Introduction
The incidence of gynecological cancers (GC) has
increased in Japan, with an estimated 30,964 newly diagnosed
patients in 2009. In recent years, the incidence of GC has
increased among younger patients, as well as among older patients
(1). The risk factors for GC differ
by organ. For example, the development of invasive cervical cancer
(CC) requires persistent infection by human papillomavirus
(2,3). Several occurrence risk factors for
endometrial cancer (EM) have been established, including excess
body weight (4) and diabetes
mellitus (DM) (5). The occurrence
risk factors for ovarian cancer (OV) include age at diagnosis,
family history of OV, infertility treatment and assisted
fertilization, obesity and metabolic syndromes (6). Recently, metabolic conditions, such as
obesity, hyperlipidemia and DM, have been attracting increasing
attention with respect to OV incidence (7,8).
Therefore, the onset of EM and OV appears to be associated with
lifestyle and behavioral factors, such as dietary habits, physical
activity, smoking and alcohol consumption.
Chronic diseases (CD) and cancer share common risk
factors, including aging and unhealthy habits, such as smoking,
poor diet, sedentary lifestyle, obesity and alcohol intake. CD
include hypertension (HT), DM, dyslipidemia (DL), heart disease
(HD) and cerebrovascular disease (CVD), and constitute >20% of
the occurrence risk factors for various cancers (9). In 2017, the Japanese Ministry of
Health, Labour and Welfare reported that 108,000 CD patients were
aged <35 years, 3,141,000 CD patients were aged 35-64 years, and
11,458,000 CD patients were aged ≥65 years among women without
cancer in Japan (10).
The development rates of CD are rapidly increased by
excess body weight (11), but there
are no published details of the effect of CD on GC. Therefore, the
aim of the present study was to investigate the correlations
between CD and GC, including CC.
Patients and methods
Study population
The present retrospective study reviewed the medical
records of 1,590 GC patients who were treated at the Department of
Obstetrics and Gynecology of Okayama University Hospital (Okayama,
Japan) between April 2004 and December 2017. The study protocol was
approved by the Institutional Review Board of Okayama University
Hospital (1904-05). Several studies reported that the threshold for
lowest risk of all-cause mortality was ~100 g alcohol/week and
<10 cigarettes/day (12,13). All patients underwent a review of
their medical history and lifestyle habits (smoking-positive:
Current smokers of ≥10 cigarettes/day; alcohol intake-positive:
Alcohol intake of ≥14 g/day), physical examination and routine
clinical staging. The patients were treated according to the Japan
Society of Gynecologic Oncology clinical guidelines (14-16).
The treatment options for gynecological cancer included surgery,
radiotherapy and/or chemotherapy, depending on tumor stage and
additional risk factors.
Data collection
The patients were asked to complete questionnaires
on their history of cardiac disease, lifestyle habits (smoking and
alcohol intake), and medications for HT, DM and DL. The
measurements included standard medical examinations, such as
height, weight, blood pressure, fasting blood glucose, hemoglobin
A1c and serum lipid profile, including triglyceride (TG), serum
high-density lipoprotein cholesterol (HDL-C) and low-density
lipoprotein cholesterol (LDL-C) levels. Patients with HT were
defined as those with blood pressure ≥140/90 mmHg, or those on
antihypertensive medication. Patients with DM were defined as those
with hemoglobin A1c ≥6.5%, or those receiving antidiabetic
medication. Patients with DL were determined as those with TG ≥150
mg/dl, HDL-C <40 mg/dl, and LDL-C ≥140 mg/dl, or those receiving
medication for DL. The presence of HD and CVD were assessed based
on self-reports. Height and weight were measured on admission,
prior to any therapeutic intervention. Body mass index (BMI) was
defined according to the 2015 World Health Organization
classification as follows: Underweight, <18.5 kg/m2;
normal weight, 18.5-24.99 kg/m2; overweight, 25.0-29.99
kg/m2; and obese, ≥30.0 kg/m2.
Statistical analysis
Data were analyzed using χ2 and
Mann-Whitney U-tests for comparisons, and by one-way analysis of
variance followed by Fisher's protected least significant
difference test for all pairwise comparisons. Survival curves were
constructed using the Kaplan-Meier method, and differences between
survival curves were examined using the log-rank test. Analyses
were performed using SPSS software, version 23.0 (IBM Corp.), with
the significance level set at 0.05.
Results
Patient characteristics
CC, EM and OV patients were aged 25-92, 23-91 and
15-84 years, respectively (median CC age, 55.0 years; median EM
age, 59.0 years; and median OC age, 57.0 years). Age was divided
into four groups: <50, 50-59, 60-69 and ≥70 years. The <50
years group included 277 CC (40.4%), 120 EM (19.6%) and 78 OV
(26.7%) patients; the 50-59 years group included 138 CC (20.2%),
200 EM (32.6%) and 87 OV (29.8%) patients; the 60-69 years group
included 133 CC (19.4%), 168 EM (27.4%) and 82 OV (28.1%) patients;
and the ≥70 years group included 137 CC (20.0%), 125 EM (20.4%) and
45 OV (15.4%) patients.
The HT, DM, DL, HD and CVD patients in the <50
years group included 9, 4, 2, 2 and 3 CC patients, respectively
(3.2, 1.4, 0.7, 0.7 and 1.1%, respectively); 12, 13, 5, 4 and 0 EM
patients, respectively (10.0, 10.8, 4.2, 3.3 and 0%, respectively);
and 1, 2, 0, 1 and 0 OV patients, respectively (1.3, 2.6, 0, 1.3
and 0%, respectively). The HT, DM, DL, HD and CVD patients in the
50-59 years group included 16, 10, 4, 2 and 2 CC patients,
respectively (11.6, 7.2, 2.9, 1.4 and 1.4%, respectively); 34, 17,
18, 6 and 5 EM patients, respectively (17.0, 8.5, 9.0, 3.0 and
2.5%, respectively); and 13, 3, 8, 2 and 0 OV patients,
respectively (14.9, 3.4, 9.2, 2.3 and 0%, respectively). The HT,
DM, DL, HD and CVD patients in the 60-69 years group included 43,
15, 14, 4 and 6 CC patients, respectively (32.3, 11.2, 10.5, 3.0
and 4.5%, respectively); 61, 20, 27, 13 and 6 EM patients,
respectively (48.8, 16.0, 21.6, 10.4 and 4.8%, respectively); and
19, 4, 8, 1 and 3 OV patients, respectively (23.2, 4.9, 9.8, 1.2
and 3.7%, respectively). Finally, the HT, DM, DL, HD and CVD
patients in the ≥70 years group included 57, 12, 14, 15 and 13 CC
patients, respectively (41.6, 8.8, 10.2, 10.9 and 9.5%,
respectively); 67, 31, 42, 8 and 6 EM patients, respectively (39.9,
18.5, 25.0, 4.8 and 3.6%, respectively); and 13, 6, 7, 2 and 3 OV
patients, respectively (28.9, 13.3, 15.6, 4.4 and 6.7%,
respectively) (Table I).
 | Table IIncidence of GC among patients with
chronic diseases in different age groups. |
Table I
Incidence of GC among patients with
chronic diseases in different age groups.
| | CC | EM | OV |
|---|
| Chronic diseases | Number | (%) | Number | (%) | Number | (%) |
|---|
| HT, age (years) |
|
<50 | 9 | 3.2 | 12 | 10 | 1 | 1.3 |
|
50-59 | 16 | 11.6 | 34 | 17 | 13 | 14.9 |
|
60-69 | 43 | 32.3 | 61 | 48.8 | 19 | 23.2 |
|
≥70 | 57 | 41.6 | 67 | 39.9 | 13 | 28.9 |
| DM, age (years) |
|
<50 | 4 | 1.4 | 13 | 10.8 | 2 | 2.6 |
|
50-59 | 10 | 7.2 | 17 | 8.5 | 3 | 3.4 |
|
60-69 | 15 | 11.2 | 20 | 16 | 4 | 4.9 |
|
≥70 | 12 | 8.8 | 31 | 18.5 | 6 | 13.3 |
| DL, age (years) |
|
<50 | 2 | 0.7 | 5 | 4.2 | 0 | 0 |
|
50-59 | 4 | 2.9 | 18 | 9 | 8 | 9.2 |
|
60-69 | 14 | 10.5 | 27 | 21.6 | 8 | 9.8 |
|
≥70 | 14 | 10.2 | 42 | 25 | 7 | 15.6 |
| HD, age (years) |
|
<50 | 2 | 0.7 | 4 | 3.3 | 1 | 1.3 |
|
50-59 | 2 | 1.4 | 6 | 3 | 2 | 2.3 |
|
60-69 | 4 | 3 | 13 | 10.4 | 1 | 1.2 |
|
≥70 | 15 | 10.9 | 8 | 4.8 | 2 | 4.4 |
| CVD, age (years) |
|
<50 | 3 | 1.1 | 0 | 0 | 0 | 0 |
|
50-59 | 2 | 1.4 | 5 | 2.5 | 0 | 0 |
|
60-69 | 6 | 4.5 | 6 | 4.8 | 3 | 3.7 |
|
≥70 | 13 | 9.5 | 6 | 3.6 | 3 | 6.7 |
The associations of each GC type [CC (n=685), EM
(n=613) and OV (n=292)] with clinical characteristics (cancer
stage, HT, DM, DL, HD, CVD, BMI, smoking and alcohol intake) were
assessed (Table II). Regarding FIGO
stage, there were significantly more EM patients with early-stage
disease compared with CC patients (P<0.001). Conversely, the
number of advanced-stage OV patients was significantly higher
compared with CC patients (P<0.001). The incidence of HT, DM, DL
and high BMI were significantly higher in EM patients compared with
CC patients (all P<0.001). Furthermore, significantly more CC
patients were smoking- and alcohol intake-positive compared with EM
and OV patients (P<0.001, P<0.001, P<0.001 and P=0.026,
respectively).
 | Table IIBaseline characteristics and CD in
patients with gynecological cancers. |
Table II
Baseline characteristics and CD in
patients with gynecological cancers.
| | CC | EM | OV |
|---|
| Baseline
characteristics | Number | (%) | P-value | Number | (%) | P-value | Number | (%) | P-value |
|---|
| Stage | | | - | | |
<0.001a | | |
<0.001a |
|
I, II | 437 | 63.8 | | 460 | 75.0 | | 139 | 47.6 | |
|
III, IV | 248 | 36.2 | | 153 | 25.0 | | 153 | 52.4 | |
| HT | | | - | | |
<0.001a | | | 0.375 |
|
Present | 125 | 18.2 | | 174 | 28.4 | | 46 | 15.8 | |
|
Absent | 560 | 81.8 | | 439 | 71.6 | | 246 | 84.2 | |
| DM | | | - | | |
<0.001a | | | 0.601 |
|
Present | 41 | 6 | | 81 | 13.2 | | 15 | 5.1 | |
|
Absent | 644 | 94 | | 532 | 86.8 | | 277 | 94.9 | |
| DL | | | - | | |
<0.001a | | | 0.075 |
|
Present | 34 | 5 | | 92 | 15.0 | | 23 | 7.9 | |
|
Absent | 651 | 95 | | 521 | 85.0 | | 269 | 92.1 | |
| HD | | | - | | | 0.126 | | | 0.272 |
|
Present | 23 | 3.4 | | 31 | 5.1 | | 6 | 2.1 | |
|
Absent | 662 | 96.6 | | 582 | 94.9 | | 286 | 97.9 | |
| CVD | | | - | | | 0.453 | | | 0.229 |
|
Present | 24 | 3.5 | | 17 | 2.8 | | 6 | 2.1 | |
|
Absent | 661 | 96.5 | | 596 | 97.2 | | 286 | 97.9 | |
| BMI,
kg/m2 | | | - | | |
<0.001a | | | 0.537 |
|
<25.0 | 549 | 80.1 | | 370 | 60.4 | | 239 | 81.8 | |
|
≥25.0 | 136 | 19.9 | | 243 | 39.6 | | 53 | 18.2 | |
| Smoking | | | - | | |
<0.001a | | |
<0.001a |
|
Negative | 524 | 76.5 | | 577 | 94.1 | | 274 | 93.8 | |
|
Positive | 161 | 23.5 | | 36 | 5.9 | | 18 | 6.2 | |
| Alcohol | | | - | | |
<0.001a | | | 0.026a |
|
Negative | 609 | 88.9 | | 580 | 94.6 | | 273 | 93.5 | |
|
Positive | 76 | 11.1 | | 33 | 5.4 | | 19 | 6.5 | |
The effect of CD was examined by determining how
many of CC, EM and OV patients in each of the four age groups had
CD. CD patients were divided into 189 CC (27.6%), 265 EM (43.2%)
and 72 OV (24.7%) patients. Patients in the <50 years group with
CD included 19 CC (6.9%), 27 EM (22.5%) and 4 OV (5.1%) patients.
CD patients in the 50-59 years group included 29 CC (21.0%), 57 EM
(28.5%) and 21 OV (24.1%) patients. CD patients in the 60-69 years
group included 60 CC (45.1%), 98 EM (58.3%), and 25 OV (30.5%)
patients. Finally, CD patients in the ≥70 years group included 81
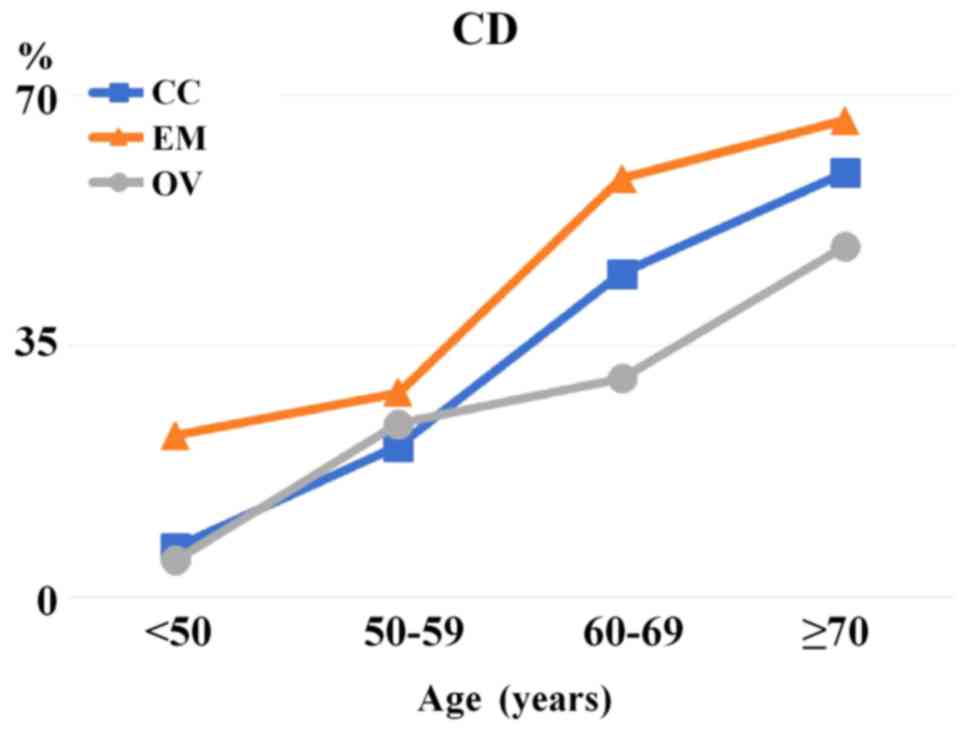
CC (59.1%), 83 EM (66.4%) and 22 OV (48.9%) patients. CC patients
aged ≥70 years and EM patients aged 60-69 and ≥70 years accounted
for >50% of those with CD. Therefore, the number of patients
with CD aged ≥70 years was 8.6-fold higher in the CC group,
3.0-fold higher in the EM group, and 9.6-fold higher in the OV
group compared with patients aged <50 years (Fig. 1).
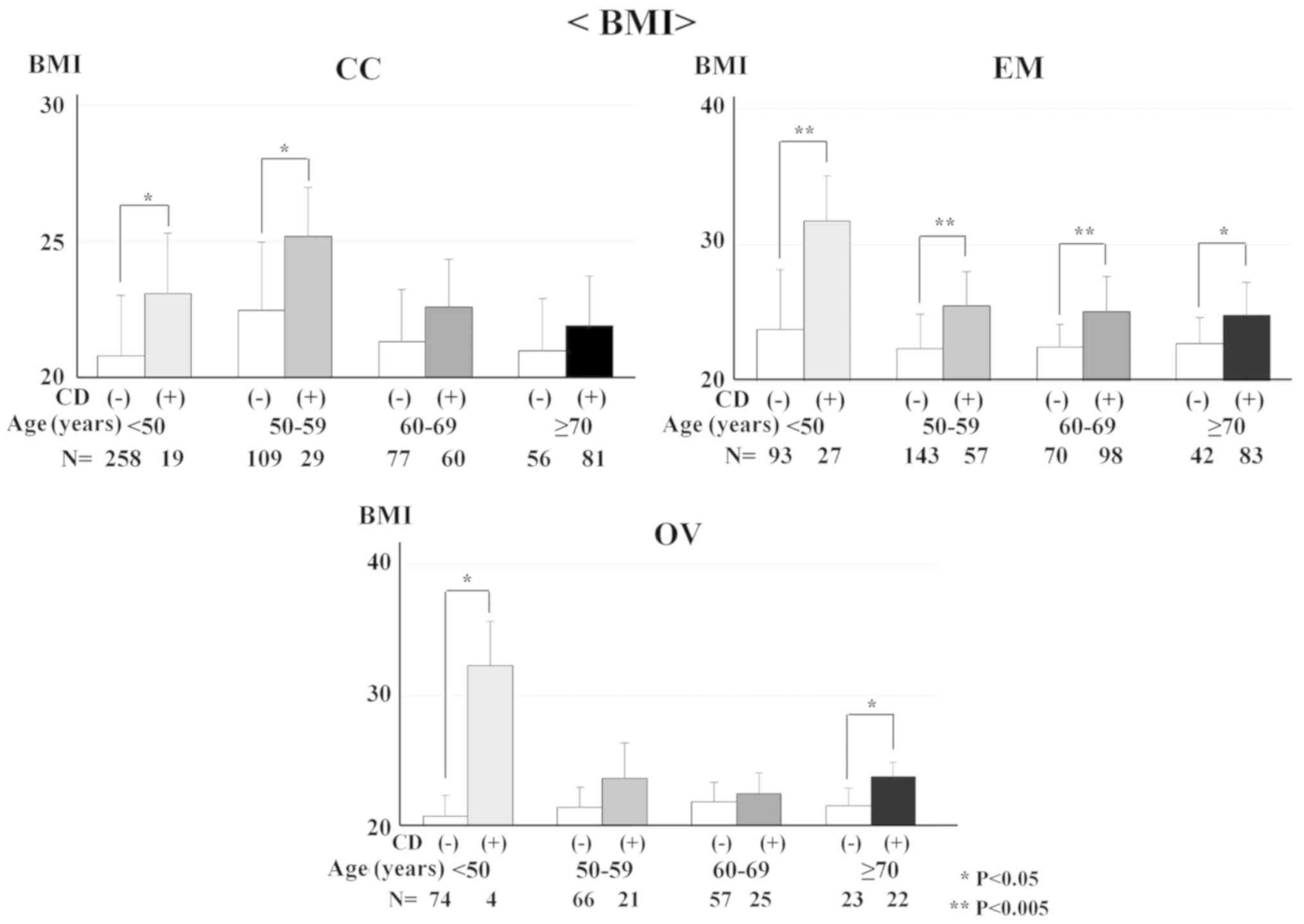
CD was examined for its association with BMI,
smoking and alcohol intake at any age in CC, EM and OV patients.
BMI and CD were significantly associated in CC patients aged <50
and 50-59 years (P=0.025 and P=0.010, respectively). BMI and CD
were significantly associated in all age groups in EM patients
(P<0.001, P<0.001, P<0.001 and P=0.014, respectively). BMI
and CD were significantly associated in OV patients aged 50-59 and
≥70 years (P=0.043 and P=0.004, respectively; Fig. 2). However, there was no association
between CD and smoking or alcohol intake at any age in CC, EM or OV
patients.
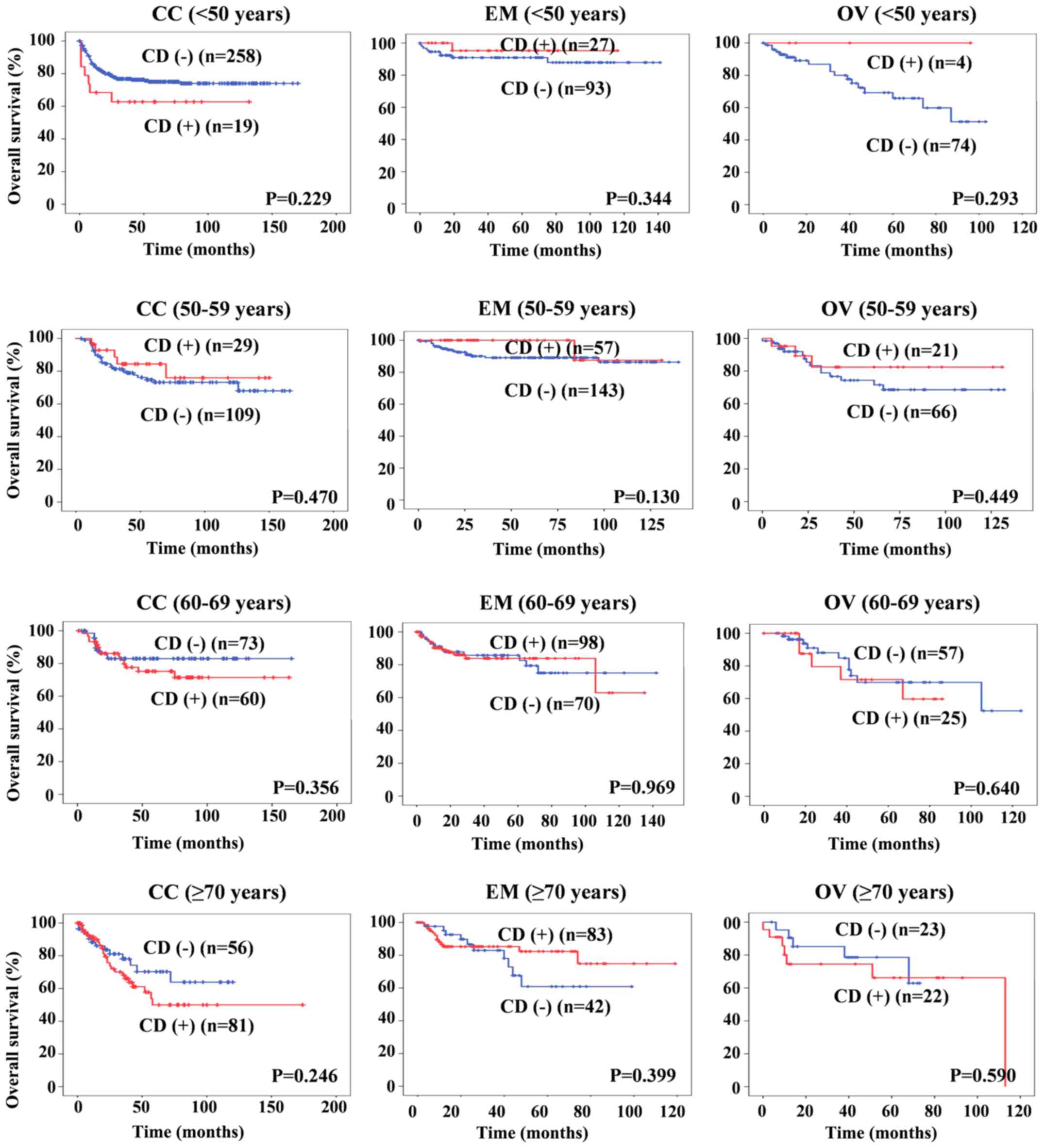
In the present study, the median overall survival
(OS) rates for patients with CC, EM and OV were 44.0, 40.0 and 32.5
months, respectively. The follow-up period was 1-174, 1-146 and
1-132 months, respectively. The OS curves for the 1,590 GC patients
according to their CD are shown in Fig.
3. There was no significant correlation between CD and survival
at any age in CC, EM or OV patients.
Discussion
The occurrence risk factors for cancer include
smoking, an unhealthy diet, obesity, sedentary lifestyle, DM, HT
and alcohol abuse, either alone or in combination. Accumulating
evidence has suggested that obesity is an important occurrence risk
factor for EM, and BMI is significantly associated with symptoms in
EM patients (17). Zhang et
al also demonstrated that DM is associated with EM (5), and several studies have reported the
association of metabolic markers of obesity, including elevated
blood glucose, TG and total cholesterol levels, with EM (18).
CD and cancer share common risk factors,
particularly those associated with an unhealthy lifestyle, such as
smoking, an unhealthy diet, physical inactivity, obesity and
alcohol intake. CD is known to contribute to >20% of occurrence
risk factors for various cancers (9); however, to the best of our knowledge,
this is the first study to describe an association between CD and
GC, including CC.
In the present study, CD, including HT, DM, DL, HD
and CVD, were examined in patients with CC, EM and OV. For all
diseases, we observed a high frequency of EM, CC and OV, in
decreasing order. For example, HT was observed in 18.2, 28.4 and
15.8% of CC, EM and OV patients, respectively; DL was observed in
5.0, 15.0 and 7.9% of CC, EM and OV patients, respectively; DM
occurred in 6.0, 13.2 and 5.1% of CC, EM and OV patients,
respectively; HD was diagnosed in 3.4, 5.1 and 2.1% of CC, EM and
OV patients, respectively; and CVD was recorded in 3.5, 2.8 and
2.1% of CC, EM and OV patients, respectively. Among the CD
patients, 27.6, 43.2 and 24.7% had CC, EM and OV, respectively. The
incidence of CD was found to increase with age in GC patients, with
CC patients aged >70 years and EM patients aged >60 years
accounting for >50% of patients with CD. Moreover, the numbers
of any disease increased with increasing age, regardless of the
number of CD.
CD were examined for their association with
lifestyle factors, such as obesity, smoking and alcohol intake, at
any age in all GC patients. In the present study, CD were more
prevalent among EM patients compared with CC and OV patients. Among
the CD patients, 27.6, 43.2 and 24.7% had CC, EM and OV,
respectively. Of note, occurrence risk factors for EM have been
established, including HT, DM and DL.
However, there was no association of smoking and
alcohol intake with CD. We also determined whether CD were
associated with outcome in GC patients, and found that the presence
of CD was not a prognostic predictor for CC, EM or OV patients.
There were certain limitations to the present study.
The number of patients was relatively small, and the examinations
were performed at a single institution. Further prospective studies
involving more patients and multiple institutions should provide
more definitive data to verify the significance of our
findings.
In conclusion, the presence of CD appears to
contribute to >24% of the occurrence risk factors for GC
patients in Japan.
Acknowledgements
The authors would like to thank all those who
contributed to the present study, particularly the statisticians
and colleagues of Okayama University Graduate School of Medicine,
Dentistry and Pharmaceutical Sciences. We appreciate their help
with data management and statistical support. We would also like to
thank Sarah Williams, PhD, and H. Nikki March, PhD for editing a
draft of this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN and KO contributed to the conception, design and
conduction of the study, and analysis and interpretation of the
data. HM, YM, JH, CO and HM contributed to data collection and the
conduction of the study. All the authors have read and approved the
final version of this manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
principles set out in the Declaration of Helsinki 1964 and all
subsequent revisions, and was approved by the Institutional Review
Board of Okayama University Hospital (IRB approval no.
1904-005).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests to disclose.
References
|
1
|
Hori M, Matsuda T, Shibata A, Katanoda K,
Sobue T and Nishimoto H: Japan Cancer Surveillance Research Group:
Cancer incidence and incidence rates in Japan in 2009: A study of
32 population-based cancer registries for the monitoring of cancer
incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 45:884–891.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Al Moustafa AE, Ghabreau L, Akil N, Rastam
S, Alachkar A and Yasmeen A: High-risk HPVs and human carcinomas in
the Syrian population. Front Oncol. 4(68)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aune D, Navarro Rosenblatt DA, Chan DS,
Vingeliene S, Abar L, Vieira AR, Greenwood DC, Bandera EV and Norat
T: Anthropometric factors and endometrial cancer risk: A systematic
review and dose-response meta-analysis of prospective studies. Ann
Oncol. 26:1635–1648. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Y, Liu Z, Yu X, Zhang X, Lü S, Chen
X and Lü B: The association between metabolic abnormality and
endometrial cancer: A large case-control study in China. Gynecol
Oncol. 117:41–46. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xie H, Hou Y, Cheng J, Openkova MS, Xia B,
Wang W, Li A, Yang K, Li J, Xu H, et al: Metabolic profiling and
novel plasma biomarkers for predicting survival in epithelial
ovarian cancer. Oncotarget. 8:32134–32146. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen Y, Zhang L, Liu W and Wang K:
Case-control study of metabolic syndrome and ovarian cancer in
Chinese population. Nutr Metab (Lond). 14(21)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nagle CM, Dixon SC, Jensen A, Kjaer SK,
Modugno F, deFazio A, Fereday S, Hung J, Johnatty SE; Australian
Ovarian Cancer Study Group, et al: Obesity and survival among women
with ovarian cancer: Results from the Ovarian cancer association
consortium. Br J Cancer. 113:817–826. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tu H, Wen CP, Tsai SP, Chow WH, Wen C, Ye
Y, Zhao H, Tsai MK, Huang M, Dinney CP, et al: Cancer risk
associated with chronic diseases and disease markers: Prospective
cohort study. BMJ. 360(k134)2018.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Ministry of Health labor and Welfare
reports. Patient survey overview in 2017 year in Japan, pp16-32,
2017 (Japanese).
|
|
11
|
Chen Y, Copeland WK, Vedanthan R, Grant E,
Lee JE, Gu D, Gupta PC, Ramadas K, Inoue M, Tsugane S, et al:
Association between body mass index and cardiovascular disease
mortality in east Asians and south Asians: Pooled analysis of
prospective data from the Asia Cohort Consortium. BMJ.
347(f5446)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wood AM, Kaptoge S, Butterworth AS,
Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M,
Burgess S, et al: Risk thresholds for alcohol consumption: Combined
analysis of individual-participant data for 599 912 current
drinkers in 83 prospective studies. Lancet. 391:1513–1523.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Whitfield JB, Heath AC, Madden PAF,
Landers JG and Martin NG: Effects of high alcohol intake,
alcohol-related symptoms and smoking on mortality. Addiction.
113:158–166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ebina Y, Mikami M, Nagase S, Tabata T,
Kaneuchi M, Tashiro H, Mandai M, Enomoto T, Kobayashi Y, Katabuchi
H, et al: Japan Society of Gynecologic Oncology guidelines 2017 for
the treatment of uterine cervical cancer. Int J Clin Oncol.
24:1–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ebina Y, Katabuchi H, Mikami M, Nagase S,
Yaegashi N, Udagawa Y, Kato H, Kubushiro K, Takamatsu K, Ino K and
Yoshikawa H: Japan Society of Gynecologic Oncology guidelines 2013
for the treatment of uterine body neoplasms. Int J Clin Oncol.
21:419–434. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Komiyama S, Katabuchi H, Mikami M, Nagase
S, Okamoto A, Ito K, Morishige K, Suzuki N, Kaneuchi M, Yaegashi N,
et al: Japan Society of Gynecologic Oncology guidelines 2015 for
the treatment of ovarian cancer including primary peritoneal cancer
and fallopian tube cancer. Int J Clin Oncol. 21:435–446.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jenabi E and Poorolajal J: The effect of
body mass index on endometrial cancer: A meta-analysis. Public
Health. 129:872–880. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Friedenreich CM, Biel RK, Lau DC, Csizmadi
I, Courneya KS, Magliocco AM, Yasui Y and Cook LS: Case-control
study of the metabolic syndrome and metabolic risk factors for
endometrial cancer. Cancer Epidemiol Biomarkers Prev. 20:2384–2395.
2011.PubMed/NCBI View Article : Google Scholar
|