Introduction
The first gastrointestinal tumor with dual
neuroendocrine and exocrine differentiation was reported by Cordier
in 1924(1). The World Health
Organization published a new classification for neuroendocrine
neoplasms (NENs) of the digestive system in 2010 that divided NENs
into four main categories: Neuroendocrine tumor (NET) G1, NET G2,
neuroendocrine carcinoma (NEC or NET G3), and mixed
adenoneuroendocrine carcinoma (MANEC). MANEC is defined as a tumor
that is composed of neuroendocrine and epithelial cells arranged in
glandular formations, and may be considered as cancer, as both
components are malignant. Occasionally, a squamous cell carcinoma
component may be detected, albeit rarely. It is generally
considered that these two components must account for at least 30%
of the tumor in cases diagnosed with mixed cancer, as only a small
number of immunohistochemically positive neuroendocrine cells found
in adenocarcinoma are not sufficient to establish a diagnosis of
mixed cancer (2).
Liver MANECs are relatively rare, with only a few
cases reported to date, mainly in the stomach (3,4),
pancreas (5), esophagus (6) and Vater ampulla (7). Due to the low incidence of hepatic
MANECs, they may pose a diagnostic challenge, as only one component
is usually identified and the diagnosis is incomplete. The
diagnosis of MANECs mainly depends on pathological examination.
Tumor architecture is the most important diagnostic characteristic
of MANECs, and the diagnosis is then confirmed by
immunohistochemical examination for chromogranin A, synaptophysin,
CD56, or neuron-specific enolase (NSE) expression (8). The treatment of MANECs is mainly
surgical, and prognosis depends on the stage and tumor type. There
are currently no definitive recommendations regarding adjuvant
chemotherapy due to the small number of such cases reported to
date. Further improvements in the diagnosis and treatment of MANEC
are needed. The purpose of the present study was to further explore
the pathogenesis of MANEC, and to provide a theoretical basis for
early diagnosis, clinical treatment and prognosis of this type of
tumor, in order improve diagnostic awareness and optimize
individualized treatment.
Case report
A 55-year-old woman presented with chest and back
pain of unknown etiology. The patient was admitted to the West
China Hospital (Chengdu, China) for further examination and
treatment. Laboratory data revealed increased levels of
α-fetoprotein (AFP), serum carbohydrate antigen (CA)125 and NSE,
which were 23.41 ng/ml (normal range <20 ng/ml), 62.26 U/ml and
127.9 ng/ml, respectively. The AFP values associated with primary
hepatocellular carcinoma are generally known to be >500 ng/ml
for 4 weeks, or 200-500 ng/ml for 8 weeks. Therefore, the diagnosis
of primary liver cancer in this patient was not considered likely.
CA125 is widely present in mesothelial tissue and is currently the
most important ovarian cancer-associated antigen. CA125 is the most
reliable diagnostic indicator for ovarian cancer, and its normal
level is <35 U/ml, whereas NSE is mainly used for the diagnosis
of neuroendocrine tumors, with a serum reference value of <12.5
ng/ml. Contrast-enhanced computed tomography (CT) examination
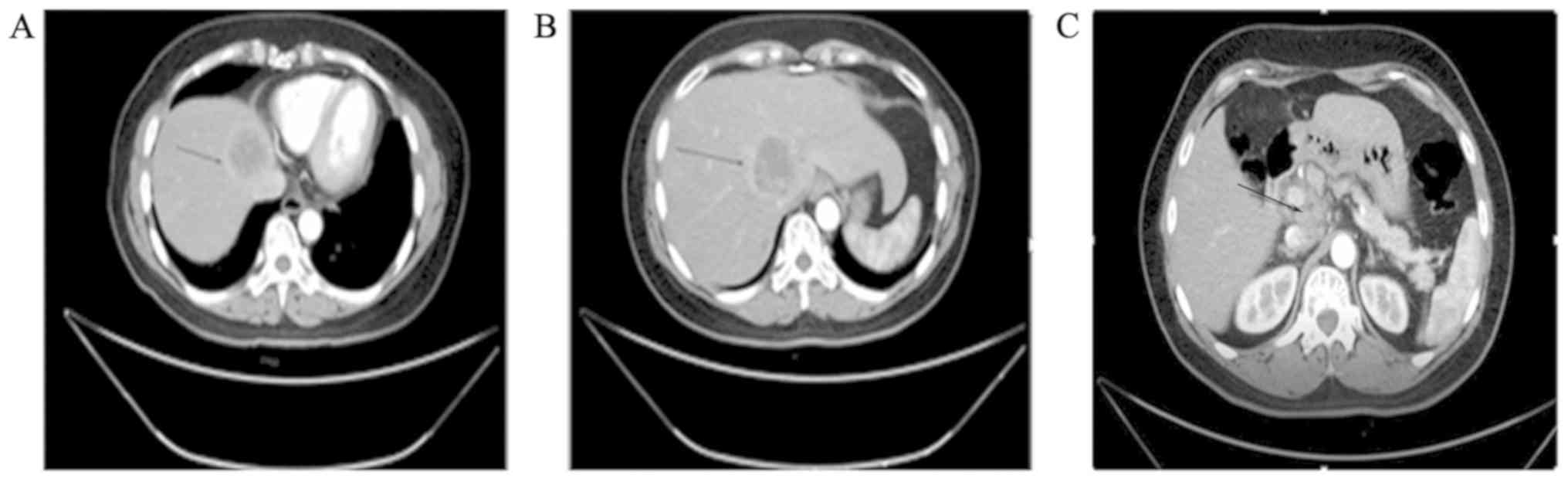
revealed low-density tumors sized 4.6 and 4.4 cm in the caudate and
left internal lobe of the liver, respectively (Fig. 1A and B). There were multiple enlarged lymph nodes
along the abdominal aorta, the hepatogastric and gastrosplenic
ligaments, and in the space between the portal vein and the
inferior vena cava (Fig. 1C). The
patient was subjected to left three hepatic resection (including
left inner lobe, left outer lobe and right anterior lobe
resection), abdominal lymph node dissection, liver tumor
radiofrequency ablation, hepatic caudate lobe resection, intestinal
adhesion release, repair of vena cava damage, portal vein repair
and hilar cholangioplasty in our hospital. The liver margin of the
surgical specimen was not invaded by cancer. Subsequent
immunohistochemical examination did not rule out the possibility of
a gastrointestinal origin, and gastrointestinal endoscopy and
positron emission tomography (PET)-CT were performed to exclude
distant metastasis. Upper gastrointestinal endoscopy revealed
chronic non-atrophic gastritis and esophagitis (grade B).
Endoscopic examination of the lower digestive tract detected polyps
of the colon, diagnosed as tubular adenomas following biopsy and
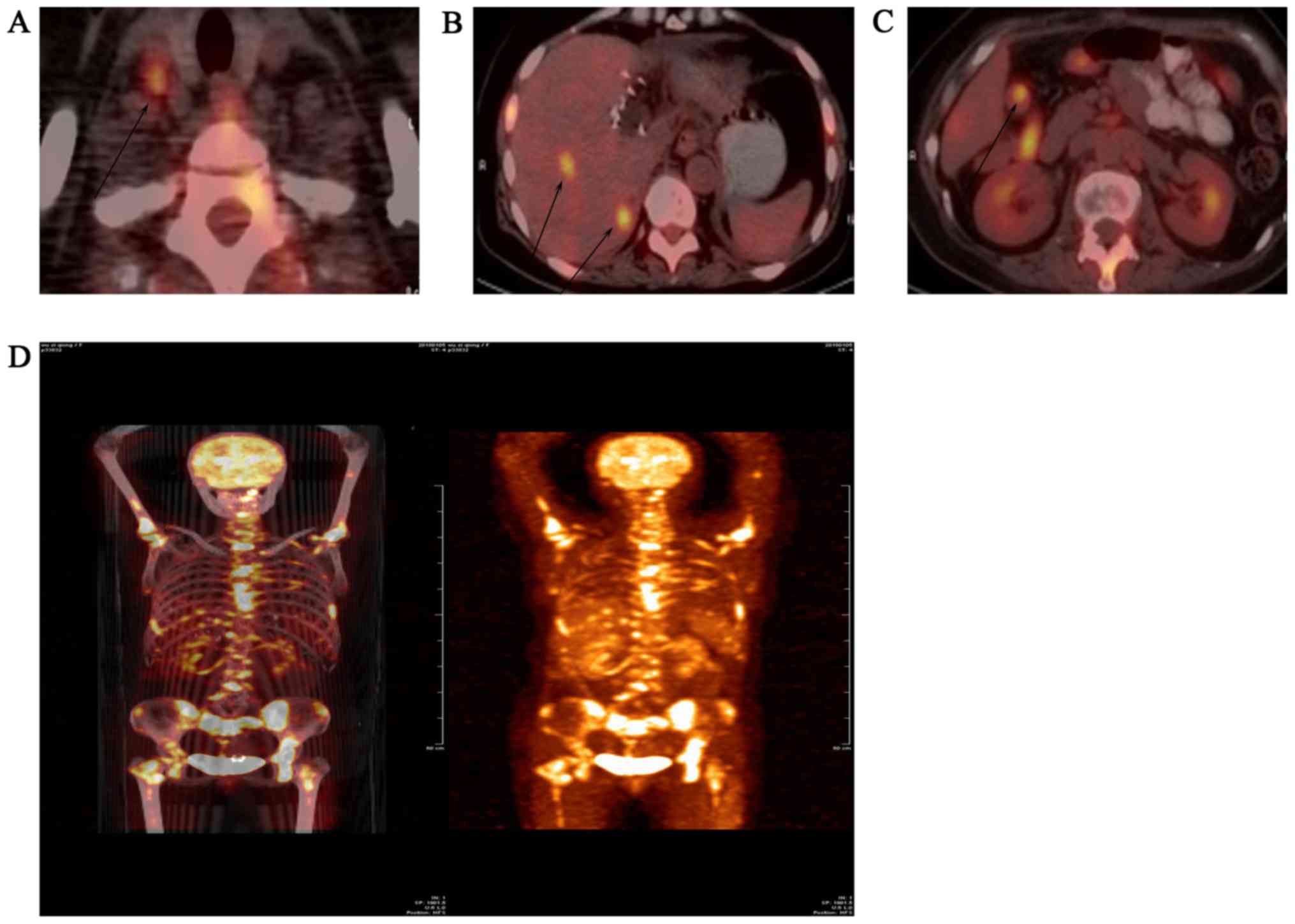
histopathological examination. The PET examination demonstrated
active glucose metabolism in the liver, cervical lymph nodes,
abdominal lymph nodes and bones, mostly due to tumor metastases
(Fig. 2). There was no evidence of
intestinal, ovarian or other metastases.
The surgical specimen was fixed in 10% neutral
formalin solution for >48 h. The tissue samples were then
embedded in paraffin for sectioning at 1 mm, and the sections were
subjected to conventional dewaxing and hydration. A multimeric
anti-rabbit/mouse IgG-HRP kit (SV0004; Boster Biological Technology
Co., Ltd.) was applied for experimental testing. The sections were
incubated with 3% H2O2 in deionized water for
5-10 min at room temperature to eliminate endogenous peroxidase
activity, and then rinsed with PBS (Boster Biological Technology
Co., Ltd.; cat. no. AR0030) 3 times for 5 min each time. The
antigen was repaired by boiling 0.01 M sodium citrate buffer
solution in a microwave oven. After adding 5% BSA blocking
solution, the sections were incubated at 37˚C for 30 min for
blocking and then dried. The sections were then incubated with
primary antibodies against CK20 (clone OVIL 12/30; Leica
Biosystems; 1:50 dilution), carcinoembryonic antigen (CEA) (clone
12-140-10; Leica Biosystems; 1:200 dilution), synaptophysin (Merck
KGaA; cat. no. MAB5258-I; 1:200 dilution), Ki-67 (BM2889; 1:100
dilution), retinoblastoma (RB; BM2184; 1:100 dilution) and p53
(BM0101; 1:50 dilution) (all from Boster Biological Technology Co.,
Ltd.) at 37˚C for 1-2 h, and then washed 3 times with PBS, 5 min
each time. HRP-labeled anti-rabbit/mouse IgG was added dropwise,
incubated at 37˚C for 30 min and rinsed with PBS 3 times, 5 min
each time. The sections were colored with DAB (the reaction time
was controlled under a microscope) and then washed thoroughly with
tap water. According to the need for hematoxylin (cat. no. AR0005;
Boster Biological Technology Co., Ltd.) re-dyeing, the dyeing time
was 0.5-2 min. The sections were then dehydrated, transparentized
and photographed under a microscope (Olympus CX31; Olympus
Corporation).
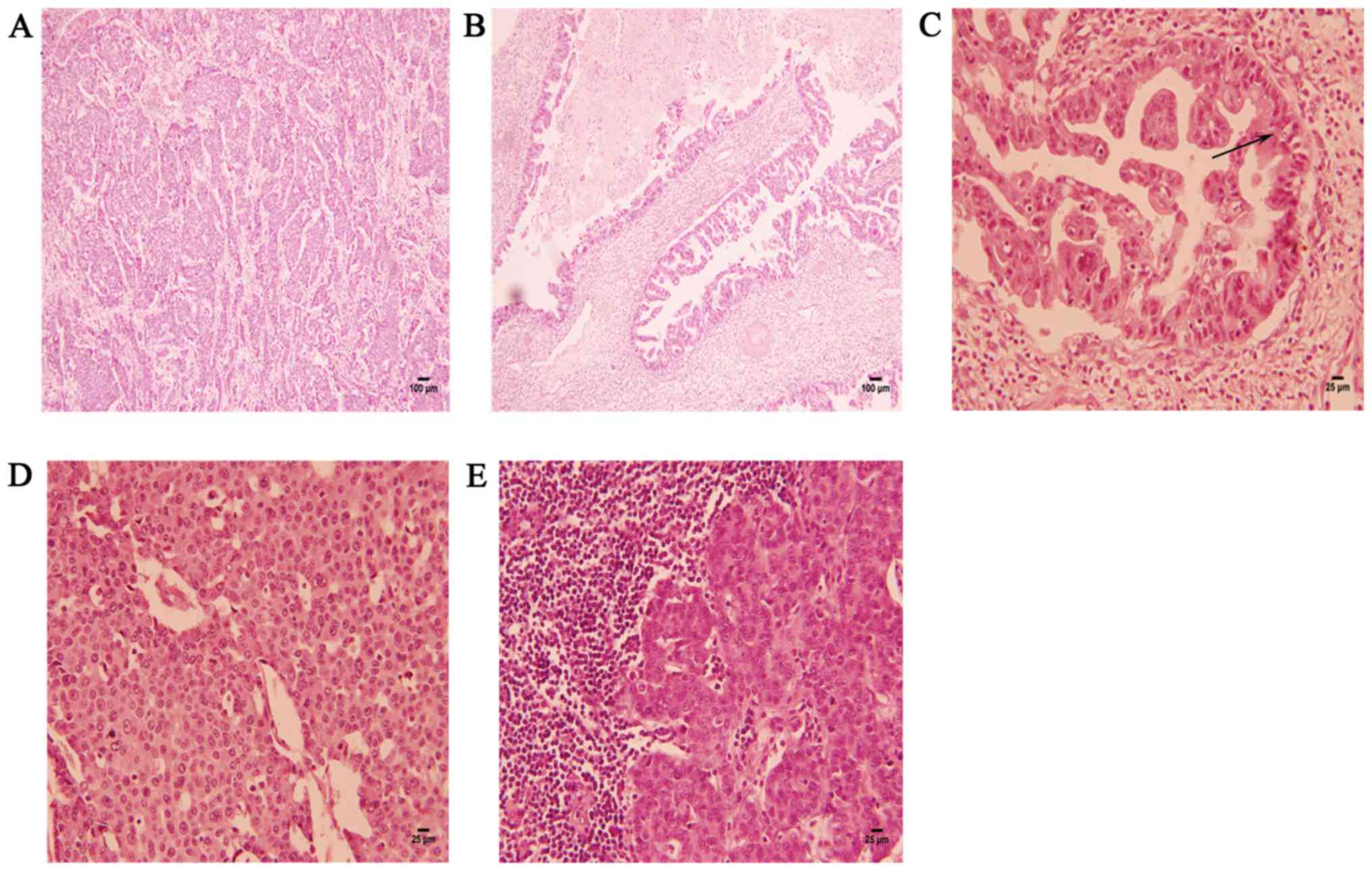
The excised liver tumor was composed of two parts.
One part was a tumor with nest-like growth in the liver parenchyma,
and the nest-like areas consisted of highly malignant large cells
(Fig. 3A). The nest-like areas
lacking acinar/glandular structures consisted of tumor cells with
salt-and-pepper nuclei, a high nucleus-to-cytoplasm ratio and
increased nuclear chromatin density (Fig. 3D). The other part was an
adenocarcinomatous component arising from the intrahepatic bile
ducts (Fig. 3B). Under high
magnification, the cells exhibited disorderly arrangement and
prominent atypia, with scattered goblet cells and a large amount of
mucus secreted in the lumen (Fig.
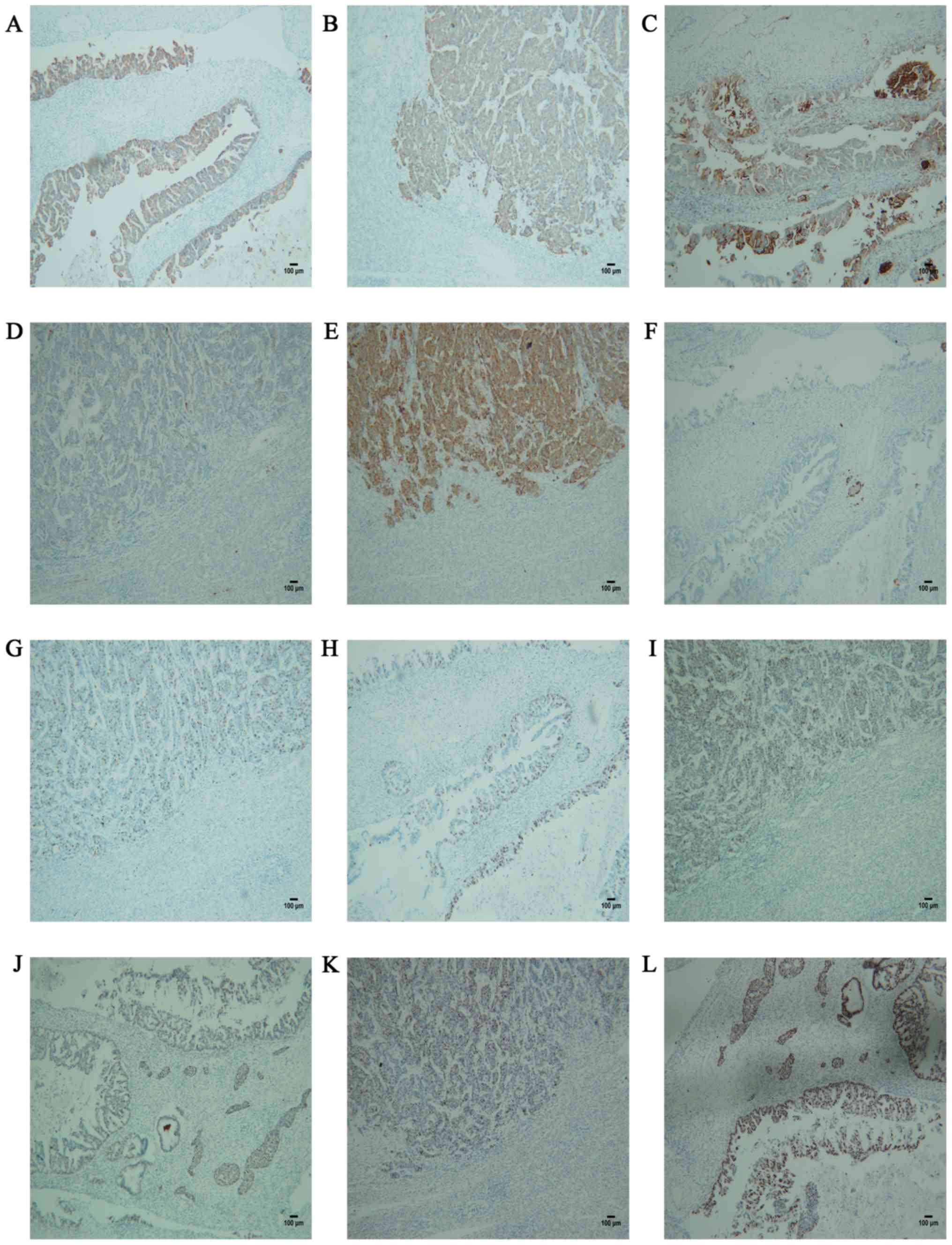
3C). The expression of cytokeratin (CK)20 and CEA, which are
positive in intrahepatic cholangiocarcinoma, confirmed the
adenocarcinomatous nature (Fig.
4A-D). Synaptophysin, which is positively expressed in hepatic
parenchymal tumors and negative in bile duct adenocarcinoma,
confirmed the NET component of MANEC and the dual differentiation
of this tumor (Fig. 4E and F). The MIB-1 proliferation index of the NET
component was significantly higher compared with that of the
adenocarcinomatous component (Fig.
4G and H). The Ki-67 positivity
rate of the NET component was ~60%, and the mitotic figure count
was ~35/10 high-power fields (HPF). Lymph node invasion by the NET
component was identified (Fig. 3E),
indicating that NET was the main determinant of the prognosis. RB
and P53 are positive in NET and adenocarcinoma. RB and P53
positivity is observed in various tumors, and suggests a poor
prognosis (Fig. 4I-L). The patient
in this case succumbed to disease progression 3 months after
surgery.
Discussion
According to the 2010 WHO classification, NETs of
the digestive system are classified as follows: NET G1 (carcinoid;
mitotic count: <2 per 10 HPF and/or ≤2% Ki-67 index); NET G2
(mitotic count: 2-20 per 10 HPF and/or 3-20% Ki-67 index); NEC
(large- or small-cell type); and MANEC (9). The Ki-67 positivity rate in the present
case was ~60%, and the mitotic figure count was ~35/10 HPF. The
neuroendocrine components contain small, intermediate and large
neuroendocrine cells.
In cytology, classification relies on
differentiation (number of mitotic divisions and Ki-67
proliferation index), which reflects the extent to which malignant
cells resemble normal cells. Tumor morphology and Ki-67
proliferation index positivity rate are considered as key
prognostic factors (10). According
to the grade of differentiation and malignancy of the two
components, MANECs are further divided into the following subtypes:
High-grade malignant MANEC, intermediate-grade malignant MANEC, and
a low-grade mixed gonadal neuroendocrine tumor (11). High-grade malignant MANECs consist of
a carcinomatous component (adenocarcinoma or squamous cell
carcinoma) and a poorly differentiated NEC component.
Intermediate-grade malignant MANECs consist of adenocarcinoma and
G1 or G2 NEN, whereas low-grade mixed gonadal neuroendocrine tumors
consist of adenoma and G1 or G2 NEN components. Based on the
Digestive System Tumor NET WHO 2010 Grading Criteria, NET G3 (NEC)
is defined as a mitotic count >20/10 HPF and/or Ki-67 >20%.
Therefore, the NET of the patient in the present case was G3
(NEC).
CK20 and CEA are positive in adenocarcinoma, and
confirmed the presence of an adenocarcinomatous component in MANEC.
Synaptophysin, which is positive in NET and negative in
adenocarcinoma, confirmed the NET component of the MANEC. RB and
P53 expression is positive in various tumors, and is suggestive of
a poor prognosis. It was previously suggested that, when MANECs
contain poorly differentiated (G3) NET components, they should be
treated as NECs (12,13). This simpler proliferation-based
ENETS/WHO 2010 classification system provides prognostic
information, but has not been validated to predict recurrence
following surgical resection.
Liver MANEC is rarely reported. The origin of MANEC
remains uncertain. There are two main theories regarding the origin
of this disease. Based on the first theory, it is hypothesized that
the two components of MANEC originate from two different cell
lines. The adenocarcinoma cells originate from pluripotent stem
cells, whereas NEC originates from embryonic neural cells. Based on
the second hypothesis, MANEC is considered to originate from
endodermal pluripotent stem cells, which are affected by hormones,
the local microenvironment and an instable genome during the
process of tumor occurrence and development, eventually leading to
a two-way or multidirectional differentiation (14-16).
However, in most cases, the adenocarcinomatous and NEC components
of MANEC are cross-mixed. Only in a few cases these two components
are closely linked without mixing (referred to as ‘colliding’
tumors), which suggests that the majority of these tumors may
originate from pluripotent stem cells and undergo multidirectional
differentiation during tumor occurrence and development. Zhang
et al (17) reported that, in
addition to the adenocarcinoma and NEC components, mixed cancer
also contains squamous cell carcinoma, which may support the
concept that these tumors originate from pluripotent stem cells.
However, it remains unclear why two tumor cells of a different
origin and behavior can coexist in one tumor, that is, that the
same tumor contains two different components of cancer cells,
adenocarcinoma or neuroendocrine cancer and the squamous cell
carcinoma.
It was previously considered that the percentage of
each component in mixed cancers determines disease progression.
However, retrospective case analyses have demonstrated that the
prognosis of MANEC depends on the main component (18), rather than the proportion of each
component. This is because this smaller percentage volume may
metastasize and significantly affect patient outcome. Most authors
argue that the characteristics of the neuroendocrine component have
a considerable impact on the clinical manifestations of MANEC
(19). In addition, in most
literature reports, lymph node and liver metastases are almost
always from NEC rather than adenocarcinoma, as NECs are usually
poorly differentiated and more aggressive compared with
adenocarcinomas. Scardoni et al (20) concluded that there are multiple
drivers of gene mutations in the neuroendocrine component, such as
the ATM, ERBB4, KDR/VEGFR2, JAK3 and TP53 genes. Although some
authors believe that clinical behavior depends on the grade of the
neuroendocrine component, others report that the characteristics of
the adenocarcinomatous part affect the outcome in cases with
well-differentiated neuroendocrine components (21,22).
Gurzu et al (8) reported that
the glandular component was predominant in lymph node metastases,
and nuclear expression of maspin in glandular structures compared
with its negativity in the neuroendocrine component confirmed the
higher aggressiveness of adenocarcinoma compared with poorly
differentiated NEC. A previous study reported that the mortality
rate by histological type was significantly higher in low
differentiation adenocarcinoma/adverse NET compared with that in
highly differentiated (adenocarcinoma/low-grade NETs (23). This suggests that the NEC component,
and not the adenocarcinoma component, is the main driver of cancer
progression in this cancer type. Others believe that adenocarcinoma
may affect the prognosis of MANECs with highly differentiated NEC.
However, in the present case, both the adenocarcinoma and NEC were
high-grade malignant tumors (the NEC component accounted for ~60%
and the adenocarcinoma for ~40%). The metastatic lymph nodes in
this case were from NEC, which suggests that NEC is more likely to
invade lymphatic vessels and plays an important role in the
prognosis of high-grade MANEC, which is mainly responsible for
disease progression. Based on most literature reports, lymph node
metastasis is almost always from NEC rather than adenocarcinoma
and, therefore, must be treated as a NEC. In addition, the higher
the positive rate of P53 expression on immunohistochemistry, the
worse the prognosis. The RB gene is widely distributed in various
tissues, and is known to inhibit cell proliferation, promote cell
differentiation, and regulate the cell cycle, but its role in MANEC
remains unclear, although it may be associated with prognosis.
Further research on the RB gene is required. Ki-67 is a marker of
cell proliferation and, the higher its positivity rate, the higher
the malignant potential of the tumor. Ki-67 is closely associated
with tumor differentiation, invasion, metastasis and prognosis.
However, further extensive clinical research and epidemiological
analysis are required. A clear understanding of the key factors
affecting cancer progression is crucial for determining standard
treatment options. However, recent WHO classifications indicate
that MANEC treatment is similar to that for common
adenocarcinoma.
Due to the rarity of MANEC, the most effective
chemotherapy regimen remains to be determined. The National
Comprehensive Cancer Network recommends the use of cisplatin or
etoposide, but the prognosis remains poor, with a median survival
time of 7-10 months at present. However, there is currently no
consensus among different investigators regarding the choice of
adjuvant chemotherapy. Some authors recommend the use of cisplatin
or carboplatin with etoposide (24),
whereas others approve the combination of cisplatin with
fluoropyrimidines in cases with metastatic adenopathy (25). Surgery remains the preferred
treatment for early liver malignant tumors. For the diagnosis of
liver malignant tumors, a bioptic specimen may be collected during
surgery. However, a liver biopsy may lead to dissemination of tumor
cells, which may cause metastasis to the peritoneum or other sites
and worsen the prognosis. Based on each patient's status, the
development of effective and individualized treatment approaches to
reduce postoperative complications requires continuous clinical
practice and experience.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJY, ZPL, TPY and XMH participated, conceived and
designed the present case report, analyzed and interpreted the data
and wrote the manuscript. ZPL, CLL and YD evaluated the patient and
participated in treatment. YD and CLL evaluated radiological
images. QYL made strict changes to the language of the manuscript
and made suggestions. NL and HL participated in the histochemical
photography and scrutinized the manuscript. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient's family provided permission to publish
the case details and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jain A, Singla S, Jagdeesh KS and
Vishnumurthy HY: Mixed adenoneuroendocrine carcinoma of cecum: A
rare entity. J Clin Imaging Sci. 3(10)2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shimada N, Miwa S, Arai T, Kitagawa N,
Akita S, Iinuma N and Ishii K: Cystic mixed adenoneuroendocrine
carcinoma of the pancreas: A case report. Int J Surg Case Rep.
52:1–4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kwok CM: Mixed adenoneuroendocrine
carcinoma of the stomach. Case Rep Gastroenterol. 9:241–245.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamauchi H, Sakurai S, Nakazawa N, Yoshida
T, Tabe Y, Saitoh K, Fukasawa T, Kiriyama S, Naitoh H and Kuwano H:
A case of mixed adenoneuroendocrine carcinoma of the stomach with
focal intestinal metaplasia and hypergastrinemia. Int Surg.
100:562–567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Minakawa K, Oka K, Nihei T, Sando N,
Oikawa H, Toda J, Hosokawa Y, Matsumoto T and Yanagisawa A:
Pancreatic endocrine tumor with partial acinar cell
differentiation. APMIS. 114:720–725. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kadhim MM, Jespersen ML, Pilegaard HK,
Nordsmark M and Villadsen GE: Mixed adenoneuroendocrine carcinoma
is a rare but important tumour found in the oesophagus. Case Rep
Gastrointest Med. 2016(9542687)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ginori A, Lo Bello G, Vassallo L and
Tripodi SA: Amphicrine carcinoma of the ampullary region. Tumori.
101:e70–e72. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gurzu S, Kadar Z, Bara T, Bara T Jr,
Tamasi A, Azamfirei L and Jung I: Mixed adenoneuroendocrine
carcinoma of gastrointestinal tract: Report of two cases. World J
Gastroenterol. 21:1329–1333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Onishi I, Kitagawa H, Harada K, Maruzen S,
Sakai S, Makino I, Hayashi H, Nakagawara H, Tajima H, Takamura H,
et al: Intraductal papillary neoplasm of the bile duct accompanying
biliary mixed adenoneuroendocrine carcinoma. World J Gastroenterol.
19:3161–3164. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Niederle MB, Hackl M, Kaserer K and
Niederle B: Gastroenteropancreatic neuroendocrine tumours: The
current incidence and staging based on the WHO and European
neuroendocrine tumour society classification: An analysis based on
prospectively collected parameters. Endocr Relat Cancer.
17:909–918. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang Q, Zhou Z, Chen J, Di M, Ji J, Yuan
W, Liu Z, Wu L, Zhang X, Li K and Shu X: Correlation of metastasis
characteristics with prognosis in gastric mixed adenoneuroendocrine
carcinoma: Two case reports. Medicine (Baltimore).
96(e9189)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
La Rosa S, Marando A, Sessa F and Capella
C: Mixed Adenoneuroendocrine carcinomas (MANECs) of the
gastrointestinal tract: An update. Cancers (Basel). 4:11–30.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Volante M, Rindi G and Papotti M: The grey
zone between pure (neuro)endocrine and non-(neuro)endocrine
tumours: A comment on concepts and classification of mixed
exocrine-endocrine neoplasms. Virchows Arch. 449:499–506.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Furlan D, Cerutti R, Genasetti A, Pelosi
G, Uccella S, La Rosa S and Capella C: Microallelotyping defines
the monoclonal or the polyclonal origin of mixed and collision
endocrine-exocrine tumors of the gut. Lab Invest. 83:963–971.
2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Paniz Mondolfi AE, Slova D, Fan W, Attiyeh
FF, Afthinos J, Reidy J, Pang Y and Theise ND: Mixed
adenoneuroendocrine carcinoma (MANEC) of the gallbladder: A
possible stem cell tumor? Pathol Int. 61:608–614. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vanacker L, Smeets D, Hoorens A, Teugels
E, Algaba R, Dehou MF, De Becker A, Lambrechts D and De Greve J:
Mixed adenoneuroendocrine carcinoma of the colon: Molecular
pathogenesis and treatment. Anticancer Res. 34:5517–5521.
2014.PubMed/NCBI
|
|
17
|
Zhang W, Xiao W, Ma H, Sun M, Chen H and
Zheng S: Neuroendocrine liver metastasis in gastric mixed
adenoneuroendocrine carcinoma with trilineage cell differentiation:
A case report. Int J Clin Exp Pathol. 7:6333–6338. 2014.PubMed/NCBI
|
|
18
|
Lee EJ, Park SM, Maeng L, Lee A and Kim
KM: Composite glandular-endocrine cell carcinomas of the stomach:
Clinicopathologic and methylation study. APMIS. 113:569–576.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Levi Sandri GB, Carboni F, Valle M, Visca
P and Garofalo A: Mixed adenoneuroendocrine gastric carcinoma: A
case report and review of the literature. J Gastric Cancer.
14:63–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Scardoni M, Vittoria E, Volante M, Rusev
B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G,
Butturini G, et al: Mixed adenoneuroendocrine carcinomas of the
gastrointestinal tract: Targeted next-generation sequencing
suggests a monoclonal origin of the two components.
Neuroendocrinology. 100:310–316. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim JJ, Kim JY, Hur H, Cho YK and Han SU:
Clinicopathologic significance of gastric adenocarcinoma with
neuroendocrine features. J Gastric Cancer. 11:195–199.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee JH, Kim HW, Kang DH, Choi CW, Park SB
and Kim SH: A gastric composite tumor with an adenocarcinoma and a
neuroendocrine carcinoma: A case report. Clin Endosc. 46:280–283.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Y, Yau A, Schaeffer D, Magliocco A, Gui
X, Urbanski S, Waghray R, Owen D and Gao ZH: Colorectal
glandular-neuroendocrine mixed tumor: Pathologic spectrum and
clinical implications. Am J Surg Pathol. 35:413–425.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Delle Fave G, Kwekkeboom DJ, Van Cutsem E,
Rindi G, Kos-Kudla B, Knigge U, Sasano H, Tomassetti P, Salazar R
and Ruszniewski P: ENETS Consensus Guidelines for the management of
patients with gastroduodenal neoplasms. Neuroendocrinology.
95:74–87. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Van Laethem JL, Carneiro F, Ducreux M,
Messman H, Lordick F, Ilson DH, Allum WH, Haustermans K, Lepage C,
Matysiak-Budnik T, et al: The multidisciplinary management of
gastro-oesophageal junction tumours: European society of digestive
oncology (ESDO): Expert discussion and report from the 16th ESMO
world congress on gastrointestinal cancer, barcelona. Dig Liver
Dis. 48:1283–1289. 2016.PubMed/NCBI View Article : Google Scholar
|