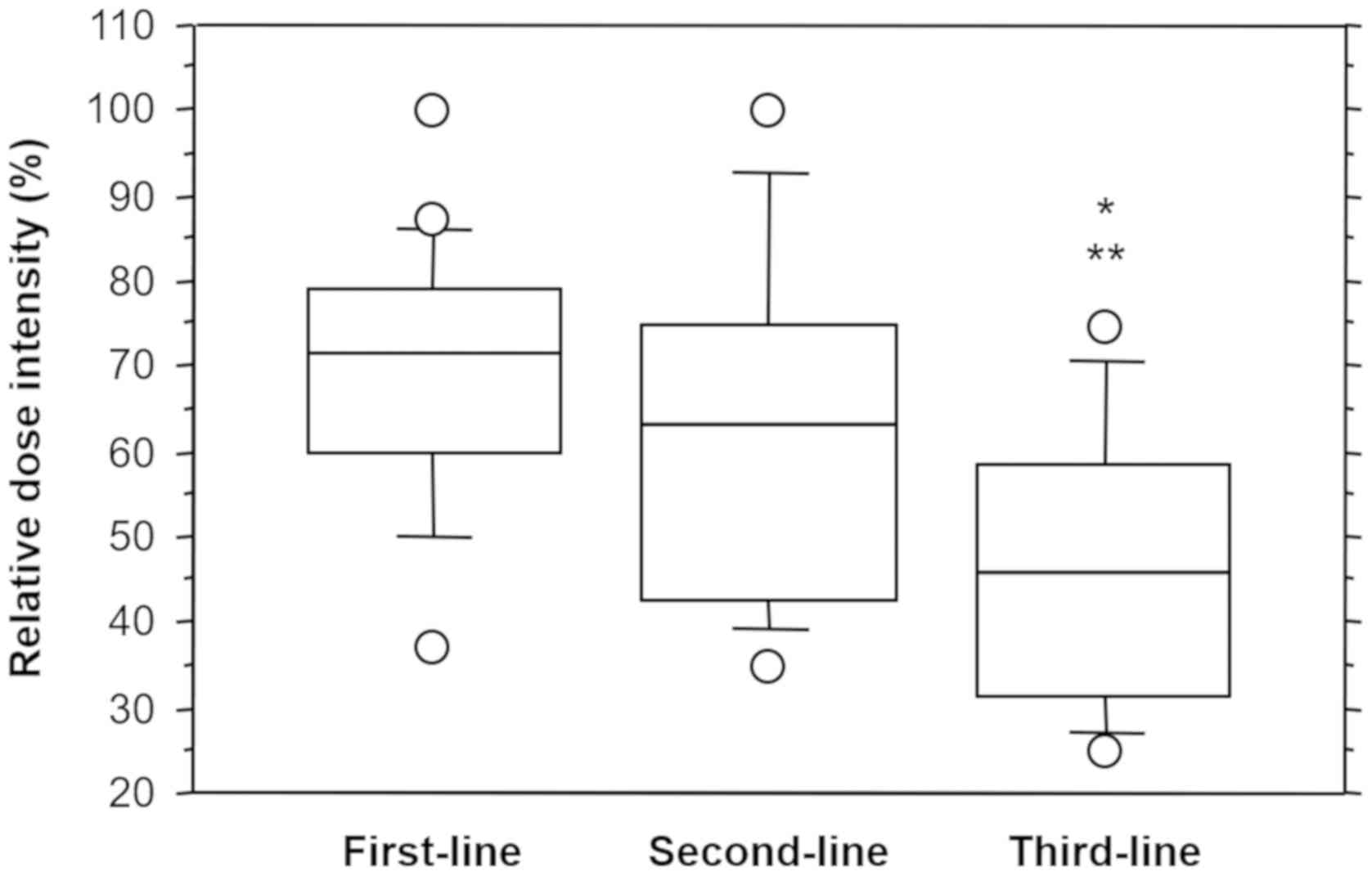
The RDI (mean ± standard deviation) was 69.3±15.0%
for first-line sunitinib, 63.2±20.2% for second-line sunitinib, and
46.1±16.3% for third-line sunitinib. Analysis of variance indicated
that third-line sunitinib had a significantly lower RDI compared
with first- and second-line sunitinib (P=0.0003 and 0.0109,
respectively; Fig. 3).
Discussion
Third-line sunitinib demonstrated clinical benefits
for selected patients with clear-cell mRCC, despite the notable
results of phase III clinical trials using first-line sunitinib
(2,3). Although sunitinib is widely approved
for the first-line treatment of clear-cell mRCC, sequential effects
after failure of second-line sorafenib have been reported (9–11). In
the present study, third-line sunitinib exhibited a DCR of 42.9%,
which was not statistically significantly different from that of
first-line (50.0%) or second-line (71.4%) sunitinib (P=0.3429). The
median PFS and OS for third-line sunitinib were 12.0 and 20.0
months, respectively. For first-line sunitinib, the PFS and OS were
9.0 and 9.0 months, respectively; for second-line sunitinib, the
PFS and OS were 19.0 and 22.0 months, respectively. These results
were not statistically significantly different (P=0.5326 and
0.2932, respectively). To the best of our knowledge, this is the
first report of a favorable outcome with the use of third-line
sunitinib. Recently, Miyake et al (12) reported the clinical significance of
third-line sunitinib for mRCC after the sequential use of
first-line cytokine therapy and second-line sorafenib. The PR rate
was 8.6%, and the median PFS and OS were 10.9 and 14.2 months,
respectively (12). These results
were similar to those of the present study, thus supporting the
clinical and oncological efficacy of third-line sunitinib.
Cross-resistance between sorafenib and sunitinib
must be addressed. Both agents are small-molecule tyrosine kinase
inhibitors that block the intracellular domain of the VEGF
receptor. These two agents were developed by similar molecular
pathways. However, previous clinical studies demonstrated that
there is no definitive cross-resistance between sorafenib and
sunitinib (9–11). Eichelberg et al (9) reported that 50.0% of patients benefited
from secondary use of sunitinib. Radiologically confirmed SD or PR
was observed in 7 (23.3%) and 8 (26.7%) patients, respectively. The
median PFS was 10.3 months (9).
Dudek et al (10) reported a
DCR of 58.6% for those who received sunitinib as a second-line
agent. The median time to progression was 78 weeks (10). Sablin et al (11) reported that disease control was
achieved in 66.2% of patients who were treated with second-line
sunitinib following failure of first-line sorafenib. These studies
included patients previously treated with various cytokine-based
therapies. The clinical benefits of third-line sunitinib in
selected patients were revealed. Resistance to single-agent
anti-angiogenic therapy may develop through compensatory mechanisms
driven by upregulation of plasma VEGF, fibroblast growth factors,
ephrin-A1 or angiopoietin-2, or through the development of
hypoxia-inducible factor-α independence by activation of
angiogenesis with IL-8, cyclooxygenase-2, prostaglandin E2, NF-κB,
RAS, PI3K/Akt, or p38 (13).
A prognostic marker for selecting patients who will
benefit the most from third-line sunitinib is needed. Dudek et
al (10) revealed that Memorial
Sloan-Kettering Cancer Center (MSKCC) risk classification (14) and best response to first-line therapy
were independently associated with OS in patients receiving
sequential therapy with sorafenib and sunitinib. Patients with poor
vs. good or intermediate MSKCC risk classification were at higher
risk for disease progression (P=0.008), and patients who had PR or
SD during initial treatment were at a slightly lower risk for
disease progression during sequential therapy (P=0.011) (10). Miyake et al (12) reported that performance status (PS),
pretreatment C-reactive protein (CRP) level and response to
second-line sorafenib were identified as significant predictors of
PFS in the univariate analysis; only response to second-line
sorafenib was demonstrated to be independently associated with PFS
in the multivariate analysis (P=0.0032). Additionally, the
univariate analysis identified significant associations of PS,
MSKCC risk classification and pretreatment CRP level with OS. Only
PS was an independent predictor of OS in the multivariate analysis
(P=0.0029) (12). We previously
demonstrated that normal pretreatment CRP levels predict better PFS
with sunitinib treatment, including cohorts receiving third-line
treatment (15). However, in the
present study, significant results were not obtained by using the
Cox proportional hazards regression analysis including variables
such as Eastern Cooperative Oncology Group PS, MSKCC risk
classification, pretreatment CRP level, response to first-line
cytokine therapy, and response to second-line sorafenib (data not
shown). Further investigation of prognostic factors to predict
response to third-line sunitinib is required.
A significantly lower RDI of third-line sunitinib
(46.1±16.3%) was a novel finding of the present study. Patients who
received third-line sunitinib experienced optimized therapeutic
efficacy from a relatively low dose. An expanded-access trial
reported that the mean RDI was 95.2% (16). However, in Japanese patients,
difficulty continuing with the initial dose of sunitinib therapy
without drug withdrawal was reported (17). Thus, the initial dose of sunitinib
may be reduced in selected patients using third-line sunitinib;
dose adjustment for each individual is important.
The median OS of third-line sunitinib was 20.0
months in the present study. The median PFS with cytokine therapy
and sorafenib was 10.0 and 5.0 months, respectively. The median
survival from the initiation of first-line cytokine therapy was
41.0 months (range, 5–133 months). Patients who received third-line
sunitinib experienced long-term survival benefits from these
sequential therapies. Several studies have demonstrated that
sequential treatment with sorafenib followed by sunitinib achieved
longer combined PFS compared with treatment with sunitinib followed
by sorafenib (18,19). These results suggest that the
sequential use of sorafenib followed by sunitinib after failure of
first-line cytokine therapy may be a suitable approach to selected
patients with mRCC.
There are currently no standard third-line
treatments for mRCC. Some patients received third-line sunitinib
after failure of first-line cytokine therapy and second-line
sorafenib, as sunitinib was approved after sorafenib. The present
study revealed that the outcomes with third-line sunitinib were
similar, or even superior, compared to those with first- and
second-line sunitinib. The major and most obvious reason is
preselection; the patients who are physically fit and have cancer
with increased aggressiveness are more likely to benefit from
subsequent lines of treatment and have a generally better OS. As
the last 12–24 months have brought about marked changes in the
first- and second-line treatment of mRCC, the role of salvage
sunitinib treatment may become more prominent.
There were certain potential limitations to the
present study. First, this was a retrospective single-institutional
study and the sample size was small, which may be a major source of
bias. Second, it was unclear which patients will benefit the most
from third-line sunitinib treatment; in order to confirm its
clinical effectiveness and safety, prospective studies on
third-line sunitinib with larger sample sizes are needed.
In conclusion, third-line sunitinib was
well-tolerated in selected patients with mRCC and it appears to be
an effective treatment option for mRCC following failure of
cytokine therapy and sorafenib. Furthermore, optimized therapeutic
efficacy was obtained with a relatively low dose of sunitinib.
However, careful selection of eligible patients is crucial.
Acknowledgements
The authors would like to thank Dr Neil M. Singer
for providing expert editorial assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF designed the present study. TH, MN, KY and MI
collected and interpreted the patients' data. TF and KM analyzed
the patients' data. TF was a major contributor to writing the
manuscript. All authors have read and approved the final version of
this manuscript for publication.
Ethics approval and consent to
participate
The present study was approved by the Kitasato
University Medical Ethics Organization (approval no. KMEO B16-156).
Patient consent was not required for the present study, as it was
conducted retrospectively.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garcia JA and Rini BI: Recent progress in
the management of advanced renal cell carcinoma. CA Cancer J Clin.
57:112–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naito S, Yamamoto N, Takayama T, Muramoto
M, Shinohara N, Nishiyama K, Takahashi A, Maruyama R, Saika T,
Hoshi S, et al: Prognosis of Japanese metastatic renal cell
carcinoma patients in the cytokine era: A cooperative group report
of 1463 patients. Eur Urol. 57:317–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
General rule for clinical and pathological
studies on renal cell carcinoma, the 4th edition, . The Japanese
Urological Association, The Japanese Society of Pathology, Japan
Radiological Society. 2011.
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Cancer Institute, . Cancer
Therapy Evaluation Program. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40July
5–2019
|
|
9
|
Eichelberg C, Heuer R, Chun FK, Hinrichs
K, Zacharias M, Huland H and Heinzer H: Sequential use of the
tyrosine kinase inhibitors sorafenib and sunitinib in metastatic
renal cell carcinoma: A retrospective outcome analysis. Eur Urol.
54:1373–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dudek AZ, Zolnierek J, Dham A, Lindgren BR
and Szczylik C: Sequential therapy with sorafenib and sunitinib in
renal cell carcinoma. Cancer. 115:61–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sablin MP, Negrier S, Ravaud A, Oudard S,
Balleyguier C, Gautier J, Celier C, Medioni J and Escudier B:
Sequential sorafenib and sunitinib for renal cell carcinoma. J
Urol. 182:29–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyake H, Kusuda Y, Harada K, Sakai I and
Fujisawa M: Third-line sunitinib following sequential use of
cytokine therapy and sorafenib in Japanese patients with metastatic
renal cell carcinoma. Int J Clin Oncol. 18:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizukami Y, Kohgo Y and Chung DC: Hypoxia
inducible factor-1 independent pathways in tumor angiogenesis. Clin
Cancer Res. 13:5670–5674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita T, Iwamura M, Ishii D, Tabata K,
Matsumoto K, Yoshida K and Baba S: C-reactive protein as a
prognostic marker for advanced renal cell carcinoma treated with
sunitinib. Int J Urol. 19:908–913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gore ME, Szczylik C, Porta C, Bracarda S,
Bjamason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano D,
et al: Safety and efficacy of sunitinib for metastatic renal-cell
carcinoma: An expanded-access trial. Lancet Oncol. 10:757–763.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawashima A, Tsujimura A, Takayama H, Arai
Y, Nin M, Tanigawa G, Yasunaga Y, Mukai M, Uemura M, Nakai Y, et
al: Importance of continuing therapy and maintaining one-month
relative dose intensity in sunitinib therapy for metastatic renal
cell carcinoma. Med Oncol. 29:3298–3305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Porta C, Procopio G, Cartenì G, Sabbatini
R, Bearz A, Chiappino I, Ruggeri EM, Re GL, Ricotta R, Zustovich F,
et al: Sequential use of sorafenib and sunitinib in advanced
renal-cell carcinoma (RCC): An Italian multicentre retrospective
analysis of 189 patient cases. BJU Int. 108:E250–E257. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stenner F, Chastonay R, Liewen H, Haile
SR, Cathomas R, Rothermundt C, Siciliano RD, Stoll S, Knuth A,
Buchler T, et al: A pooled analysis of sequential therapies with
sorafenib and sunitinib in metastatic renal cell carcinoma.
Oncology. 82:333–340. 2012. View Article : Google Scholar : PubMed/NCBI
|