Introduction
Early gastric cancer (EGC) is defined by the
Japanese Gastric Cancer Association as cancer that is confined to
the mucosa or submucosa, regardless of the presence of lymph node
metastasis with a 5-year survival rate ≥85% (1-4).
However, when EGC with bone metastasis develops into disseminated
bone marrow carcinosis combined with disseminated intravascular
coagulation, prognosis is generally poor. Bone metastasis is common
in patients with advanced breast, lung, kidney and prostate cancer
but not in patients with gastric cancer. In gastric cancer, the
incidence of bone metastasis is higher in advanced cancer than in
early lesions (5).
Fluorodeoxyglucose positron emission tomography
(FDG-PET) is considered to be useful for staging malignant tumors,
including lung, esophagus, and colon cancer, and for identifying
metastatic lesions. However, in patients with gastric cancer,
FDG-PET is not useful for the detection of regional lymph nodes or
T1 tumors (6).
We present the case of a small EGC with bone
metastasis, which was diagnosed by esophagogastroduodenoscopy (EGD)
and FDG-PET. Written informed consent was obtained from the patient
for the publication of the case details and associated images.
Case report
In 2014, a 63-year-old Japanese man was referred to
our hospital with back pain and anorexia of 2 weeks' evolution. He
had no relevant personal or family history. His height was 169 cm,
weight was 71 kg. His laboratory results were as follows: lactate
dehydrogenase (LDH), 779 IU/l; alkaline phosphatase, 347 IU/l;
serum calcium level, 12.6 mg/dl; and carcinoembryonic antigen and
carbohydrate antigen 19-9 levels, 34.11 and 5.64 ng/ml,
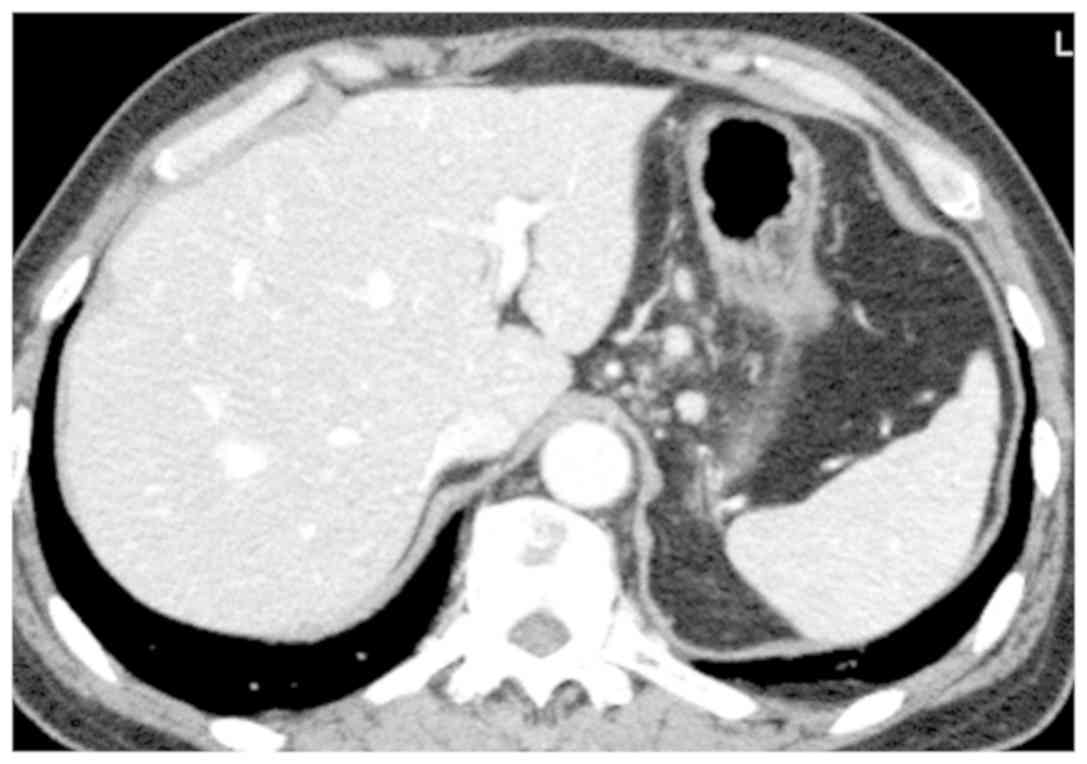
respectively. Enhanced CT revealed slight lymph node enlargement in
the lesser curvature of the stomach (Fig. 1). Magnetic resonance imaging
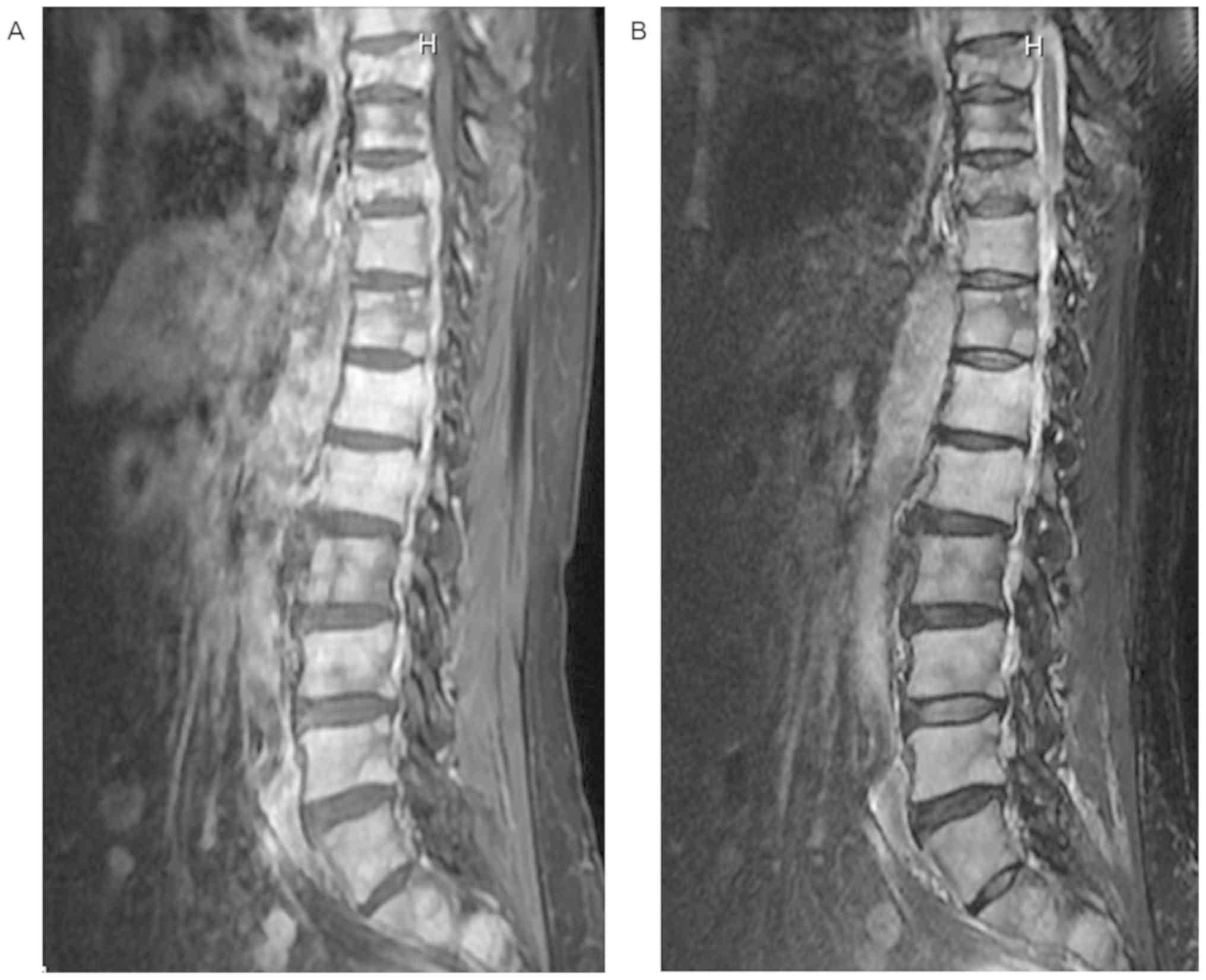
demonstrated multiple metastatic lesions in the thoracic and spinal
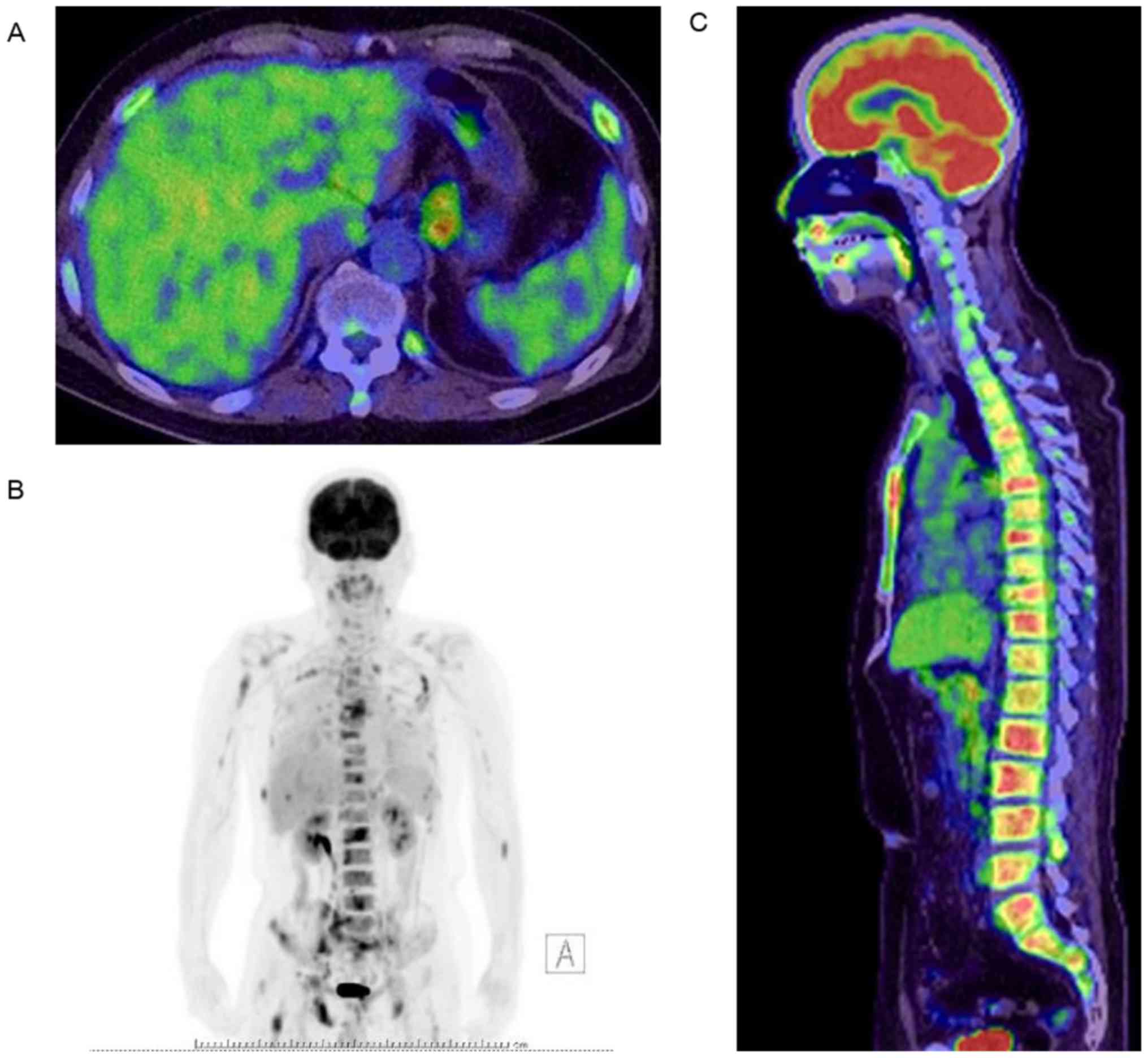
bone (Fig. 2). FDG-PET was performed
and revealed focal uptake in the lesser curvature of the stomach
and the spinal, pelvic, and thigh bone but did not detect uptake in
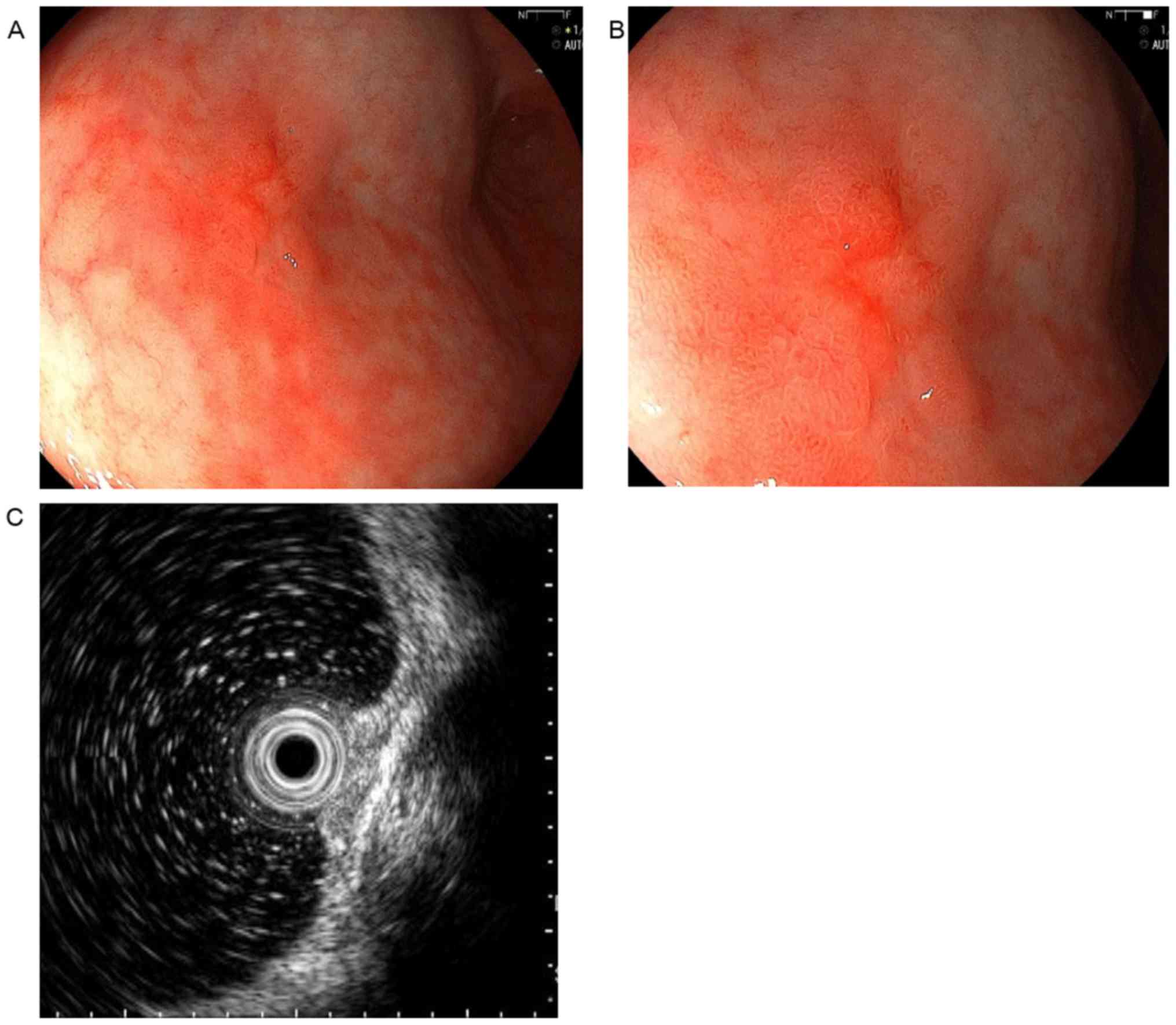
the stomach (Fig. 3). EGD revealed a
10 mm slightly elevated lesion with a central depression in the
middle-third of the stomach. Endoscopic ultrasonography (EUS)
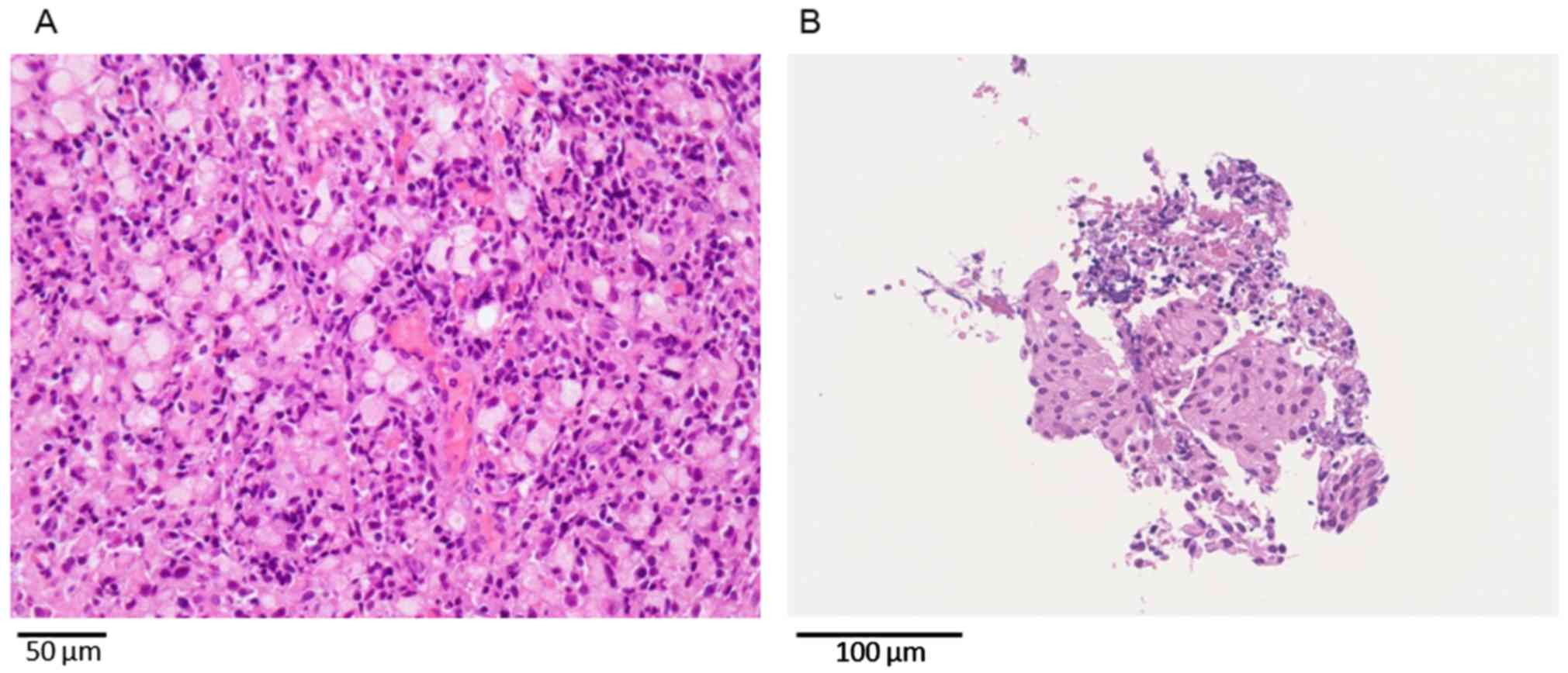
confirmed that the tumor was confined to the mucosa (Fig. 4). A biopsy specimen acquired from the
lesion indicated signet-ring cell carcinoma, and histological
biopsy from the lumbar spine revealed cell aggregation such as that
noted in signet-ring cell carcinoma (Fig. 5). The final diagnosis was confirmed
to be EGC with synchronous multiple bone metastasis. The patient
was treated using elcatonin and zoledronic acid for hypercalcemia,
and chemotherapy (S-1 + cisplatin) for the gastric cancer was
initiated. S-1 (100 mg/body/day) was given orally twice daily for
the first 3 weeks of a 5-week course. CDDP was given as an
intravenous infusion of 100 mg/body/day on day 8 of each course.
After the initiation of therapy, his LDH and serum calcium levels
decreased and pain was alleviated. However, two months after the
initiation of therapy, his LDH level increased again and therefore
he was administered second-line chemotherapy using nab-paclitaxel.
After being administered two courses of nab-paclitaxel (440 mg/body
on day 1, every 21 days), he complained of dyspnea. CT revealed
interstitial pneumonia (broad ground-glass opacity in both lungs);
thus, since we diagnosed drug-induced pneumonia caused by
nab-paclitaxel, we discontinued nab-paclitaxel and initiated
prednisolone. These chemotherapies were authorized by Japanese
Gastric Cancer Association and Pharmaceuticals and Medical Devices
Agency of Japan (7). Although
interstitial pneumonia improved, the patient's general condition
worsened during that time, and we were unable to administer a new
anticancer drug. And the patient died 120 days after consultation
at our hospital.
Discussion
Bone metastasis is common in prostate, breast, lung,
kidney and thyroid cancers, but it is rare in gastric cancer.
Yoshikawa et al reported that bone metastasis occurred in
0.7-1.4% of gastric cancer patients (8). Park et al reported that bone
recurrence after curative resection of gastric cancer was detected
in 1.8% of the cases, and the incidence of bone recurrence was
significantly higher in advanced gastric cancer (3.5%) than in
early lesions (0.4%) (5). Choi et
al reported that the incidence of bone metastasis was 45.3% in
gastric cancer cases, including patients with non-curative
resection, and Mori et al stated that bone metastasis
occurred in 15.9% of gastric cancer cases in autopsy studies
(9,10). Thus, there are a variety of reports
on the incidence of bone metastasis with gastric cancer, and the
rate of bone metastasis may be higher than we believed. Kobayashi
et al suggested that a reason that asymptomatic bone
metastasis could be underestimated is that examination by bone
scintigraphy is not a routine clinical practice (11). In addition, Park et al,
reported that the rate of bone metastasis varied depending on the
progression of gastric cancer (5).
Gurzu et al (12) reviewed the literature on metastasis
with early gastric cancer in PubMed from 1983 to 2015. Only 79
cases of EGC with bone metastasis had been published. The time
interval between the diagnosis of EGC and bone metastasis was
reported in 47 cases out of 79 cases, and 17 cases (36.2%) involved
synchronous bone metastasis (12).
Our case of early gastric cancer with synchronous bone metastasis
found taking the back pain as an opportunity, was considerably
rare.
Park et al also reported that the risk
factors for recurrence of bone metastasis after curative resection
of gastric cancer included depth of invasion and lymph node
metastasis (5). Similarly, Kobayashi
et al reviewed Japanese case reports to investigate the
characteristics of bone metastasis from EGC, and they suggested
that risk factors for bone metastasis from EGC included
depressed-type signet-ring cell carcinoma, poorly differentiated
carcinoma, and/or the likely involvement of lymph node metastasis
(11). In our case, although
histological examination with specimen removal was not performed,
we diagnosed early gastric cancer by EGD and EUS. Our case was that
of a slightly elevated lesion with a central depressed type
signet-ring cell carcinoma with suspected lymph node metastasis,
matching the characteristics described above.
Bone scintigraphy is frequently used for the
diagnosis of bone metastasis. Recently, FDG-PET has been reported
to be useful for the detection of primary malignant lesions as well
as for distant metastasis including bone metastasis. However, the
detection of gastric cancer by FDG-PET is believed to be
determinant, given that the results are based on physiological
accumulation in a stomach wall and the depth and histopathological
type of the tumor. Dassen et al reported that the
sensitivity of FDG-PET was 26-63% for early gastric cancer and
93-98% for advanced gastric cancer (13). In addition, it is thought that
FDG-PET has a lower sensitivity in detect diffuse type, mucinous
adenocarcinoma or signet-ring cell carcinoma than other
histological types, due to lower glucose transporter 1 (GLUT1)
expression. The lesion is visually recognized as accumulation of a
malignant neoplasm on FDG-PET. Cellular FDG uptake is related to
GLUT1 expression, and GLUT1 is frequently overexpressed in
malignant tissue. Kawamura et al analyzed GLUT1 expression
in gastric neoplasms and reported that 30% of gastric cancers were
positive for GLUT1 expression. In addition, signet-ring cell
carcinoma and mucinous adenocarcinoma showed very low positive
values for GLUT1 expression (2 and 6%, respectively) (14). Yamada et al and Alakus et
al evaluated whether cohesive carcinoma (papillary
adenocarcinoma, tubular adenocarcinoma, and solid-type poorly
differentiated adenocarcinoma) were more easily detectable than
non-cohesive carcinoma, signet-ring cell carcinoma, and non-solid
type poorly differentiated adenocarcinoma. They found that GLUT1
expression was the most important factor for determining the degree
of FDG uptake (15,16).
Previous studies have shown that the sensitivity and
specificity of FDG-PET for the detection of bone metastasis in
gastric cancer were 30-100% and 25-99%, respectively (17-19).
Shimada et al reviewed recent publications to evaluate the
clinical utility of FDG-PET for gastric cancer and reported that
FDG-PET had high specificity for detecting distant metastasis
including bone metastasis, but it was not useful for the detection
of regional lymph nodes or T1 tumors (6). We believe that FDG-PET is useful for
bone metastasis cases because it is possible to detect a distant
metastasis including a bone metastasis or a malignant tumor
anywhere in the body except for early gastric carcinoma.
EGC with signet-ring cell carcinoma has a
possibility of metastasizing to the bone, even if the tumor is
small; as Kang et al reported, EGC that presented the small
flat erosions with bone marrow metastasis, and it has features that
are difficult to identify by FDG-PET (20). Therefore, when bone metastasis is
detected but a primary lesion is not detected by FDG-PET, early
gastric cancer should be anticipated, and a careful EDG should be
performed.
Chemotherapy or radiation treatment for pain
reduction are common treatments for gastric cancer with bone
metastasis. Hironaka et al reported the effectiveness of
sequential methotrexate and 5-fluorouracil for gastric cancer with
bone metastasis (21). However, in
the 2008 S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS) study, the median
overall survival was 13.0 months with S-1/cisplatin (CDDP) therapy,
and the standard first-choice treatment is S-1/CDDP therapy for
advanced gastric cancer (22). The
response rate of 3-weekly doses of nab-paclitaxel as second-line
chemotherapy for advanced gastric cancer was reported to be 27.8%,
but recently, weekly nab-paclitaxel was non-inferior to weekly
solvent-based paclitaxel in terms of overall survival (23,24).
The usefulness of ramucirumab was recently reported.
Wilke et al reported that the combination of ramucirumab
with paclitaxel significantly increased overall survival compared
with placebo plus paclitaxel (25).
There have been some reports that S-1/CDDP or S-1 plus oxaliplatin
is effective for gastric cancer with bone metastasis, and these
regiments might be promising for gastric cancer with bone
metastasis (26-28).
In our case, S-1/CDDP therapy was initiated without radiotherapy,
owing to multiple bone metastasis. After the S-1/CDDP, the
patient's LDH and serum calcium levels had decreased and pain had
also alleviated, which effectively controlled his condition. But
the effective period with S-1/CDDP was short; thus, the
effectiveness of second-line therapy with nab-paclitaxel could not
be confirmed. He could not receive third-line chemotherapy due to
drug-induced interstitial pneumonia, and he died 120 days after
consultation at our hospital. We believe that it is important to be
able to continue chemotherapy to improve prognosis for patients
with gastric cancer with bone metastasis, and further and larger
clinical trials in the future will be important to achieving this
goal.
We must be aware that there are cases of bone
metastasis with signet-ring cell carcinoma from the time of the
initial diagnosis, even at an early stage. Back pain are presented
at the time of diagnosis or after treatment, PET-CT or whole-body
bone scintigraphy should be performed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets are available from the corresponding
author on reasonable request.
Authors' contributions
IF and KM diagnosed, investigated and managed the
patient. IF and TM contributed to the writing of the manuscript. TT
and JH acquired the data, contributed clinical advice and
critically reviewed the manuscript. TM, KM and SO acquired the data
in the diagnostic imaging and pathological examination. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the case details and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma. 3rd English edition.
Gastric Cancer. 14:101–112. 2011.
|
|
2
|
Shiraishi N, Inomata M, Osawa N, Yasuda K,
Adachi Y and Kitano S: Early and late recurrence after gastrectomy
for gastric carcinoma. Univariate and multivariate analyses.
Cancer. 89:255–261. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nakajima T: Gastric cancer treatment
guidelines in Japan. Gastric Cancer. 5:1–5. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Santoro E, Garofalo A, Scutari FA, Carlini
M, Zanarini T and Santoro E: Multicenter retrospective study of
3,024 patients operated on for stomach cancer in Italy.
Epidemiology, surgical treatment and survival. Ann Gastroenterol
Hepatol (Paris). 27:167–171. 1991.(In French). PubMed/NCBI
|
|
5
|
Park JM, Song KY, O JH, Kim WC, Choi MG
and Park CH: Bone recurrence after curative resection of gastric
cancer. Gastric Cancer. 16:362–369. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shimada H, Okazumi S, Koyama M and
Murakami K: Japanese gastric cancer association task force for
research promotion: Clinical utility of
18F-fluoro-2-deoxyglucose positron emission tomography
in gastric cancer. A systematic review of the literature. Gastric
Cancer. 14:13–21. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2014 (Ver.4). Gastric
Cancer. 20:1–19. 2017.
|
|
8
|
Yoshikawa K and Kitaoka H: Bone metastasis
of gastric cancer. Jpn J Surg. 13:173–176. 1983.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Choi CW, Lee DS, Chung JK, Lee MC, Kim NK,
Choi KW and Koh CS: Evaluation of bone metastasis by Tc-99m MDP
imaging in patients with stomach cancer. Clin Nucl Med. 20:310–314.
1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mori W, Adachi Y, Okabe H and Oota K: An
analysis of 755 autopsied cases of malignant tumors. A statistical
study of their metastasis. Gan No Rinsho. 9:351–374. 1963.(In
Japanese).
|
|
11
|
Kobayashi M, Okabayashi T, Sano T and
Araki K: Metastatic bone cancer as a recurrence of early gastric
cancer-characteristics and possible mechanisms. World J
Gastroenterol. 11:5587–5591. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gurzu S, Jung I and Kadar Z: Aberrant
metastatic behavior and particular features of early gastric
cancer. APMIS. 123:999–1006. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dassen AE, Lips DJ, Hoekstra CJ, Pruijt
JFM and Bosscha K: FDG-PET has no definite role in preoperative
imaging in gastric cancer. Eur J Surg Oncol. 35:449–455.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kawamura T, Kusakabe T, Sugino T, Watanabe
K, Fukuda T, Nashimoto A, Honma K and Suzuki T: Expression of
glucose transporter-1 in human gastric carcinoma: Association with
tumor aggressiveness, metastasis, and patient survival. Cancer.
92:634–641. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yamada A, Oguchi K, Fukushima M, Imai Y
and Kadoya M: Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose
positron emission tomography in gastric carcinoma: Relation to
histological subtypes, depth of tumor invasion, and glucose
transporter-1 expression. Ann Nucl Med. 20:597–604. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alakus H, Batur M, Schmidt M, Drebber U,
Baldus SE, Vallböhmer D, Prenzel KL, Metzger R, Bollschweiler E,
Hölscher AH and Mönig S: Variable 18F-fluorodeoxyglucose uptake in
gastric cancer is associated with different levels of GLUT-1
expression. Nucl Med Commun. 31:532–538. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoshioka T, Yamaguchi K, Kubota K,
Saginoya T, Yamazaki T, Ido T, Yamaura G, Takahashi H, Fukuda H and
Kanamaru R: Evaluation of 18F-FDG PET in patients with advanced,
metastatic, or recurrent gastric cancer. J Nucl Med. 44:690–699.
2003.PubMed/NCBI
|
|
18
|
Ma DW, Kim JH, Jeon TJ, Lee YC, Yun M,
Youn YH, Park H and Lee SI: 18F-fluorodeoxyglucose
positron emission tomography-computed tomography for the evaluation
of bone metastasis in patients with gastric cancer. Dig Liver Dis.
45:769–775. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawanaka Y, Kitajima K, Fukushima K, Mouri
M, Doi H, Oshima T, Niwa H, Kaibe N, Sasako M, Tomita T, et al:
Added value of pretreatment (18)F-FDG PET/CT for staging of
advanced gastric cancer: Comparison with contrast-enhanced MDCT.
Eur J Radiol. 85:989–995. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kang SH, Kim JI, Moon HS, Kang HM, Kim SH,
Seong JK, Lee BS, Jeong HY, Song KS, Noh SM, et al: Overt bone
marrow metastasis from early gastric cancer. Endoscopy. 40:E34–E35.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hironaka SI, Boku N, Ohtsu A, Nagashima F,
Sano Y, Muto M, Fujii T, Tajiri H and Yoshida S: Sequential
methotrexate and 5-fluorouracil therapy for gastric cancer patients
with bone metastasis. Gastric Cancer. 3:19–23. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sasaki Y, Nishina T, Yasui H, Goto M, Muro
K, Tsuji A, Koizumi W, Toh Y, Hara T and Miyata Y: Phase II trial
of nanoparticle albumin-bound paclitaxel as second-line
chemotherapy for unresectable or recurrent gastric cancer. Cancer
Sci. 105:812–817. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shitara K, Takashima A, Fujitani K, Koeda
K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K,
et al: Nab-paclitaxel versus solvent-based paclitaxel in patients
with previously treated advanced gastric cancer (ABSOLUTE): An
open-label, randomised, non-inferiority, phase 3 trial. Lancet
Gastroenterol Hepatol. 2:277–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Woo Y, Fujisaki S, Takashina M, Tomita R,
Sakurai K and Takayama T: A case of metastases to the bone, skin,
and ovary from gastric cancer occurring more than eight years after
distal gastrectomy. Gan To Kagaku Ryoho. 44:1571–1573. 2017.(In
Japanese). PubMed/NCBI
|
|
27
|
Takishita C, Yajima K, Iwasaki Y, Ohashi
M, Iwanaga T and Oohinata R: A case of early gastric cancer with
multiple synchronous bone metastases treated complete response with
S-1+CDDP. Gan To Kagaku Ryoho. 41:2611–2614. 2014.(In Japanese).
PubMed/NCBI
|
|
28
|
Choi YJ, Kim DH, Han HS, Han JH, Son SM,
Kim DS and Yun HY: Long-term survival after gastrectomy and
metastasectomy for gastric cancer with synchronous bone metastasis.
World J Gastroenterol. 24:150–156. 2018.PubMed/NCBI View Article : Google Scholar
|