Introduction
Gastric cancer (GC) is one of the most common
malignant tumors of the digestive system. The 2018 Global Cancer
Report shows that more than half of the 18.1 million new cancer
cases and 9.6 million cancer-related deaths worldwide occur in
Asia. GC ranks as the sixth most common newly diagnosed cancer,
with an incidence of 5.7%; however, the mortality rate is as high
as 8.2%, second only to mortality due to lung cancer (1). Although comprehensive treatment based
on surgery in recent years has further improved the treatment of
patients with GC, the overall prognosis of patients with GC is
still poor (2,3). Therefore, it is particularly important
to effectively predict the survival of patients with GC and to
develop individualized treatment options. In the past, combined
with the comprehensive evaluation of intraoperative and
postoperative pathology, tumor biomarkers, such as the pathological
stage, depth of invasion, number of involved lymph nodes, and
presence or absence of surrounding tissues, are the key factors
used to determined the prognosis of patients (4). However, the prognosis of patients with
GC is related not only to tumor biomarkers but also to the body's
inflammation and immunity.
In recent years, the importance of preoperative
nutritional status and immunity in patients with GC has been
recognized (5,6). The preoperative nutritional status and
immunity of patients with GC determine whether the patient can
undergo surgery and are some of the important factors in the
evaluation of postoperative complications, postoperative recurrence
and metastasis, chemotherapy tolerance and long-term prognosis
(7-10).
Li et al (11) showed that
the implementation of effective preoperative nutrition and immune
supportive treatments can improve the quality of life and long-term
prognosis of patients with GC. The prognostic nutritional index
(PNI) is a simple and convenient indicator for assessing
preoperative nutritional status and immunity in patients (12). In recent years, it has been used for
the clinical evaluation of prognosis in patients with a variety of
cancers. However, few studies have explored the value of the
preoperative PNI compared with the value of traditional
inflammatory markers in the prognosis of patients after GC radical
surgery. This study investigated the value of the preoperative PNI
compared with the value of traditional inflammatory indicators in
the prognostic evaluation of patients undergoing GC radical surgery
to provide a reference for clinical diagnosis and treatment.
Patients and methods
Patients
We retrospectively assessed all patients with
resectable GC who were treated between January 1, 2010, and January
15, 2013, at the Department of Gastrointestinal Surgery of The
First Affiliated Hospital of Bengbu Medical College (Bengbu,
China). The following inclusion criteria were applied: i) GC was
preoperatively confirmed by pathology and histopathology; ii)
patient had not undergone neoadjuvant radiotherapy and chemotherapy
before surgery; iii) had no infectious diseases before blood
collection; iv) was confirmed by imaging to have no distant
metastasis; v) patient underwent radical gastrectomy; vi) follow-up
occurred during or after March, and vii) patient died of GC or
gastric cancer-related diseases. The following exclusion criteria
were applied: i) Patient could not tolerate surgery due to severe
liver and kidney dysfunction; ii) had an active infection; iii) had
autoimmune disease; iv) had other malignant tumors and end-stage
diseases, and v) patient was missing clinical data. Finally, 170
patients were included in the study. Clinical data, such as sex,
age, recent weight loss, histological type, tumor location, tumor
size, TNM stage, and pathological stage, were collected. The
results of the first blood collection of patients admitted to the
hospital included neutrophil count, lymphocyte count, monocyte
count, and serum albumin concentration. The NLR, LMR, and PNI were
calculated. The NLR was calculated as the neutrophil count divided
by the lymphocyte count. The LMR was calculated as the lymphocyte
count divided by the monocyte count. The PNI was equal to the serum
albumin (g/L) + 5* lymphocyte absolute count
(109/L) (12). Surgical
procedures were performed according to the Japanese Gastric Cancer
Treatment Guidelines (13). The
pathological staging of all enrolled patients was performed
according to the eighth edition of the AJCC guidelines for
histopathological findings (14).
This study was approved by the Ethics Committee of the First
Affiliated Hospital of Bengbu Medical College. All patients
provided signed informed consent.
Follow-up
All patients were followed up with a regular
clinical visit and by telephone. A postoperative follow-up
assessment was performed every 3 months for 5 years and then every
6 months during years 3-5. Follow-up included a physical
examination; laboratory and imaging examinations; laboratory tests,
including routine blood tests, routine biochemical tests, and tumor
markers; and imaging examinations, including a chest X-ray and an
enhanced abdominal CT examination. The clinical follow-up time was
from the date of surgery to the time of death or the deadline,
which was December 2018.
Statistical analysis
Data analysis was performed using SPSS Statistics
23.0 software (ver. 23.0; IBM Corp.). Measurement data were
expressed as mean ± SD. Differences between groups were analyzed by
one-way analysis of variance (ANOVA) and Student's t-tests. The
χ2 test was used to compare the count data, and Fisher's
exact test was used when the sample size was <5. The diagnostic
value of each inflammatory index for cancer-related death 5 years
after radical gastrectomy was analyzed by receiver operating
characteristic (ROC) curve analysis. The areas under the curves and
the Z values were compared to determine the discriminatory ability
of each inflammatory index. The overall survival (OS) time was the
number of days from the day of surgery until the day of death. The
survival rate was expressed by the Kaplan-Meier curve, and the
log-rank χ2 test was used for comparison between the
groups. Multivariate analysis of the 5 year survival rate after GC
surgery was performed with the Cox proportional hazard regression
model. All statistical tests were bilateral, and the significance
was set to P<0.05.
Results
The ROC curve was used to determine
the cut-off value
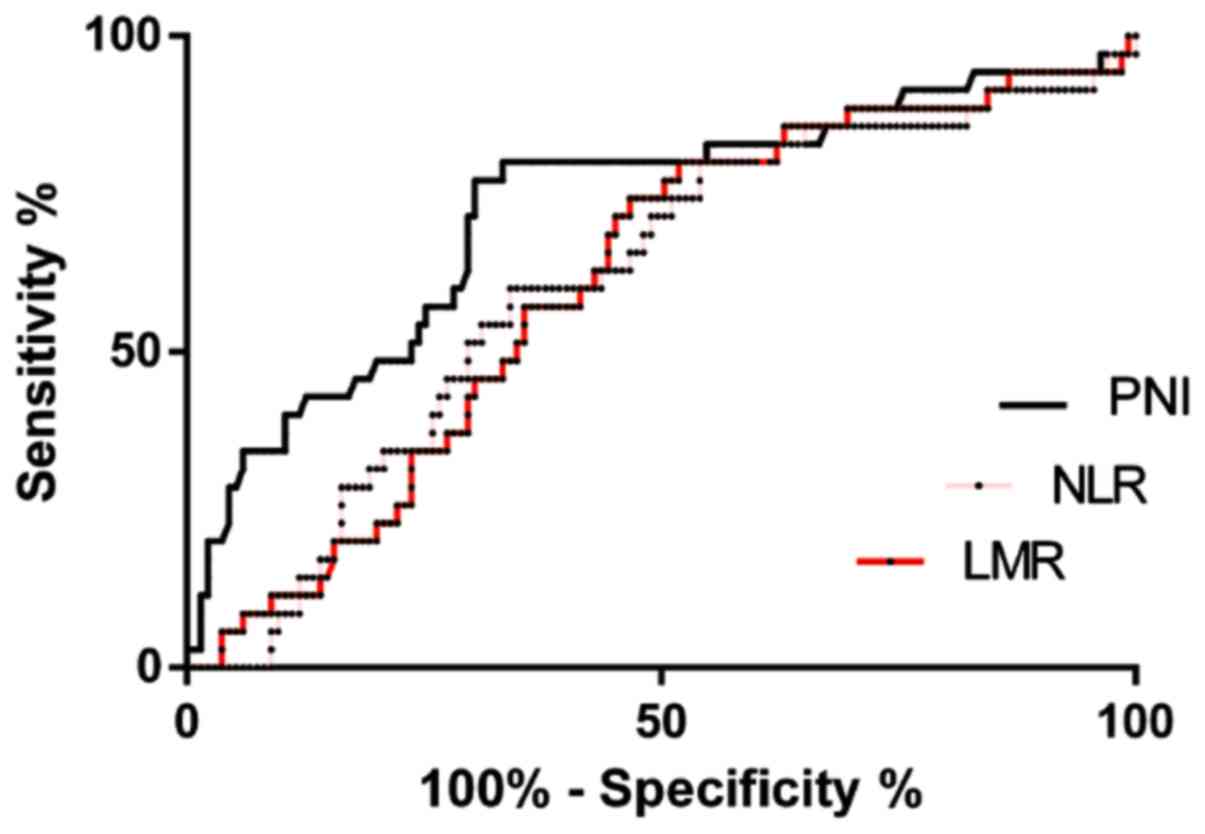
The areas under the PNI, NLR, and LMR curves were
0.725, 0.609 and 0.609, respectively. When the PNI was 46.030, the
Youden index was at the maximum, with a sensitivity of 77.14% and a
specificity of 69.69%. When the NLR was 2.464, the Youden index was
at the maximum, with a sensitivity of 80.00% and a specificity of
45.93%. When the LMR was 3.279, the Youden index was at the
maximum, with a sensitivity of 80.00% and a specificity of 48.15%.
In addition, the ROC curve showed that the area under the PNI curve
was 0.725, which was significantly higher than those of the NLR and
LMR (P<0.05) (Fig. 1 and Table I).
 | Table IComparison of the receiver operating
curves of inflammation indicators. |
Table I
Comparison of the receiver operating
curves of inflammation indicators.
| Parameter | AUC | 95% CI | Cut-off | Sensitivity (%) | Specificity (%) | Z-value | P-value |
|---|
| PNI | 0.725 | 0.623-0.826 | 46.03 | 77.14 | 69.69 | - | - |
| NLR | 0.609 | 0.507-0.712 | 2.464 | 80.00 | 45.93 | 2.228 | 0.026a |
| LMR | 0.609 | 0.510-0.708 | 3.279 | 80.00 | 48.15 | 2.714 | 0.006a |
Clinicopathological
characteristics
The general clinical features of 170 patients with
GC are shown in Table II. There
were 127 males and 43 females. The average age of the patients was
61.14±11.47 years old. The most common pathological type of tumor
was adenocarcinoma, and the most common tumor site was the cardia.
With regard to TNM staging, there were 15 patients with T1-T2
tumors, 155 patients with T3-T4 tumors, 77 patients with N0-N1
tumors, 93 patients with N2-N3 tumors, 53 patients with phase I/II
tumors, and 117 patients with phase III tumors. PNI was
significantly associated with sex, histological type, tumor size, N
stage, pathological stage, the NLR, and the LMR (P<0.05)
(Table II).
 | Table IIGeneral clinical characteristics of
patients with gastric cancer. |
Table II
General clinical characteristics of
patients with gastric cancer.
| Parameter | PNI<46.03
(n=102) | PNI≥46.03 (n=68) | χ2/F | P-value |
|---|
| Sex | | | | |
|
Male | 82 | 45 | | |
|
Female | 20 | 23 | 4.3630 | 0.0367a |
| Age (years) | | | | |
|
<60 | 35 | 28 | | |
|
≥60 | 67 | 40 | 0.8238 | 0.3641a |
| Recent weight loss
(kg) | | | | |
|
<5 | 63 | 46 | | |
|
≥5 | 39 | 22 | 0.6136 | 0.4334a |
| Histological
type | | | | |
|
Adenocarcinoma | 82 | 63 | | |
|
Other | 20 | 5 | 4.8851 | 0.0271a |
| Tumor location | | | | |
|
Gastric
cardia | 44 | 22 | | |
|
Gastric
body | 27 | 18 | | |
|
Gastric
antrum | 31 | 28 | 2.5892 | 0.2740b |
| Tumor size
(cm) | | | | |
|
<5 | 43 | 41 | | |
|
≥5 | 59 | 27 | 5.3691 | 0.0205a |
| T stage | | | | |
|
T1-T2 | 6 | 9 | | |
|
T3-T4 | 96 | 59 | 2.7421 | 0.0977a |
| N stage | | | | |
|
N0-N1 | 33 | 44 | | |
|
N2-N3 | 69 | 24 | 17.241 |
<0.0001a |
| Pathological
stage | | | | |
|
I-II | 20 | 33 | | |
|
III | 82 | 35 | 15.911 |
<0.0001a |
| NLR | | | | |
|
<2.464 | 40 | 61 | | |
|
≥2.464 | 62 | 7 | 43.131 |
<0.0001a |
| LMR | | | | |
|
<3.279 | 63 | 9 | | |
|
≥3.279 | 39 | 59 | 12.581 | 0.0004a |
Subsistence analysis
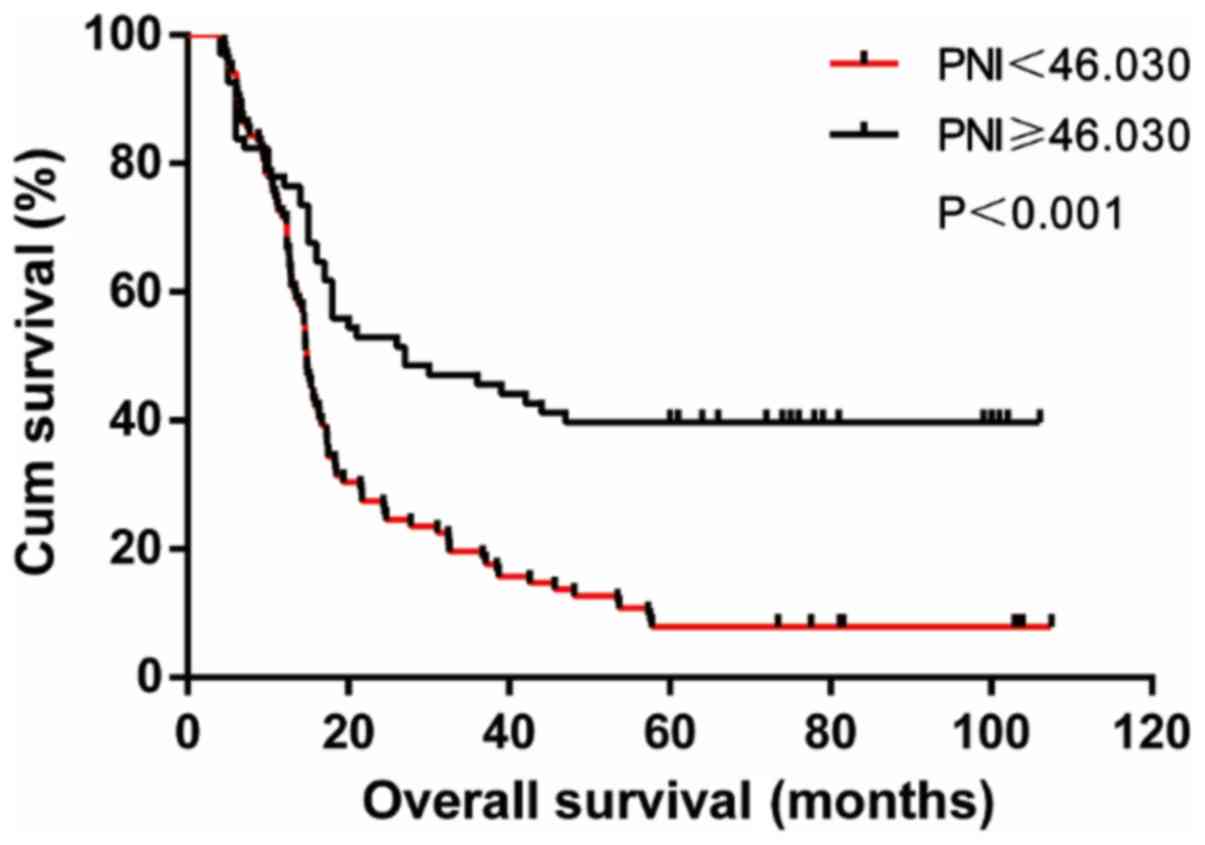
The average follow-up time for the entire group was
31.7 months (4.6-107.5) months. The postoperative survival rate of
patients with PNI <46.03 was significantly lower than that of
patients with PNI ≥46.03 (23.7 months vs. 34.3 months, log-rank
χ2=19.700, P<0.001) (Fig.
2).
Univariate analysis and multivariate
analysis of postoperative survival in gastric cancer
Univariate analysis showed that tumor location,
tumor size, T stage, N stage, pathological stage, the NLR, the LMR,
and the PNI may be risk factors for survival after GC radical
resection (P<0.01). The significant results obtained by
univariate analysis were included in the Cox regression model.
Finally, T3-T4 stage (HR=5.267; 95% CI, 1.878-14.770), N2-N3 stage
(HR=1.731; 95% CI, 1.036-2.895), pathological grade III (HR=8.386;
95% CI, 4.415-15.929) and PNI≥46.03 (HR=1.513; 95% CI, 1.015-2.256)
were identified as independent risk factors affecting the survival
of GC patients after radical surgery (Table III).
 | Table IIIUnivariate analysis and multivariate
analysis of the 5-year survival of patients after radical
gastrectomy. |
Table III
Univariate analysis and multivariate
analysis of the 5-year survival of patients after radical
gastrectomy.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Parameter | n | MST (months) | P-value | HR | 95% CI | aP-value |
|---|
| Sex | | | | | | |
|
Male | 127 | 16.3 | | | | |
|
Female | 43 | 17.6 | 0.728 | - | - | - |
| Age (years) | | | | | | |
|
<60 | 63 | 17.5 | | | | |
|
≥60 | 107 | 15.9 | 0.204 | - | - | - |
| Recent weight loss
(kg) | | | | | | |
|
<5 | 109 | 17.4 | | | | |
|
≥5 | 61 | 15.6 | 0.214 | - | - | - |
| Histological
type | | | | | | |
| Adenocarcinoma | 145 | 17.1 | | | | |
|
Other | 25 | 15.2 | 0.136 | - | - | - |
| Tumor location | | | | | | |
|
Gastric
cardia | 66 | 15.75 | | | | |
|
Gastric
body | 45 | 14.7 | | | | |
|
Gastric
antrum | 59 | 27.4 | 0.007 | 0.821 | (0.663-1.016) | 0.070 |
| Tumor size
(cm) | | | | | | |
|
<5 | 84 | 20.45 | | | | |
|
≥5 | 86 | 14.75 | <0.001 | 1.026 | (0.705-1.494) | 0.892 |
| T stage | | | | | | |
|
T1-T2 | 15 | 77.6 | | | | |
|
T3-T4 | 155 | 15.7 | <0.001 | 5.267 | (1.878-14.770) | 0.002 |
| N stage | | | | | | |
|
N0-N1 | 77 | 47.8 | | | | |
|
N2-N3 | 93 | 12.7 | <0.001 | 1.731 | (1.036-2.895) | 0.036 |
| Pathological
stage | | | | | | |
|
I-II | 53 | 72.5 | | | | |
|
III | 117 | 14.1 | <0.001 | 8.386 | (4.415-15.929) | 0.000 |
| NLR | | | | | | |
|
<2.464 | 101 | 20.6 | | | | |
|
≥2.464 | 69 | 14.6 | 0.005 | - | - | - |
| LMR | | | | | | |
|
<3.279 | 72 | 15.15 | | | | |
|
≥3.279 | 98 | 21.05 | 0.003 | - | - | - |
| PNI | | | | | | |
|
<46.03 | 102 | 14.8 | | | | |
|
≥46.03 | 68 | 27.5 | <0.001 | 1.513 | (1.015-2.256) | 0.042 |
Discussion
GC has been attracting attention in the academic
community due to its high morbidity and mortality rates (1). Although surgery has improved the 5 year
survival rate of patients with GC, the prognosis is still not
optimistic. Gullo et al (15)
showed that the prognosis of GC as a highly heterogeneous tumor is
closely related to many factors. In addition to the traditional TNM
staging system, it is also closely related to the body's immunity,
inflammation, and nutrition.
Most patients with GC suffer from nausea, vomiting,
abdominal pain, abdominal distension and other symptoms that affect
the appetite and diet of the patient. On the other hand, the rapid
growth of malignant tumor cells causes the body to consume a large
amount of nutrients, resulting in the lack of the synthesis of
nutrients, which causes tumor necrosis and the production of toxic
substances, leading to metabolic disorders in the body. Therefore,
most patients often have different degrees of malnutrition at the
time of treatment (16,17). Malnutrition can lead to the loss of
optimal treatment timing for patients and the need to delay
treatment, which can lead to disease progression. In addition,
malnutrition leads to decreased T cell function and the
deterioration of the intestinal environment, further aggravating
the progression of tumor-associated inflammation (18). In 1863, since Virchow first proposed
the association between inflammation and cancer, the role of
inflammation in tumorigenesis, tumor development and metastasis has
been continuously explored (19).
Mantovani et al (20) showed
that tumor-associated inflammation is characterized by the swelling
of inflammatory cells and the production and release of
inflammatory factors in tumor tissues. Inflammatory cells and
immune cells, such as neutrophils, lymphocytes, and monocytes, in
the peripheral blood of patients with tumor-associated inflammation
are considered to be important factors leading to tumor
development, invasion and metastasis (21-24).
Existing studies have shown that the NLR and LMR play important
roles in determining the prognosis of cancer patients (25-27).
The PNI was first established by Japanese scholars
and was originally used to assess the preoperative nutritional
status, surgical risk and postoperative complications in surgical
patients (12). The PNI is
calculated as an indicator of the nutritional status of the body
and is based on the level of serum albumin and the number of
lymphocytes (12). As the main
component of plasma protein, serum albumin plays an important role
in maintaining the colloid osmotic pressure of the body; on the
other hand, it can also reflect the nutritional status of the body.
Ouyang et al (28) showed
that preoperative serum albumin levels are associated with
prognosis in patients with GC. The immune response of lymphocytes
to the tumor has been gradually applied to the prognostic
evaluation of tumor patients in recent years (29). The PNI has been used recently for the
prognostic evaluation of various tumors, including colorectal
cancer (30), liver cancer (31), and pancreatic cancer (32).
Our results show that the preoperative PNI is
superior to traditional inflammatory markers for the evaluation of
prognosis after GC radical surgery. This may be because the
preoperative PNI (in contrast with the traditional inflammatory
indicators: The NLR and LMR) can reflect the nutritional status of
the body and the body's inflammatory and immune response. A high
preoperative PNI and good OS in GC patients suggest that active
perioperative nutritional support for GC patients may be a new
method of improving patient outcomes. Multivariate analysis
indicated that the T stage, N stage, pathological grade and PNI
were independent risk factors for survival after radical
gastrectomy. These results further show that the preoperative PNI
may be superior to traditional inflammatory indicators as a
potential indicator of the prognosis of patients after radical
gastrectomy.
The limitations of this study are as follows: First,
the study was a retrospective analysis, and the sample size is
small; large-scale, multicenter, prospective studies should be
designed. Second, we were not able to collect data on indicators of
systemic infections in our research, such as the level of CRP, so
we cannot rule out systemic inflammation or the effect of stress on
the conclusions of this paper. Because this study was a
retrospective study, we only collected data on the patients'
survival, but did not have access to complete and effective data on
disease-free survival. Therefore, it was not possible to analyze
these important prognostic indicators. Finally, the follow-up time
was relatively, and a study with a longer-term follow-up period
should be designed. In future studies, we will further address the
deficiencies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Program
on Natural Scientific Research from the Department of Education of
Anhui Province, China (grant no. KJ2017A219).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML conceived the present study. LW, YM and TC
collected the data. LW and DS analyzed the data. LW, LZ and SG
wrote the manuscript. LZ and SG supervised the study, performed the
statistical analysis and data interpretation, drafted and
critically revised the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Medical Research Ethics Committee of the First Affiliated Hospital
of Bengbu Medical College (Bengbu, China). All patients provided
signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lin GT, Chen QY, Zheng CH, Li P, Xie JW,
Wang JB, Lin JX, Lu J, Cao LL, Lin M, et al: Lymph node
noncompliance affects the long-term prognosis of patients with
gastric cancer after laparoscopic total gastrectomy. J Gastrointest
Surg. 1(10)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Machlowska J, Pucułek M, Sitarz M,
Terlecki P, Maciejewski R and Sitarz R: State of the art for
gastric signet ring cell carcinoma: From classification, prognosis,
and genomic characteristics to specified treatments. Cancer Manag
Res. 11:2151–2161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siewert JR, Böttcher K, Stein HJ and Roder
JD: Relevant prognostic factors in gastric cancer: Ten-year results
of the German gastric cancer study. Ann Surg. 228:449–461.
1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Braga M, Gianotti L, Vignali A and Di
Carlo V: Immunonutrition in gastric cancer surgical patients.
Nutrition. 14:831–835. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shi H, Jiang Y, Cao H, Zhu H, Chen B and
Ji W: Nomogram based on systemic immune-inflammation index to
predict overall survival in gastric cancer patients. Dis Markers.
2018(1787424)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fujiya K, Kawamura T, Omae K, Makuuchi R,
Irino T, Tokunaga M, Tanizawa Y, Bando E and Terashima M: Impact of
malnutrition after gastrectomy for gastric cancer on long-term
survival. Ann Surg Oncol. 25:974–983. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun J, Wang D, Mei Y, Jin H, Zhu K, Liu X,
Zhang Q and Yu J: Value of the prognostic nutritional index in
advanced gastric cancer treated with preoperative chemotherapy. J
Surg Res. 209:37–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou J, Hiki N, Mine S, Kumagai K, Ida S,
Jiang X, Nunobe S, Ohashi M, Sano T and Yamaguchi T: Role of
prealbumin as a powerful and simple index for predicting
postoperative complications after gastric cancer surgery. Ann Surg
Oncol. 24:510–517. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rosania R, Chiapponi C, Malfertheiner P
and Venerito M: Nutrition in patients with gastric cancer: An
update. Gastrointest Tumors. 2:178–187. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li JH, Han L, Du TP and Guo MJ: The effect
of low-nitrogen and low-calorie parenteral nutrition combined with
enteral nutrition on inflammatory cytokines and immune functions in
patients with gastric cancer: A double blind placebo trial. Eur Rev
Med Pharmacol Sci. 19:1345–1350. 2015.PubMed/NCBI
|
|
12
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi = 85.
1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
13
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer 20: 1-19, 2017.
|
|
14
|
In H, Solsky I, Palis B, Langdon-Embry M,
Ajani J and Sano T: Validation of the 8th edition of the AJCC TNM
staging system for gastric cancer using the national cancer
database. Ann Surg Oncol. 24:3683–3691. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gullo I, Carneiro F, Oliveira C and
Almeida GM: Heterogeneity in gastric cancer: From pure morphology
to molecular classifications. Pathobiology. 85:50–63.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fukuda Y, Yamamoto K, Hirao M, Nishikawa
K, Maeda S, Haraguchi N, Miyake M, Hama N, Miyamoto A, Ikeda M, et
al: Prevalence of malnutrition among gastric cancer patients
undergoing gastrectomy and optimal preoperative nutritional support
for preventing surgical site infections. Ann Surg Oncol. 22 (Suppl
3):S778–S785. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ryu SW and Kim IH: Comparison of different
nutritional assessments in detecting malnutrition among gastric
cancer patients. World J Gastroenterol. 16:3310–3317.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fujiya K and Terashima M: ASO author
reflections: Malnutrition after gastrectomy and its impact on
survival. Ann Surg Oncol. 25:729–730. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow. Lancet. 357:539–545. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang X, Shi H, Yuan X, Jiang P, Qian H
and Xu W: Tumor-derived exosomes induce N2 polarization of
neutrophils to promote gastric cancer cell migration. Mol Cancer.
17(146)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pylaeva E, Harati MD, Spyra I, Bordbari S,
Strachan S, Thakur BK, Höing B, Franklin C, Skokowa J, Welte K, et
al: NAMPT signaling is critical for the proangiogenic activity of
tumor-associated neutrophils. Int J Cancer. 144:136–149.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang C, Li Z, Li N, Li Y, Chang A, Zhao
T, Wang X, Wang H, Gao S, Yang S, et al: Interleukin 35 expression
correlates with microvessel density in pancreatic ductal
adenocarcinoma, recruits monocytes, and promotes growth and
angiogenesis of xenograft tumors in mice. Gastroenterology.
154:675–688. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Inoue D, Sekiguchi S, Yamagata W, Maeda G,
Yamada D, Fujiwara S, Itou S, Kurihara M, Hijioka Y, Shimoji K, et
al: Elevation of neutrophil-to-lymphocyte ratio before first-line
chemotherapy predicts a poor prognosis for second-line chemotherapy
in gastric cancer. Oncology. 96:140–146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Szor DJ, Roncon Dias A, Pereira MA, Ramos
MFKP, Zilberstein B, Cecconello I and Ribeiro U Jr:
Neutrophil-Lymphocyte ratio is associated with prognosis in
patients who underwent potentially curative resection for gastric
cancer. J Surg Oncol. 117:851–857. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pan YC, Jia ZF, Cao DH, Wu YH, Jiang J,
Wen SM, Zhao D, Zhang SL and Cao XY: Preoperative
lymphocyte-to-monocyte ratio (LMR) could independently predict
overall survival of resectable gastric cancer patients. Medicine
(Baltimore). 97(e13896)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ouyang X, Dang Y, Zhang F and Huang Q: Low
serum albumin correlates with poor survival in gastric cancer
patients. Clin Lab. 64:239–245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rho SY, Hwang HK, Chong JU, Yoon DS, Lee
WJ and Kang CM: Association of preoperative total lymphocyte count
with prognosis in resected left-sided pancreatic cancer. ANZ J
Surg. 89:503–508. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Noh GT, Han J, Cho MS, Hur H, Min BS, Lee
KY and Kim NK: Impact of the prognostic nutritional index on the
recovery and long-term oncologic outcome of patients with
colorectal cancer. J Cancer Res Clin Oncol. 143:1235–1242.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Z, Wang J and Wang P: The prognostic
value of prognostic nutritional index in hepatocellular carcinoma
patients: A meta-analysis of observational studies. PLoS One.
13(e0202987)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li S, Tian G, Chen Z, Zhuang Y and Li G:
Prognostic role of the prognostic nutritional index in pancreatic
cancer: A meta-analysis. Nutr Cancer. 71:207–213. 2019.PubMed/NCBI View Article : Google Scholar
|