Introduction
In cases of hepatocellular carcinoma (HCC),
measurement of tumor markers α-fetoprotein (AFP) and prothrombin
induced by vitamin K absence or antagonist-II (PIVKA-II) is widely
performed worldwide because such measurement is complementary to
imaging for both diagnosis and evaluation of the effects of
therapy. In addition, various studies have shown these same tumor
markers to be clinically promising in terms of predicting vascular
invasion or HCC recurrence (1-9).
Determining the presence or absence of microvascular invasion in
cases of HCC is very important when treatment strategies are being
considered, but detecting microvascular invasion remains difficult
despite advancements in various imaging techniques. Further,
whether tumor marker levels can be used to predict the presence of
microvascular invasion is unknown. We, as members of the
Association for Clinical Research on Surgery, conducted a
retrospective study in which we attempted to clarify whether
microvascular invasion can be predicted preoperatively on the basis
of a tumor marker gradient in patients who have not undergone
previous treatment for HCC.
Patients and methods
Patient selection
Included in the study were 292 patients, each of
whom had undergone curative hepatectomy as initial treatment for
HCC at one of the five Association for Clinical Research on Surgery
(ACRoS) member hospitals (Tokyo Women's Medical University, Tokyo
Medical and Dental University, Yokohama City University, Keio
University, or St. Marianna University School of Medicine hospital)
between January 2004 and December 2014. Each of these hospitals is
a high-volume liver surgery center, and the patients were
identified through a search of hospital records. Curative
hepatectomy was defined as hepatectomy in which all existing tumors
were resected macroscopically. Patients included in the study met
the following criteria: Treatment was for a solitary tumor <5 cm
in diameter and diagnosed histopathologically as HCC after surgery,
and AFP and/or PIVKA-II was measured more than twice during the
3-month period, with the first measurement obtained at the time of
the patient's first visit and the second or last measurement
obtained at the time of admission to the hospital for surgery.
Patients not included in the study were those for whom vascular
invasion was diagnosed by imaging, those who had undergone prior
treatment for HCC, those with remarkably low hepatic functional
reserve (Child-Pugh score >8 points), those who were using
warfarin regularly, and those whose decision-making ability was
deemed compromised.
The study was approved by the Committee for Medical
Ethics and clinical studies at each of the five universities,
including that of St. Marianna University School of Medicine
(Kawasaki, Japan) (approval no. 2803). Each applicable patient
received opt-out consent.
Diagnosis of HCC
The HCC had been diagnosed preoperatively according
to the protocol established at each of the five universities. The
diagnosis in all cases was based on computed tomography (CT) and
magnetic resonance imaging (MRI) findings and on tumor marker
concentrations. None of the patients had undergone needle biopsy.
In all cases, contrast CT and/or contrast MRI had been performed,
and the number of tumors and tumor diameters were judged from the
images obtained.
Measurement of AFP and PIVKA-II
Serum AFP concentrations had been measured by latex
agglutination immunoassay (LPIA-A700 kit; Daia-iatron), and serum
PIVKA-II concentrations had been measured by enzyme immunoassay
with a monoclonal antibody specific for PIVKA-II (PIVKA-II kit;
Eisai). A gradient was calculated for each marker (AFP grad and
PIVKA-II grad) by dividing the difference between the
preoperatively measured concentrations by the number of days
between measurements. However, because the gradient variances were
large, logarithmic conversion was performed, and the logarithmic
conversion ratios (log AFP grad and log PIVKA-II grad) were used
for analysis.
Surgery and pathological
evaluation
The hepatectomy had been deemed curative in all 292
patients, and there were no in-hospital deaths. Macroscopic tumor
type and pathological features (tumor size, vascular invasion,
lymph node metastasis, distant organ metastasis) had been assessed
according to the General Rules for the Clinical and Pathological
Study of Primary Liver Cancer of the Liver Cancer Study Group of
Japan (10).
Microvascular invasion was diagnosed definitively
when invasion of the tumor into the portal vein, hepatic vein,
and/or bile duct was found during postoperative pathological
examination of the surgical specimen.
Statistical analysis
Differences in categorical variables, e.g., sex,
viral status, and Child-Pugh class, between patients with and
without microvascular invasion were analyzed by means of chi-square
test or Fisher's exact test, and differences in continuous
variables, e.g., markers of liver function, such as the AFP
concentration, were analyzed by means of Student's t-test.
Association between continuous variables (e.g., age, ICG-R15, tumor
size, concentrations of the tumor markers such as AFP and PIVKA-II,
and tumor marker grad) and microvascular invasion was tested by
means of stepwise logistic regression analysis, and receiver
operating characteristic (ROC) curves were drawn to determine
optimum cut-off AFP grad and PIVKA-II grad values for predicting
microvascular invasion of HCC. JMP software (version 12; SAS
Institute Inc.) was used for all statistical analyses, and
P<0.05 was considered significant.
Results
Clinical characteristics of the patients are shown
per group in Table I. There was no
significant between-group difference in the sex ratio, age, hepatic
functional reserve, or viral status. There was a difference,
however, in the AFP concentrations.
 | Table IPatient characteristics, per study
group. |
Table I
Patient characteristics, per study
group.
| Characteristic | Without vascular
invasion (n=222) | With vascular
invasion (n=70) | P-value |
|---|
| Sex ratio
(male:female) | 168:54 | 54:16 | 0.8013 |
| Age [median (IQR)
years] | 70 (62-74) | 70.5 (62-75.3) | 0.4188 |
| Child-Pugh class | | | 0.2850 |
|
A | 210 | 65 | |
|
B | 11 | 3 | |
|
Unknown | 1 | 2 | |
| ICG-R15 [median (IQR)
%] | 13.31 (9-20.1) | 16 (10-20.6) | 0.4777 |
| Viral status | | | 0.2397 |
|
HBV | 48 | 14 | |
|
HCV | 110 | 31 | |
|
NonB
NonC | 64 | 25 | |
| Tumor markers | | | |
|
AFP
(ng/ml) | 8 (3.8-33) | 38.5 (6.8-347.3) | 0.0019 |
|
PIVKA-II
(mAu/ml) | 36.5 (19-126.8) | 77 (24.8-417) | 0.2825 |
The postoperative pathological diagnoses are shown
per group in Table II. Although
small nodular tumors with obscure margins and simple nodular tumors
were found in many of the patients with microvascular invasion,
there was no significant between-group difference in the
macroscopic tumor types or the presence of LN metastasis.
 | Table IIFinal pathological diagnoses, per
study group. |
Table II
Final pathological diagnoses, per
study group.
| Variables | Without vascular
invasion (n=222) | With vascular
invasion (n=70) | P-value |
|---|
| Macroscopic tumor
type | | | a |
|
Obscure | 14 | 0 | |
|
SN | 139 | 35 | |
|
SNEG | 37 | 19 | |
|
Multinodular | 27 | 15 | |
|
Massive | 0 | 1 | |
|
Invasiove | 1 | 0 | |
|
Other | 4 | 0 | |
| Size [median (IQR)
cm] | 2.7 (2-3.5) | 3 (2.475-3.625) | 0.037 |
| LN metastasis | | | a |
|
Positive | 0 | 1 | |
|
Negative | 216 | 68 | |
|
Unknown | 6 | 1 | |
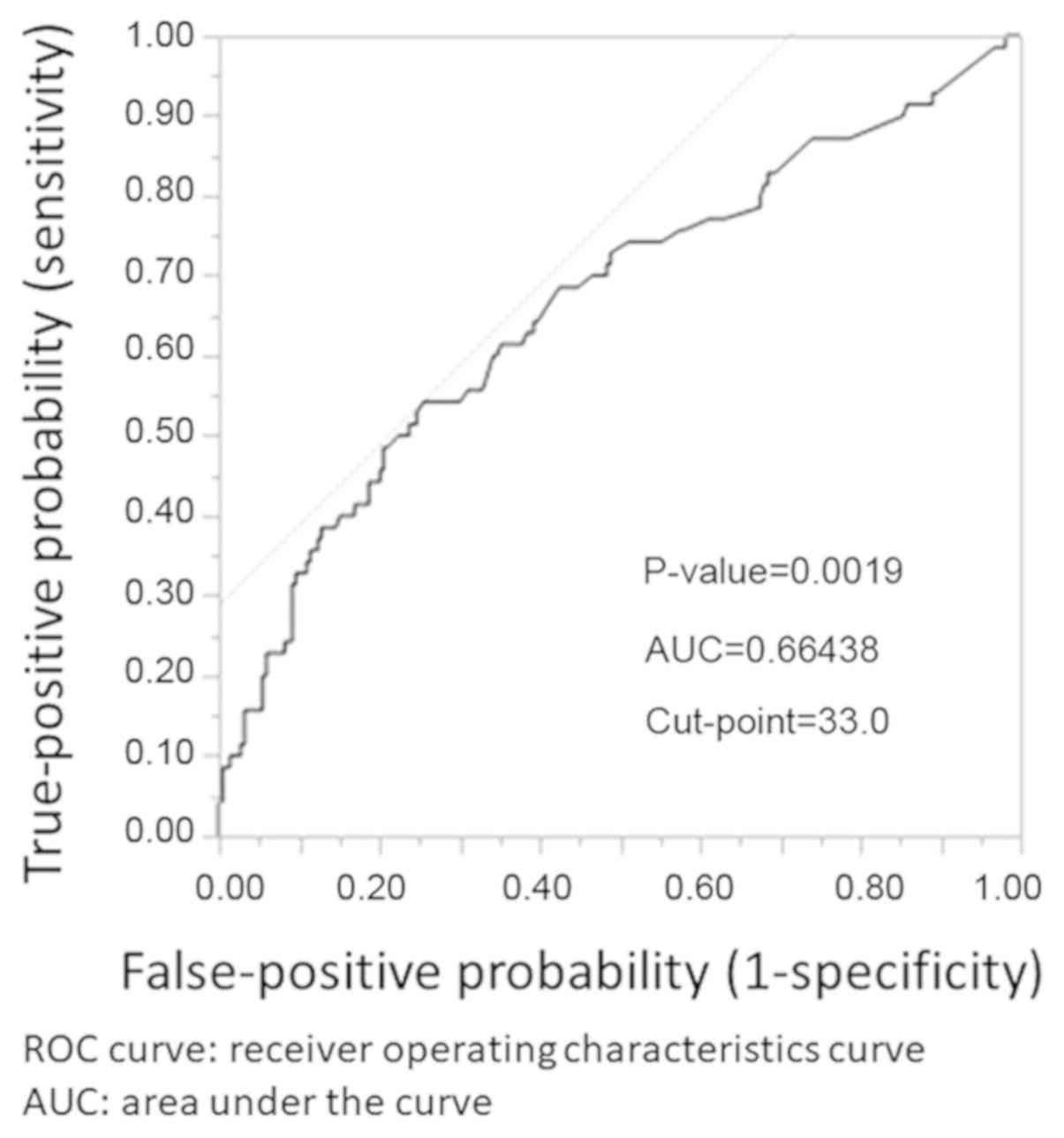
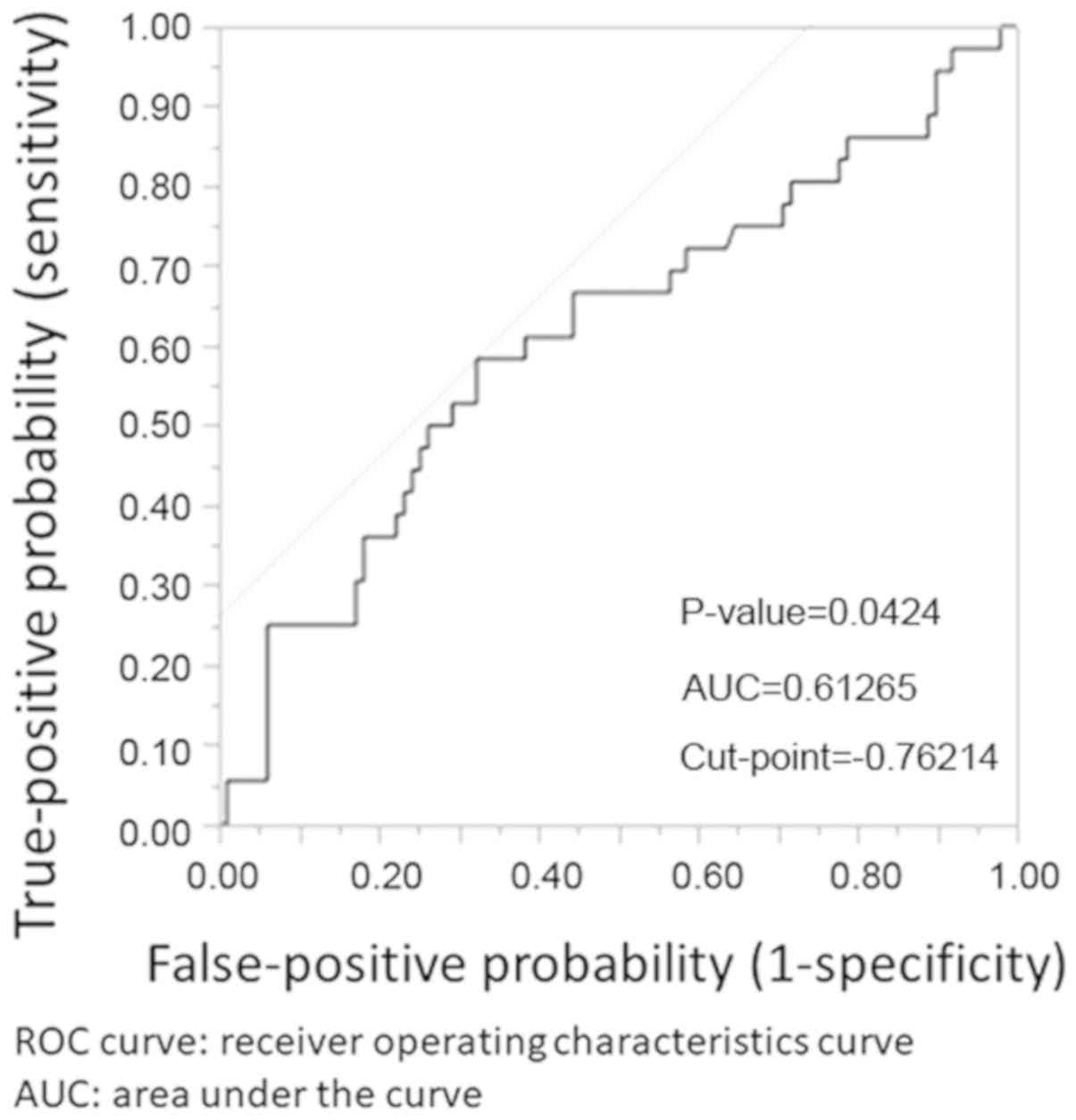
Stepwise logistic regression analysis showed the AFP
concentration and log AFP grad to be influential variables. ROC
curves obtained for the AFP concentration and log AFP grad are
shown in Figs. 1 and 2, respectively. The cut-off values obtained
by means of the Youden index were 33 ng/ml and -0.76214,
respectively. Log AFP grad -0.76214 approximates to AFP grad
0.4665.
Discussion
Liver cancer is one of the five major cancers that
occur in Japan, with HCC accounting for the majority of primary
liver cancers (11). Therefore,
treatment that improves either prognosis or the quality of life of
patients with liver cancer has become an important public policy
focus. Treatment of HCC has progressed worldwide, with hepatectomy,
ablation, and catheterization currently standing as the pillars of
HCC treatment. In selecting the ideal treatment modality for a
patient and to obtain the best possible outcome, both the degree of
cancer progression and hepatic functional reserve must be taken
into consideration. Important factors pertaining to the degree of
cancer progression are the number and size of tumors and the
presence or absence of microvascular invasion. Newly developed
imaging devices and systems and advances in detection methods have
made accurate diagnosis possible, but identifying microvascular
invasion remains difficult. Formerly, we attempted to determine
progression status by predicting pathological factors, particularly
vascular invasion, on the basis of the macroscopic type of tumor
identified on preoperative CT or MR images (12,13).
Microvascular invasion was correctly identified in only ~70% of
cases, however. The treatment strategy for HCC is selected not only
on the basis of the cancer stage but also on that of the patient's
hepatic functional reserve. Background viral hepatitis often
results in poor hepatic functional reserve, and the presence or
absence microvascular invasion is an important consideration in
determining whether to prioritize curability or hepatic functional
reserve. Thus, it is important to ascertain the presence or absence
of vascular invasion in the 30% of cases for which this cannot be
done on the basis of images obtained.
Several clinical studies of tumor markers have been
reported (2,3,5-7,9).
PIVKA-II was shown in these studies to be a useful prognostic
factor in cases of HCC unrelated to Hepatitis B virus (HBV) or
Hepatitis C Virus (HCV). HCC tumors are known to develop rapidly
after microvascular invasion, and PIVKA-II is predicted to increase
accordingly. It is from this perspective that we designed our
study. Initially, we planned to examine AFP and PIVKA-II doubling
time, but we judged doubling time to be an inappropriate variable
because variation in this time is great and such times are not
normally distributed. For this reason, we examined logarithmic
tumor marker (AFP, PIVKA-II) gradient values as predictor
variables.
Medical insurance in Japan does not cover
measurement of tumor markers more than once within the same month.
Therefore, gathering a suitable number of cases for this study was
expected to be difficult. To solve this problem, we identified
pertinent cases treated over a period of 10 years at each of five
high-volume centers, and the number of cases that we included
(n=292) allowed for valid statistical comparisons.
Of all clinical factors that we investigated, only
tumor size and AFP values differed significantly between patients
with and without microvascular invasion of the HCC. We cannot
clearly explain why AFP rather than PIVKA-II was found to be
associated with microvascular invasion, and we did not investigate
the underlying mechanism responsible for this association. However,
the proportion of patients with an AFP concentration higher than
the reference value was greater than the proportion of patients
with a PIVKA-II concentration higher than the reference value, and
this might, at least in part, explain our finding. Because we
assumed when we designed the study that the size and number of
tumors are related to microvascular invasion, we included only
cases of a solitary tumor <5 cm. Significant differences in
microvascular invasion were observed under these conditions, so we
can say with confidence that the size of the tumor is an important
factor in judging whether microvascular invasion has occurred. Even
so, as indicated above, median size of the tumors was quite similar
between our two groups, and thus it cannot be said that tumor size
is a useful indicator of microvascular invasion when the diameter
is around 3 cm. However, we identified cut-off values for AFP and
log AFP grad (33 mg/ml and -0.76214 ng/ml, respectively) that can
be used to predict the presence or absence of microvascular
invasion at ≥60% accuracy. Use of a logarithmic value may seem
cumbersome, but log AFP grad -0.76214 approximates to AFP grad
0.466, so the problem of practicality is easily resolved. When AFP
increases by 5 ng/ml or more within 10 days, it can be taken as an
indicator of microvascular invasion with a predictive accuracy of
60%. Use of the cut-off values of both AFP and log AFP grad yields
a predictive accuracy of ~70% in cases in which imaging was not
informative with respect to microvascular invasion.
Overall, our study data suggest that the presence or
absence of microvascular invasion in cases of HCC can, before
surgery, be predicted on the basis of the AFP concentration and AFP
gradient when these are used in conjunction with preoperative
imaging. We note however, that that some patients will have an HCC
that does not secrete AFP, and thus prediction of microvascular
invasion on the basis of this tumor marker will not be possible in
all cases.
Acknowledgements
The authors would like to thank Professor Eisuke
Inoue of the Department of Medical Education at St. Marianna
University School of Medicine for providing advice regarding the
statistical analysis performed in this study. Authors would also
like to thank Professor Tina Tajima of the Department of Medical
Education at St. Marianna University School of Medicine for advice
regarding the proofreading of this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SKoi conceived and designed the research. SKoi, SY,
SM, KT, TM, TH and SKob performed data acquisition, data analysis
and manuscript preparation. MS, IE, MT, MY and TO assisted with
data analysis and statistical analysis. The final version of the
manuscript has been approved by all authors.
Ethics approval and consent to
participate
The study was approved by the each Ethics Committee
of ACRoS member's hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaibori M, Ishizaki M, Matsui K and Kwon
A: Clinicopathologic characteristics of patients with non-B non-C
hepatitis virus hepatocellular carcinoma after hepatectomy. Am J
Surg. 204:300–307. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaibori M, Matsui Y, Yanagida H, Yokoigawa
N, Kwon AH and Kamiyama Y: Positive status of alpha-fetoprotein and
des-gamma-carboxy prothrombin: Important prognostic factor for
recurrent hepatocellular carcinoma. World J Surg. 28:702–707.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alejandro AG and Cervantes JG: Diagnostic
value of des-gamma carboxy prothrombin as compared with alpha feto
protein in hepatocellular carcinoma: A meta-analysis. J
Gastroenterol. 2:67–73. 2006.
|
|
4
|
Kaibori M, Saito T, Matsui Y, Uchida Y,
Ishizaki M and Kamiyama Y: A review of the prognostic factors in
patients with recurrence after liver resection for hepatocellular
carcinoma. Am J Surg. 193:431–437. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Inagaki Y, Tang W, Makuuchi M, Hasegawa K,
Sugawara Y and Kokudo N: Clinical and molecular insights into the
hepatocellular carcinoma tumour marker des-γ-carboxyprothrombin.
Liver Int. 31:22–35. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kaibori M, Ishizaki M, Matsui K and Kwon
AH: Predictors of microvascular invasion before hepatectomy for
hepatocellular carcinoma. J Surg Oncol. 102:462–468.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hemida K, Kamal W, Riad GS, Adel NA, Ayoub
MS and Fekry D: Evaluation of Pivka-II as a predictor marker for
portal vein obstruction in hepatocellular carcinoma patients. J Am
Sci. 8:162–170. 2012.
|
|
8
|
Hirokawa F, Hayashi M, Miyamoto Y, Asakuma
M, Shimizu T, Komeda K, Inoue Y and Uchiyama K: Outcomes and
predictors of microvascular invasion of solitary hepatocellular
carcinoma. Hepatol Res. 44:846–853. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zakhary NI, Khodeer SM, Shafik HE, Camelia
A and Abdel Malak CA: Impact of PIVKA-II in diagnosis of
hepatocellular carcinoma. J Adv Res. 4:539–546. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liver Cancer Study Group of Japan: The
general rules for the clinical and pathological study of primary
liver cancer. 2nd edition. Kanehara & Co., Ltd., Tokyo,
2003.
|
|
11
|
Cancer Registry and Statistics: Cancer
information service, National cancer center. Japan, 2017.
|
|
12
|
Koizumi S, Kobayashi S, Asakura T, Nakano
H, Morimoto T, Koike J and Otsubo T: Preoperative diagnosis of
macroscopic type of hepatocellular carcinoma by multi-detector-row
computed tomography is useful for prediction of the occurrence of
pathological progression factors in the tumor. J St. Marianna Univ.
2:9–16. 2011.
|
|
13
|
Ariizumi S, Kitagawa K, Kotera Y,
Takahashi Y, Katagiri S, Kuwatsuru R and Yamamoto M: A non-smooth
tumor margin in the hepatobiliary phase of gadoxetic acid disodium
(Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts
microscopic portal vein invasion, intrahepatic metastasis, and
early recurrence after hepatectomy in patients with hepatocellular
carcinoma. J Hepatobiliary Pancreat Sci. 18:575–585.
2011.PubMed/NCBI View Article : Google Scholar
|