Introduction
The number of people with osteoporosis has increased
as the population ages. Osteoporosis is often treated with
teriparatide (1), which has been
demonstrated to promote bone healing and prevent fragility
fractures in both rats and humans (2-9).
Along with its numerous clinical advantages, teriparatide has some
less well-known contraindications, such as a history of radiation
therapy (10), the presence of
primary malignant and metastatic bone tumors (11), and Paget's disease (12), all conditions under which
teriparatide may induce osteosarcoma.
Initial preclinical studies in rats revealed that
teriparatide increases the risk of osteosarcoma development. Vahle
et al reported that rats given daily injections of
recombinant human parathyroid hormone develop proliferative bone
lesions, and some rats develop osteosarcoma (13). Watanabe et al reported that
teriparatide can induce osteosarcoma in rats, depending on the dose
and duration of treatment (14).
Vahle et al reported a safe teriparatide dose for rats in
2004(15). Two cases of osteosarcoma
following the administration of teriparatide have been reported in
the USA. However, in one case, the causality between teriparatide
and the osteosarcoma could not be established (10). In addition, in the other case the
patient was treated with radiation therapy before teriparatide
administration; therefore, it is unclear whether the teriparatide
administration or radiation therapy were associated with
osteosarcoma onset (11). In the
present case, the patient had never received any radiation therapy
and there was no history of Paget's disease. To date, there are no
reported cases of definite teriparatide-induced osteosarcoma in
humans in the USA (12,16) or Japan (17), to the best of our knowledge.
The present study presents the case of an elderly
patient with severe osteoporosis in which teriparatide may have
accelerated the growth of a pre-existing malignant tumor. This case
serves as a caveat against the misdiagnosis of a pathological
fracture as a normal fracture in elderly patients, particularly
before teriparatide administration. Therefore, care should be taken
to diagnose femoral fractures in elderly patients.
Case report
A 76-year-old Japanese woman was doing farm work on
a ladder and fell 50 cm to the ground. The patient felt pain in her
right thigh and was unable to stand. She then visited National
Hospital Organization Hirosaki Hospital (Hirosaki, Japan) in
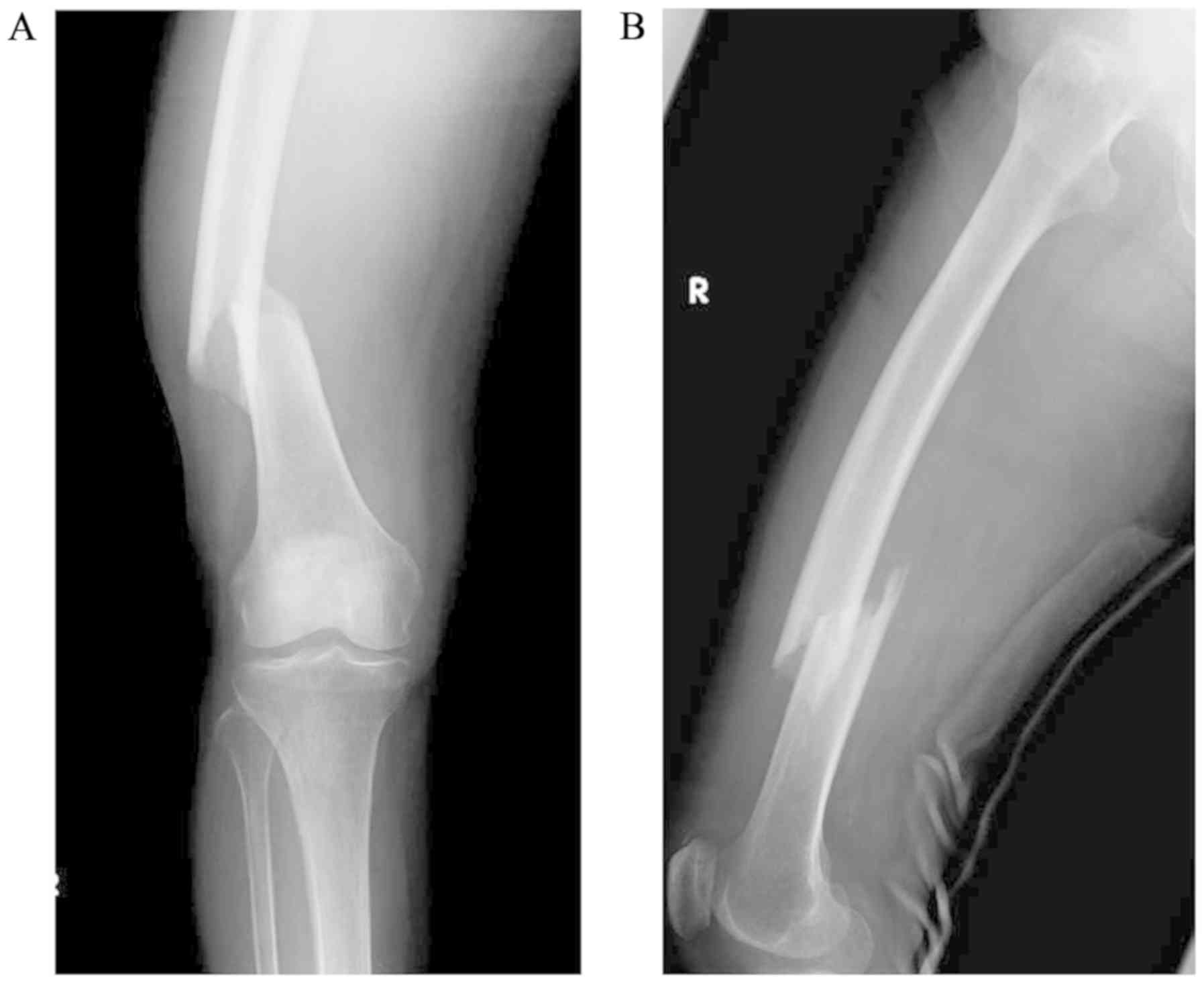
September, 2016 and was diagnosed with a right femoral-shaft
fracture (Fig. 1). The patient had
no history of illness and had never undergone radiotherapy in the
past. The laboratory data, including C-reactive protein (CRP),
alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels,
were within normal range, and the fracture was treated immediately
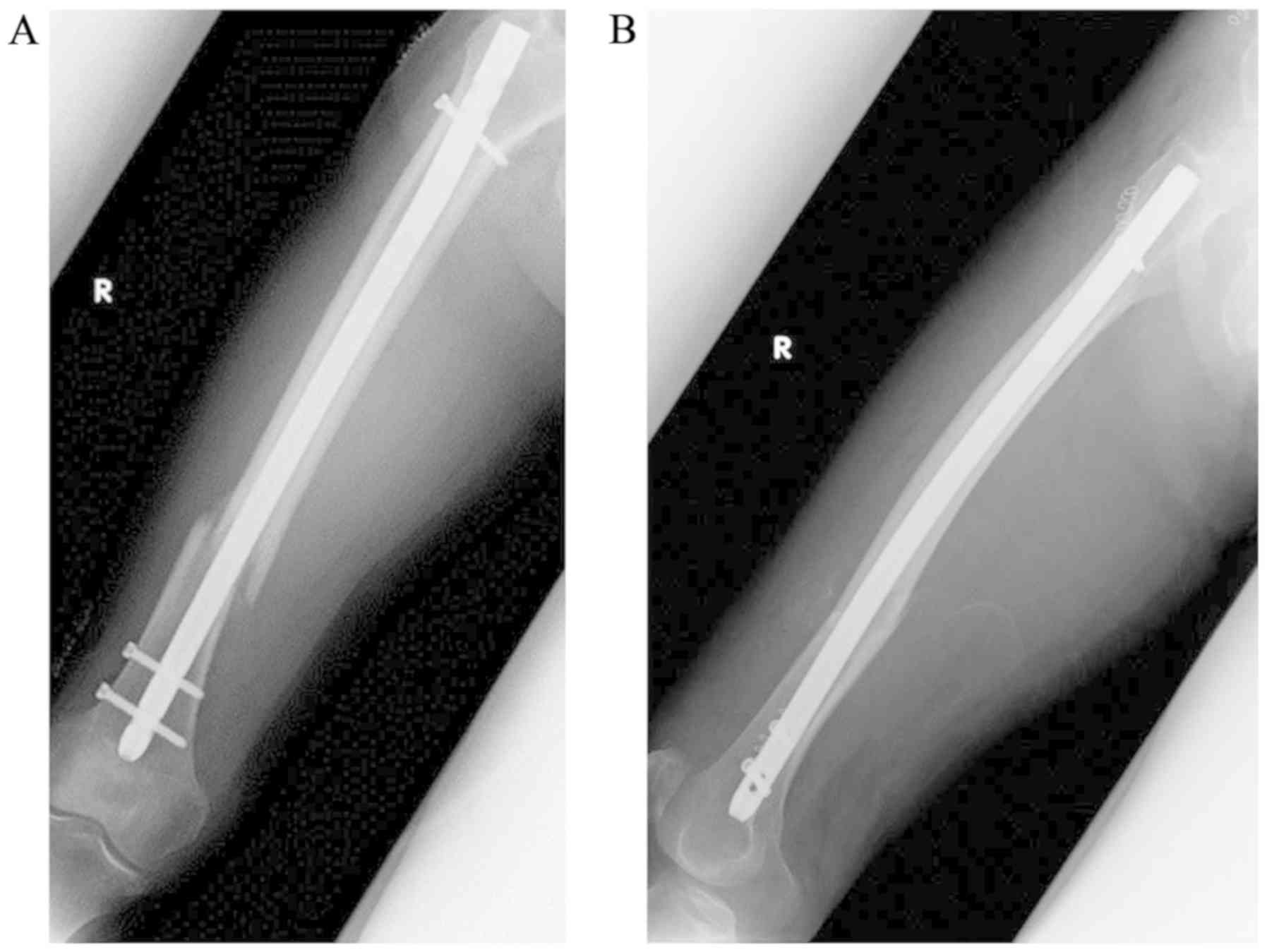
by intramedullary nail fixation (Fig.
2). A postoperative bone density test identified severe
osteoporosis. The patient was treated with a daily regimen of
teriparatide (20 µg/day); however, the drug was discontinued after
2 months due to the onset of nausea. A total of 6 months after the
initial surgery, the patient visited Hirosaki University Hospital
on April, 2017 with abnormal swelling of the right thigh. At
presentation, the right thigh had a circumference approximately
twice as large as that of the left thigh, and the right knee had a
limited range of motion. Blood tests revealed that the CRP, ALP and
LDH levels were slightly elevated, but all tumor markers, including
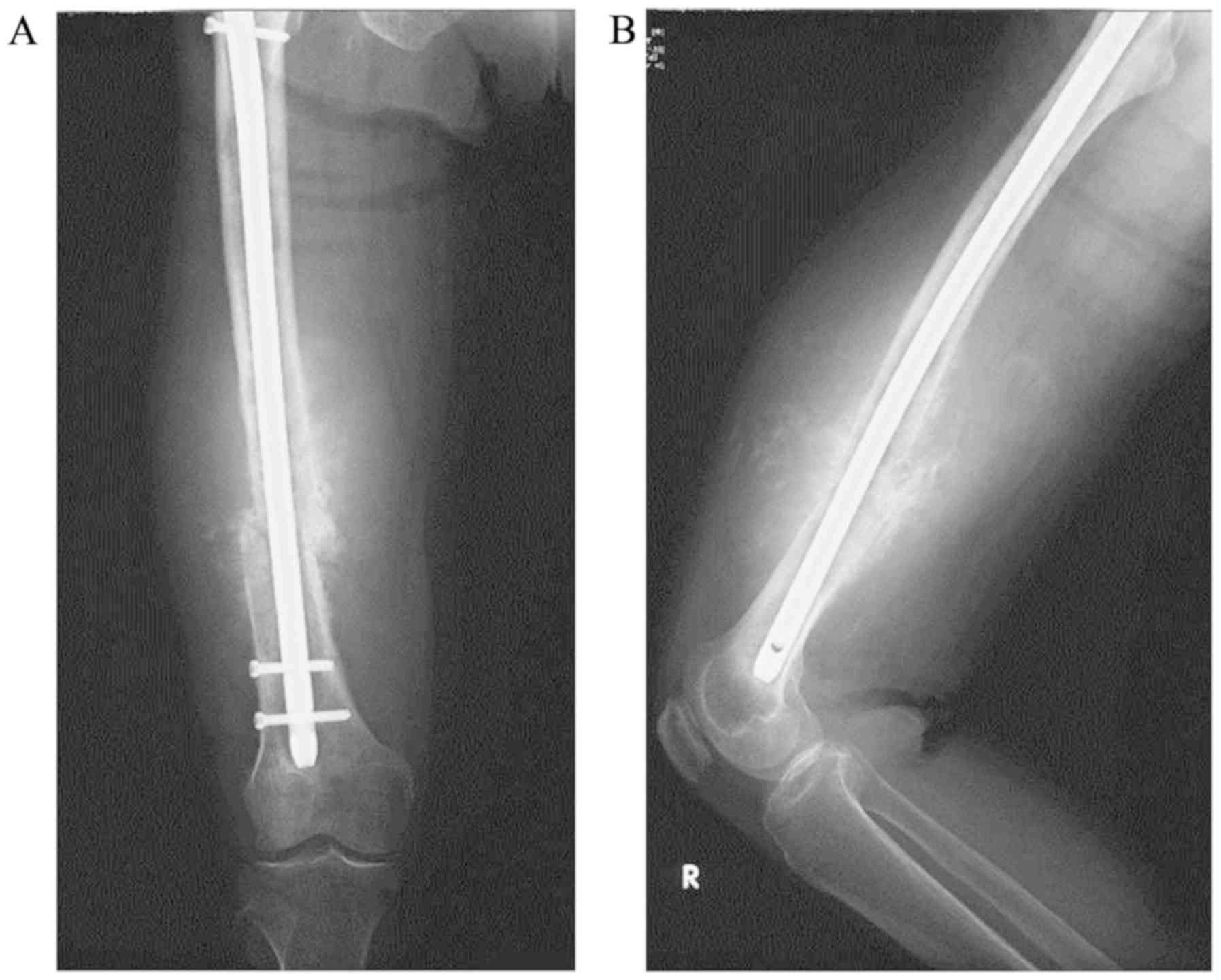
AFP, CA125, CA19-9, CEA and SCC were negative. Plain radiography
demonstrated incomplete bone union of the right femoral diaphysis
and a periosteal reaction with a sunburst-like appearance around
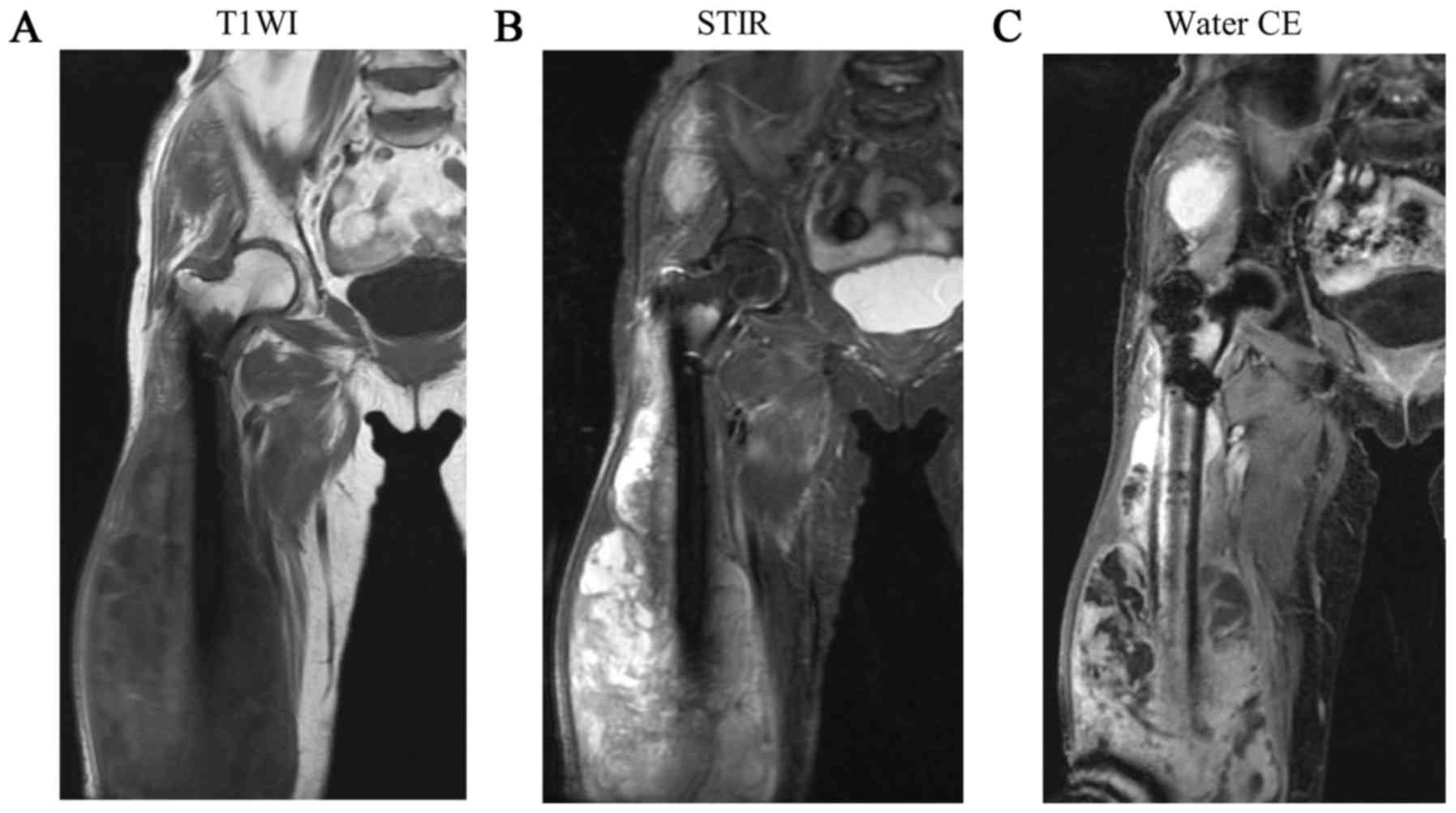
the fracture (Fig. 3). Magnetic
resonance imaging (MRI) revealed a soft tissue mass around the
femur, with a low-intensity to iso-intense signal on T1-weighted
images and a mixed low to high signal intensity on STIR images
(Fig. 4). The soft tissue mass also
exhibited diffuse and heterogeneous contrast enhancement. Another
mass with similar characteristics was identified in the gluteus
medius muscle, in a region that would lie along the pathway of the
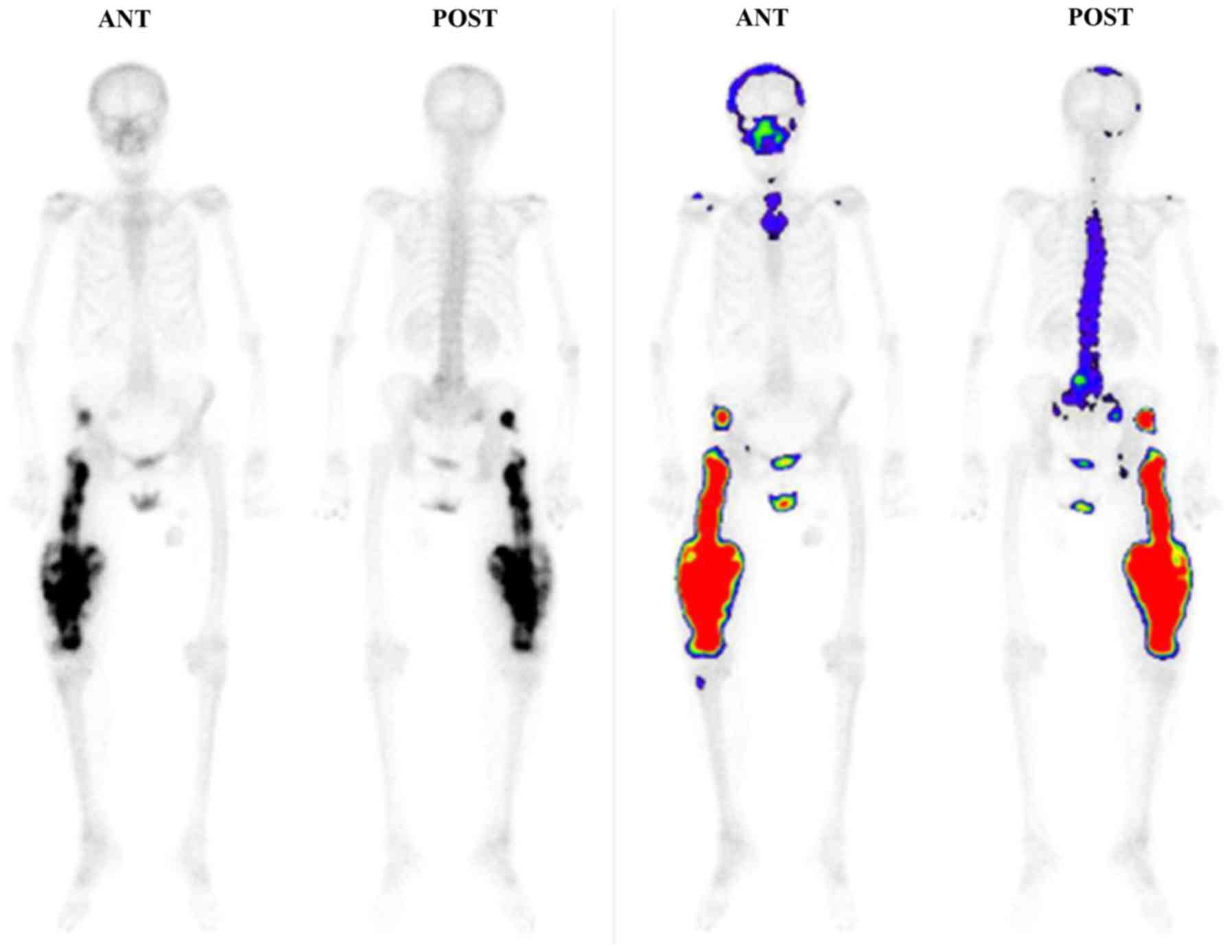
intramedullary nail insertion. No significant accumulation was
observed on a whole-body bone scintigraph, except for the right
femoral and right gluteus medius muscle regions (Fig. 5). A high-grade malignant mesenchymal
bone tumor was diagnosed by needle biopsy. The patient underwent
right hip disarticulation with resection of the gluteus medius
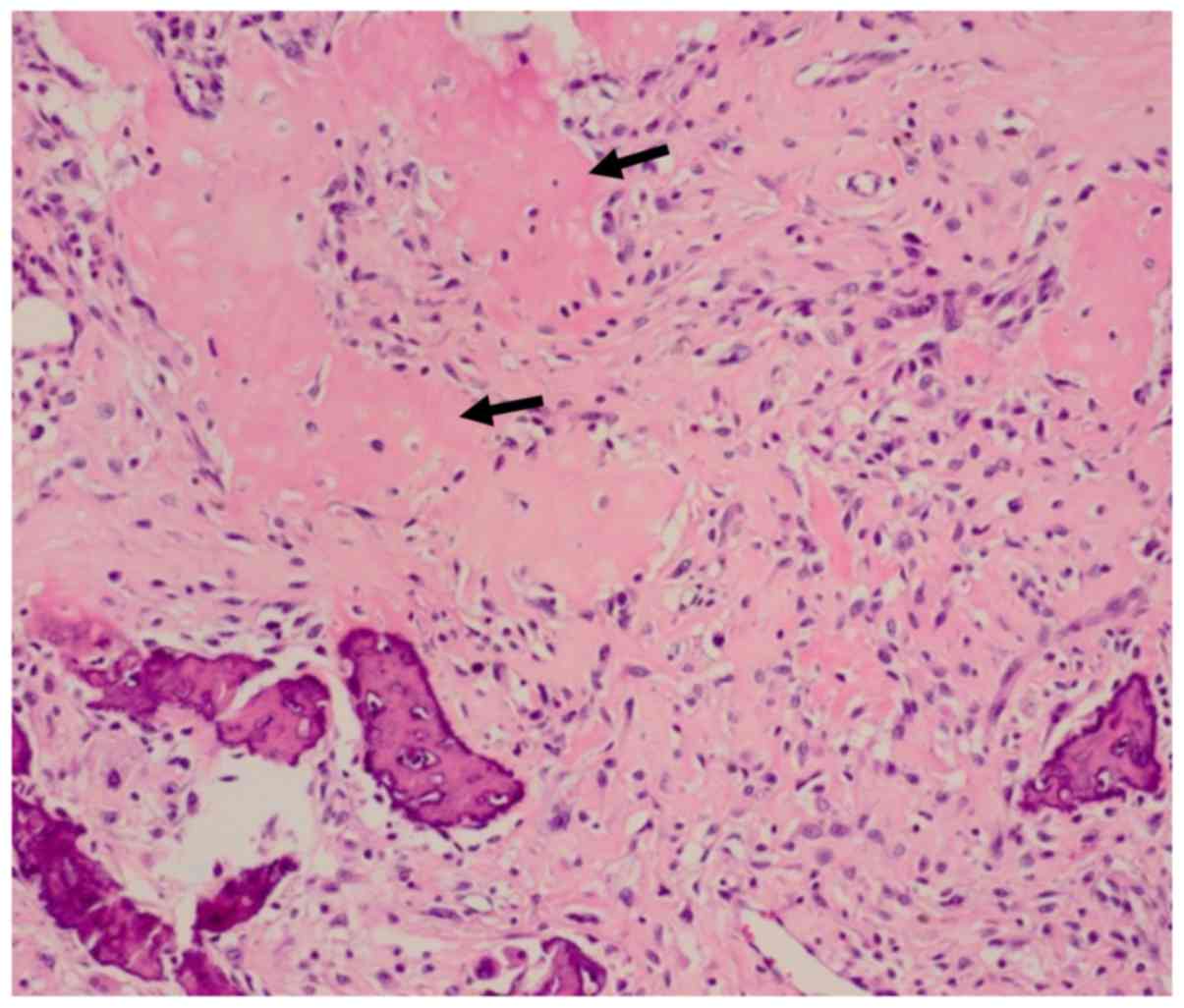
muscle. The cells were rich in polymorphisms, and strong
heteromorphic tumor cells forming osteoids were observed (Fig. 6). The definitive pathological
diagnosis was an osteoblastic osteosarcoma of the right femur.
Adjuvant chemotherapy was not performed due to the patient's
advanced age. The patient provided informed consent.
Discussion
The present case provides an important reminder that
teriparatide may accelerate the growth of a pre-existing malignant
tumor in an elderly patient. A previous study demonstrated that
teriparatide increases the risk of osteosarcoma in rats, according
to the dose and duration of administration (15). In the USA, two patients with
osteosarcoma after teriparatide administration have been reported
(10,11). In addition, other case reports have
described four patients with primary hyperparathyroidism in whom
chronically elevated parathyroid hormone levels induced
osteosarcoma (18-20).
The period of teriparatide administration was only 2 months, which
is a limitation of this case as the recommended administration of
teriparatide in osteoporosis is up to 24 months (16). The short administration period makes
it unlikely that the osteosarcoma arose from a non-pathological
fracture. Although the initial radiographs did not reveal any
malignant bone lesions in the present case, there may have been a
diffuse permeating malignant lesion; it is likely that that would
have accelerated the growth of a pre-existing malignant tumor.
The present case study also emphasizes the
importance of diagnosing femoral fractures in the elderly, due to
the possibility of pathological fractures from malignant disease.
Epidemiologically, diaphyseal femoral fractures are not as common
as proximal femoral fractures (21,22). In
the present case, a pathological fracture should have been
considered because the fracture resulted from a fall from a
relatively low height, and because the patient mentioned that they
had experienced pain in the right thigh 1 week before the injury.
These atypical clinical elements suggest that the femoral
diaphyseal fracture was a pathological fracture. However, at the
initial presentation, the plain radiographs did not reveal any
periosteal reaction, osteolytic or osteoblastic change around the
fracture site, or other abnormalities that may have made it easier
to recognize a pathological fracture. As elderly individuals have a
high risk of malignant disease, atypical clinical elements should
prompt the clinician to consider the possibility of a pathological
fracture. In such cases, clinicians should not hesitate to perform
CT scans and/or an MRI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TO, SO, MY, YI participated in the treatment of the
patient. AK performed the pathological diagnosis. All the authors
have read and approved the final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soen S, Fujiwara S, Takayanagi R, Kajimoto
K, Tsujimoto M, Kimura S, Sato M, Krege JH and Enomoto H:
Real-world effectiveness of daily teriparatide in Japanese patients
with osteoporosis at high risk for fracture: Final results from the
24-month Japan Fracture Observational Study (JFOS). Curr Med Res
Opin. 33:2049–2056. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alkhiary YM, Gerstenfeld LC, Krall E,
Westmore M, Sato M, Mitlak BH and Einhorn TA: Enhancement of
experimental fracture-healing by systemic administration of
recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg
Am. 87:731–741. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aspenberg P and Johansson T: Teriparatide
improves early callus formation in distal radial fractures. Acta
Orthop. 81:234–236. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Della Rocca GJ, Crist BD and Murtha YM:
Parathyroid hormone: Is there a role in fracture healing? J Orthop
Trauma. 24 (Suppl 1):S31–S35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hodsman AB, Bauer DC, Dempster DW, Dian L,
Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski
WP, et al: Parathyroid hormone and teriparatide for the treatment
of osteoporosis: A review of the evidence and suggested guidelines
for its use. Endocr Rev. 26:688–703. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Im GI and Lee SH: Effect of teriparatide
on healing of atypical femoral fractures: A systemic review. J Bone
Metab. 22:183–189. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Neer RM, Arnaud CD, Zanchetta JR, Prince
R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S,
Genant HK, et al: Effect of parathyroid hormone (1-34) on fractures
and bone mineral density in postmenopausal women with osteoporosis.
N Engl J Med. 344:1434–1441. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Puzas JE, Houck J and Bukata SV:
Accelerated fracture healing. J Am Acad Orthop Surg. 14:S145–S151.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rowshan HH, Parham MA, Baur DA, McEntee
RD, Cauley E, Carriere DT, Wood JC, Demsar WJ and Pizarro JM:
Effect of intermittent systemic administration of recombinant
parathyroid hormone (1-34) on mandibular fracture healing in rats.
J Oral Maxillofac Surg. 68:260–267. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Subbiah V, Madsen VS, Raymond AK, Benjamin
RS and Ludwig JA: Of mice and men: Divergent risks of
teriparatide-induced osteosarcoma. Osteoporos Int. 21:1041–1045.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Harper KD, Krege JH, Marcus R and Mitlak
BH: Osteosarcoma and teriparatide? J Bone Miner Res.
22(334)2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Andrews EB, Gilsenan AW, Midkiff K,
Sherrill B, Wu Y, Mann BH and Masica D: The US postmarketing
surveillance study of adult osteosarcoma and teriparatide: Study
design and findings from the first 7 years. J Bone Miner Res.
27:2429–2437. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vahle JL, Sato M, Long GG, Young JK,
Francis PC, Engelhardt JA, Westmore MS, Linda Y and Nold JB:
Skeletal changes in rats given daily subcutaneous injections of
recombinant human parathyroid hormone (1-34) for 2 years and
relevance to human safety. Toxicol Pathol. 30:312–321.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Watanabe A, Yoneyama S, Nakajima M, Sato
N, Takao-Kawabata R, Isogai Y, Sakurai-Tanikawa A, Higuchi K,
Shimoi A, Yamatoya H, et al: Osteosarcoma in Sprague-Dawley rats
after long-term treatment with teriparatide [human parathyroid
hormone (1-34)]. J Toxicol Sci. 37:617–629. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vahle JL, Long GG, Sandusky G, Westmore M,
Ma YL and Sato M: Bone neoplasms in F344 rats given teriparatide
[rhPTH(1-34)] are dependent on duration of treatment and dose.
Toxicol Pathol. 32:426–438. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gilsenan A, Harding A, Kellier-Steele N,
Harris D, Midkiff K and Andrews E: The Forteo Patient Registry
linkage to multiple state cancer registries: Study design and
results from the first 8 years. Osteoporos Int. 29:2335–2343.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nishikawa A, Ishida T, Taketsuna M,
Yoshiki F and Enomoto H: Safety and effectiveness of daily
teriparatide in a prospective observational study in patients with
osteoporosis at high risk of fracture in Japan: Final report. Clin
Interv Aging. 11:913–925. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Smith J, Huvos AG, Chapman M, Rabbs C and
Spiro RH: Hyperparathyroidism associated with sarcoma of bone.
Skeletal Radiol. 26:107–112. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Betancourt M, Wirfel KL, Raymond AK, Yasko
AW, Lee J and Vassilopoulou-Sellin R: Osteosarcoma of bone in
apatient with primary hyperparathyroidism: A case report. J Bone
Miner Res. 18:163–166. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jutte PC, Rosso R, de Paolis M, Errani C,
Pasini E, Campanacci L, Bacci G, Bertoni F and Mercuri M:
Osteosarcoma associated with hyperparathyroidism. Skeletal Radiol.
33:473–476. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Court-Brown CM and Caesar B: Epidemiology
of adult fractures: A review. Injury. 37:691–697. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Thorngren KG, Hommel A, Norrman PO,
Thorngren J and Wingstrand H: Epidemiology of femoral neck
fractures. Injury. 33 (Suppl 3):C1–C7. 2002.PubMed/NCBI View Article : Google Scholar
|