Introduction
Lung cancer is known for its poor prognosis, and
chemotherapy is mainly conducted as therapy in patients with
postoperative recurrence. Therapeutic options have varied with the
increase in the development of novel anticancer drugs (1). However, this has not led to a dramatic
improvement in the survival rate, particularly for patients who are
not eligible for molecular targeted therapy. One of the problems of
chemotherapy is adverse drug reactions, which damage the host's
body and weaken their immune system. As a result, the therapy is
difficult to safely continue and leads to cancer cachexia. In
particular, attention should be paid when chemotherapy is applied
for recurrence after lung cancer surgery because it may cause fatal
side effects due to decreased physical fitness, such as decreased
pulmonary function. To improve the success of postoperative
recurrent lung cancer treatment, managing chemotherapy-induced
adverse drug reactions and immune function is necessary.
Kampo medicine has been used as hozai
(supportive drug) in Japan for the reduction of anticancer
drug-induced adverse reactions (2-5).
Recently, there have been some reports that Kampo medicine enhances
immune function and anticancer action and that its combined use
with chemotherapy boosts the therapeutic effect of anticancer drugs
(6-8).
In a pharmacological study investigating the enhancement of the
anticancer effect or improvement of the immune system, ginsenosides
in ginseng, one of the components used in Kampo medicine,
demonstrated a decrease in adverse drug reactions of anticancer
drugs and reinforced the therapeutic effect (9-11).
Among several Kampo medicines, Juzentaihoto (TJ-48)
is used to improve the following symptoms in people with poor
physical strength: Physical strength decline after illness or
surgery, fatigue/malaise, loss of appetite, night sweat, coldness
of hands and feet, and anemia. In studies on the basic mechanism of
TJ-48's effect, its effects have been reported through experimental
models such as the stimulation of natural killer activity,
anticancer cytokine production, or enhancement of blood synthesis
(12-15).
However, there is no appropriate method in conventional clinical
practice to evaluate the actual effect of TJ-48 for improving the
prognosis of patients with cancer since it has been considered as
hozai and is used with other anticancer drugs (7). To solve this problem, we focused on
the relationship between cancer chemotherapy and the effect of
TJ-48 by evaluating nutritional status.
In this study, to investigate the effect of TJ-48 on
patients with chemotherapy, we conducted prospective clinical
research to assess the effects of chemotherapy with or without
TJ-48 in patients with postoperative recurrent non-small cell lung
cancer (NSCLC).
Patients and methods
Patients
This prospective study was conducted with the
approval of the Human Ethics Committee of Akita Red Cross Hospital
(approval no. H26-7). Written informed consent was obtained from
all patients before study enrollment. A total of 45 patients with
postoperative recurrent NSCLC scheduled for first-line chemotherapy
were enrolled in this study. There are 13 EGFR mutation positive
patients included in this study. Table
I shows the patients' characteristics. Of the 45 patients, 23
were administered TJ-48 (Chemo + TJ-48 group) and 22 received
chemotherapy alone (Chemo alone group). Progression-free survival
(PFS) was compared between these two groups. PFS was defined as the
period from the start of chemotherapy to exacerbation, i.e.,
increase in neoplasm, novel metastasis, and the rise in tumor
markers. Tumor size was evaluated with computed tomography using
the Response Criteria in Solid Tumors version 1.1(16). The patients were examined by blood
analysis and chest X-ray every 2 weeks during chemotherapy. The
patients were also examined with computed tomography 1 month after
chemotherapy was started.
 | Table ICharacteristics of 45 patients with
postoperative recurrence of non-small cell lung cancer. |
Table I
Characteristics of 45 patients with
postoperative recurrence of non-small cell lung cancer.
| Variable | Chemo + TJ-48
(n=23) | Chemo alone
(n=22) | P-value |
|---|
| Age (years; median,
range) | 65 (38-87) | 68 (42-80) | 0.52 |
| Sex
(male/female) | 11/12 | 19/3 | 0.01 |
| Histologic type
(Ad/Scc/Large) | 20/2/1 | 15/5/2 | 0.31 |
| Chemotherapy
(platinum doublet/single/molecular) | 6/15/2 | 6/15/1 | 0.85 |
| Adverse events
(None/<G2/>G3) | 14/8/1 | 4/12/6 | 0.01 |
| Surgical procedure
(VATS/Standard thoracotomy) | 21/2 | 16/6 | 0.13 |
| Type of surgery
(Lobe/Pneumo/Seg) | 23/0/0 | 18/2/2 | 0.05 |
| Pathological stage at
surgery (I/II/III/IV) | 5/9/7/2 | 7/5/10/0 | 0.19 |
| Metastatic organ
(Brain/Lung/Liver/Bone/Othersa) | 4/11/1/1/6 | 5/6/3/2/6 | 0.56 |
TJ-48
The patients received TJ-48 t.i.d. (7.5 g/day)
before each meal for 2 weeks before the start of chemotherapy until
they were diagnosed with disease progression. The components
containing TJ-48 are shown in Table
II. To investigate the influence of TJ-48 on immune status,
serum albumin, and total lymphocytes were measured by peripheral
blood examination at the time of the start of TJ-48 administration,
chemotherapy, and 2 months after chemotherapy.
 | Table IIList of ingredients in Juzentaihoto
(TJ-48) extract granules (7.5 g). |
Table II
List of ingredients in Juzentaihoto
(TJ-48) extract granules (7.5 g).
| Extract: Plant
species | Component ratio
(%) |
|---|
| Astragalus Root:
Astragalus membranaceus Bunge | 10.5 |
| Cinnamon Bark:
Cinnamomum cassia Blume | 10.5 |
| Rehmannia Root:
Rehmannia glutiosa Libosch. Var. purpurea Makino | 10.5 |
| Peony Root:
Paeonia lactiflora Pallas | 10.5 |
| Cnidium Rhizome:
Cnidium officinale Makino | 10.5 |
| Atractylodes Lancea
Rhizome: Atractylodes lancea De Candole | 10.5 |
| Japanese Angelica
Root: Angelica acutiloba Kitagawa | 10.5 |
| Ginseng: Panax
ginseng C.A. Mey. | 10.5 |
| Poria Sclerotium:
Poria cocos Wolf | 10.5 |
| Glycyrrhiza:
Glycyrrhiza uralensis Fisher | 5.5 |
Statistical analysis
A univariate analysis regarding the patients'
characteristics was performed by unpaired t-test using the Fisher's
exact test for sex, surgical procedure, and G test for other
factors. We analyzed the prognostic difference between the two
groups using the Kaplan-Meier method and log-rank tests. We also
evaluated the change in body weight by unpaired t-test (Welch
t-test), prognostic nutrition index (PNI) calculated by serum
albumin, and total lymphocytes using a paired t-test, with
P<0.05 considered significant. To assess the body weight loss
ratio, the ratio of body weight within 1 month before and 2 months
after chemotherapy was used. The body weight loss ratio was
calculated as follows: 1-(postoperative body weight/preoperative
body weight). When receiver operating characteristic curves were
drawn for the PNI and body weight loss ratio, the cutoff values
were 46.7 and 3.0, respectively. PNI was calculated as 10x serum
albumin (g/dl) + 0.005x total lymphocyte (/mm3), as
described previously (17). A
multivariate analysis was performed using the Cox proportional
hazard model. Statistical analyses were performed using JMP IN
10.0.2 software program (SAS Institute).
Results
Effect of TJ-48 on chemotherapy
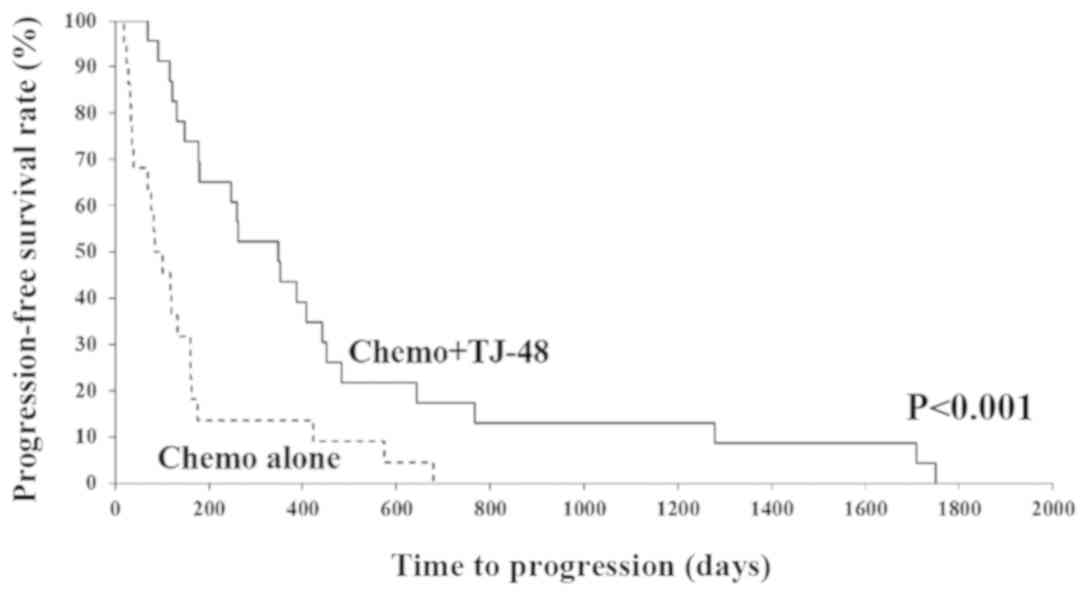
We compared the PFS rate between the Chemo + TJ-48
and Chemo alone groups (Fig. 1). We
found that the PFS rate in the Chemo + TJ-48 group was
significantly higher than that in the Chemo alone group
(P<0.001). There were 13 EGFR mutation positive patients in this
study. In cases without mutation, the average time to progression
was 355.8 days with TJ-48, which was significantly longer than 89.2
days without TJ-48. On the other hand, in cases with EGFR mutation,
there was an also significant prolongation with 684.3 days with
TJ-48 against 361.8 days without TJ-48 (data not shown).
TJ-48 reduces adverse events caused by
chemotherapy
We observed various grades of adverse events during
chemotherapy (Table I). The
following courses of chemotherapy were administered: Platinum
doublet (five cases of carboplatin + pemetrexed, three cases of
cisplatin + pemetrexed, three cases of carboplatin + paclitaxel,
and one case of carboplatin + gemcitabine), single agents (20 cases
of pemetrexed, five cases of docetaxel, three cases of Tegafur, and
two cases of S-1), and EGFR-TKI (two cases of gefitinib and one
case of erlotinib). Adverse events were observed in nine (39.1%)
cases in the Chemo + TJ-48 group and 18 (81.8%) cases in the Chemo
alone group. A statistically significant difference was noted
between the two groups (P<0.01). Furthermore, adverse events of
≥G3 were observed in only one (4.3%) case in the Chemo + TJ-48
group, whereas six (27.3%) cases occurred in the Chemo alone
group.
Effect of TJ-48 on nutritional
status
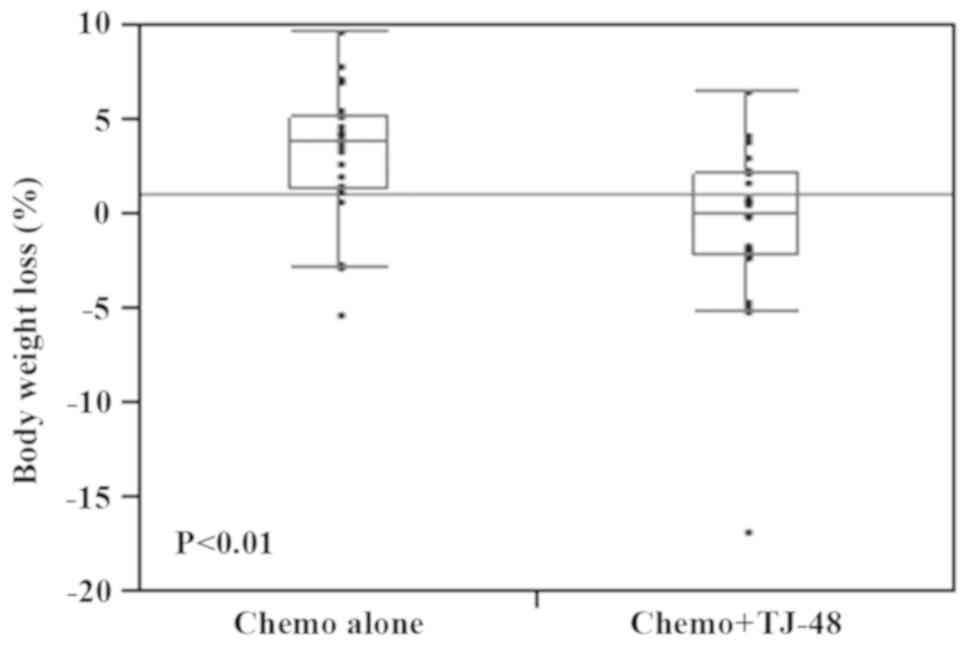
To examine whether TJ-48 influenced nutritional
status, we measured the body weight change and PNI before and after
therapy. We found that statistically significant body weight loss
was observed in the Chemo alone group compared with the Chemo +
TJ-48 group (P<0.01; Fig. 2).
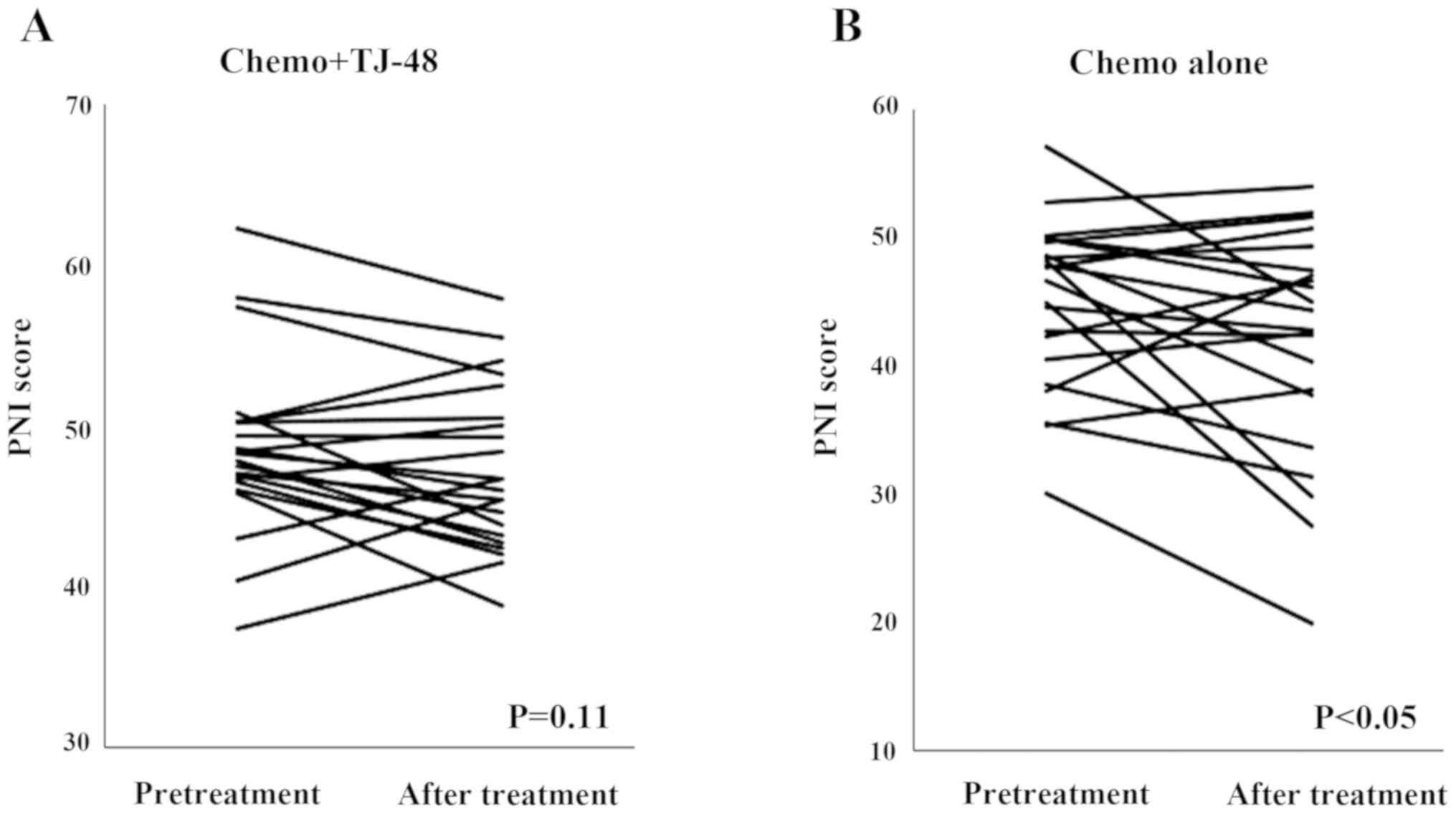
The PNI score was statistically significantly decreased in the
Chemo alone group (P<0.05), whereas there was no change in the
Chemo + TJ-48 group (P=0.11; Fig.
3).
Multivariate analysis for PFS
We next performed univariate and multivariate
analyses for PFS; the results are shown in Table III. In the univariate analysis for
PFS, female (HR, 0.45; 95% CI, 0.23-0.85), TJ-48 administration
(HR, 0.36; 95% CI, 0.18-0.65), PNI on pretreatment (HR, 0.48; 95%
CI, 0.25-0.92), and body weight loss ratio (HR, 2.74; 95% CI,
1.40-5.34) were statistically significant. In the multivariate
analysis for these factors, only TJ-48 administration was
significantly correlated with PFS (HR, 0.47; 95% CI,
0.23-0.93).
 | Table IIIUnivariate and multivariate analysis
for progression-free survival. |
Table III
Univariate and multivariate analysis
for progression-free survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Female | 0.45 | 0.23-0.85 | 0.01a | 0.57 | 0.27-1.14 | 0.11 |
| Adenocarcinoma | 0.57 | 0.28-1.23 | 0.14 | | | |
| >70 years | 1.37 | 0.70-2.57 | 0.35 | | | |
| Juzentaihoto
administration | 0.36 | 0.18-0.65 |
<0.01a | 0.47 | 0.23-0.93 | 0.03a |
| Pretreatment PNI
≥46.7b | 0.48 | 0.25-0.92 | 0.03a | 0.71 | 0.36-1.39 | 0.31 |
| Body weight loss
ratio ≥3.0b | 2.74 | 1.40-5.34 |
<0.01a | 1.69 | 0.80-3.52 | 0.17 |
Discussion
Surgical outcomes for NSCLC are relatively positive
in the early stages; however, in advanced lung cancer such as stage
III, the prognosis remains poor (18). In advanced cancer, the probability
of recurrence after surgery is extremely high, so a treatment
strategy after postoperative recurrence is important for improving
prognosis. Regarding the use of anticancer drugs in the surgical
field, lung cancer treatment includes preoperative chemotherapy,
postoperative adjuvant chemotherapy for advanced cancer, and
treatment of postoperative recurrence. Postoperative recurrence
treatment, in particular, is more likely to cause adverse events
compared with other situations because of a decrease of
postoperative lung function and loss of physical fitness due to
recurrence.
Conversely, with the advance of anticancer drugs,
which is represented by the emergence of molecular target drugs and
immune checkpoint inhibitors, the diversification of chemotherapy
is progressing (19-22).
In chemotherapy, both the drug effects and the appearance of
adverse events are important problems. Conventional cytotoxic
anticancer drugs have several adverse events such as
gastrointestinal symptoms, blood toxicity, neurotoxicity, and
interstitial pneumonia. Furthermore, the development of
interstitial pneumonia is an adverse event that affects the outcome
of molecular targeted drugs and immune checkpoint inhibitors
(23,24).
Recently, in response to the occurrence of adverse
events, steroids have been used for immune-related adverse events
with immune checkpoint inhibitors (25,26).
Since steroids have both an anti-inflammatory effect and an
immunosuppressive effect, they may be negative for cancer
treatment. In addition, when side effects appear, quality of life
decreases, leading to a decline in immunity and a worse prognosis.
Two contradictory elements are necessary to improve prognosis: Not
reducing physical fitness and using effective anticancer drugs;
however, adverse events and their treatment have a negative impact
on the former factor. Therefore, it is important to select a
treatment suitable for physical strength in the treatment strategy,
and in lung cancer chemotherapy, treatment with a single agent or
two agents has conventionally been performed.
However, multidrug therapy is recommended as a
recent treatment for unresectable NSCLC (27,28).
As is well known, there are no items focused on postoperative
recurrence cases in the lung cancer clinical practice guidelines,
and treatment is selected according to the unresectable cancer.
However, it is difficult to include recurrence cases after surgery
as unresectable cancer because the patient's condition is very
different, as described above. It is important to continue
treatment without difficulty while maintaining QOL in the treatment
strategy for postoperative recurrence.
In this study, the role of Kampo medicines used as a
side effect countermeasure was examined from different angles, and
TJ-48 combination chemotherapy was shown to be effective in
improving prognosis. This result suggests the possibility of a new
Kampo treatment.
In recent years, studies using nutritional status to
predict the prognosis of lung cancer treatment have been reported
to be useful in predicting prognosis (29-38).
For example, there are studies on the correlation between body
weight loss rate and prognosis and on preoperative body mass index
or prealbumin levels for predicting prognosis (36-38).
There are also reports of nutritional indicators; among them, PNI
is reported to be a simple and useful indicator (29-35).
However, no reports have shown that prognosis after recurrence has
been improved by preventing a decrease in physical fitness or
malnutrition. Some immunonutrition studies have reported that
preoperative immunonutrient administration contributes to the
suppression of postoperative complications, but there is no report
on the long-term prognostic improvement (39).
This study showed for the first time that the
decrease in physical strength due to side effects of anticancer
drugs exacerbates prognosis and that TJ-48 prevents malnutrition
and contributes to the improvement of prognosis. Currently, the
treatment strategy for postoperative recurrence is debatable, and
TJ-48 combination therapy is considered an effective choice to
improve prognosis.
Kampo has not been actively used thus far partially
because there was a misconception that it takes time to be
effective. In addition, the effect was not evaluated, and it was
used indiscriminately. Furthermore, there are prescription
indications specific to Kampo, represented by ‘sho,’ which
is one of the reasons why general clinicians hesitate to prescribe
it. The ‘sho’ is, in an easy-to-understand manner, ‘a thing
that represents the person's condition (constitutional, physical
strength, resistance, and symptom differences).’ Even if Kampo,
which is said to have few side effects, does not meet the
‘sho,’ there may be an adverse event; however, even if it is
not possible to evaluate the ‘sho,’ it can be used
appropriately for the indications, taking into account age and
performance status. Therefore, prescriptions that match the
constitution will likely be possible. To prevent the abuse of
Kampo, it is important to observe the condition after prescribing
and not to prescribe it without proper evaluation. Although no
effective evaluation of the effects of Kampo medicine on
cancer-bearing patients has been reported thus far, the nutritional
evaluation shown in this study may be useful. Above all, weight
change should be evaluated because it is simple and possible at
home.
There is some limitation for this study. First, this
study demonstrated that TJ-48 improves prognosis by preventing
nutritional deterioration by decreasing adverse events of
anti-cancer drugs. Unfortunately, because of clinical study, we did
not perform any experimental examination such as in vitro
mechanistic analysis. We are very interested in the direct
anticancer effects of TJ-48, but I look forward to future research.
Second, the presence or absence of EGFR mutation is an important
factor in lung cancer treatment. There were 13 EGFR mutation
positive patients in this study. In cases without mutation, the
average time to progression was 355.8 days with TJ-48, which was
significantly longer than 89.2 days without TJ-48. On the other
hand, in cases with EGFR mutation, there was an also significant
prolongation with 684.3 days with TJ-48 against 361.8 days without
TJ-48. However, in this study, the sample size is small and the
statistical power of the sub-analysis is weak, so we think that it
is necessary to further increase the sample size to analyze in
detail in the future.
In conclusion, we demonstrated that in the severe
prognosis of postoperative recurrent lung cancer, the use of TJ-48
in combination with anticancer drug treatment reduced the incidence
of side effects, and as a result, a decrease in physical fitness
was prevented, thereby improving the prognosis using a nutritional
assessment and multivariate analysis. The use of TJ-48 can be an
effective means to maximize the effects of chemotherapy and
contribute to improved prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HK made substantial contributions to conception and
design, acquisition of data, and writing of the manuscript. YS
analyzed and interpreted the patient data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This prospective study was conducted with the
approval of the Human Ethics Committee of Akita Red Cross Hospital
(approval no. H26-7). Written informed consent was obtained from
all patients prior to study enrollment.
Patient consent for publication
Identifying information was not be included in the
manuscript and written informed consent for the publication of any
associated data was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yano T, Okamoto T, Fukuyama S and Maehara
Y: Therapeutic strategy for postoperative recurrence in patients
with non-small cell lung cancer. World J Clin Oncol. 5:1048–1054.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ohnishi S and Takeda H: Herbal medicines
for the treatment of cancer chemotherapy-induced side effects.
Front Pharmacol. 6(14)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Inoue T, Takagi H, Owada Y, Watanabe Y,
Yamaura T, Fukuhara M, Muto S, Okabe N, Matsumura Y, Hasegawa T, et
al: The efficacy of the Kampo medicine rikkunshito for
chemotherapy-induced anorexia (RICH trial): Study protocol for a
randomized controlled trial. Trials. 18(485)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mori K, Kondo T, Kamiyama Y, Kano Y and
Tominaga K: Preventive effect of Kampo medicine (Hangeshashin-to)
against irinotecan-induced diarrhea in advanced non-small-cell lung
cancer. Cancer Chemother Pharmacol. 51:403–406. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ichiki M, Wataya H, Yamada K, Tsuruta N,
Takeoka H, Okayama Y, Sasaki J and Hoshino T: Preventive effect of
kampo medicine (hangeshashin-to, TJ-14) plus minocycline against
afatinib-induced diarrhea and skin rash in patients with non-small
cell lung cancer. OncoTargets and Therapy. 10:5107–5113.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ikemoto T, Shimada M, Iwahashi S, Saito Y,
Kanamoto M, Mori H, Morine Y, Imura S and Utsunomiya T: Changes of
immunological parameters with administration of Japanese Kampo
medicine (Juzen-Taihoto/TJ-48) in patients with advanced pancreatic
cancer. Int J Clin Oncol. 19:81–86. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Amitani M, Amitani H, Sloan RA, Suzuki H,
Sameshima N, Asakawa A, Nerome Y, Owaki T, Inui A and Hoshino E:
The translational aspect of complementary and alternative medicine
for cancer with particular emphasis on Kampo. Front Pharmacol.
6(150)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takeno N, Inujima A, Shinohara K, Yamada
M, Shibahara N, Sakurai H, Saiki I and Koizumi K: Immune adjuvant
effect of Juzentaihoto, a Japanese traditional herbal medicine, on
tumor vaccine therapy in a mouse model. Int J Oncol. 47:2115–2122.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li QF, Shi SL, Liu QR, Tang J, Song J and
Liang Y: Anticancer effects of ginsenoside Rg1, cinnamic acid, and
tanshinone IIA in osteosarcoma MG-63 cells: Nuclear matrix
downregulation and cytoplasmic trafficking of nucleophosmin. Int J
Biochem Cell Biol. 40:1918–1929. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
He BC, Gao JL, Luo X, Luo J, Shen J, Wang
L, Zhou Q, Wang YT, Luu HH, Haydon RC, et al: Ginsenoside Rg3
inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li L, Wang Y, Qi B, Yuan D, Dong S, Guo D,
Zhang C and Yu M: Suppression of PMA-induced tumor cell invasion
and migration by ginsenoside Rg1 via the inhibition of
NF-κB-dependent MMP-9 expression. Oncol Rep. 32:1779–1786.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kamiyama H, Takano S, Ishikawa E, Tsuboi K
and Matsumura A: Anti-angiogenic and immunomodulatory effect of the
herbal medicine ‘Juzen-taiho-to’ on malignant glioma. Biol Pharm
Bull. 28:2111–2116. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsuda T, Maekawa K, Asano K and
Hisamitsu T: Suppressive effect of Juzen-Taiho-To on lung
metastasis of B16 melanoma cells in vivo. Evid Based Complement
Alternat Med. 2011(743153)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saiki I: A Kampo medicine
‘Juzen-taiho-to’-prevention of malignant progression and metastasis
of tumor cells and the mechanism of action. Biol Pharm Bull.
23:677–688. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Onishi Y, Yamaura T, Tauchi K, Sakamoto T,
Tsukada K, Nunome S, Komatsu Y and Saiki I: Expression of the
anti-metastatic effect induced by Juzen-taiho-to is based on the
content of Shimotsu-to constituents. Biol Pharm Bull. 21:761–765.
1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shirakabe A, Hata N, Kobayashi N, Okazaki
H, Matsushita M, Shibata Y, Nishigoori S, Uchiyama S, Asai K and
Shimizu W: The prognostic impact of malnutrition in patients with
severely decompensated acute heart failure, as assessed using the
Prognostic Nutritional Index (PNI) and Controlling Nutritional
Status (CONUT) score. Heart Vessels. 33:134–144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sawabata N, Asamura H, Goya T, Mori M,
Nakanishi Y, Eguchi K, Koshiishi Y, Okumura M, Miyaoka E and Fujii
Y: Japanese Joint Committee for Lung Cancer Registry: Japanese Lung
Cancer Registry Study: First prospective enrollment of a large
number of surgical and nonsurgical cases in 2002. J Thorac Oncol.
5:1369–1375. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hong W, Wu Q, Zhang J and Zhou Y:
Prognostic value of EGFR19-del and 21-L858R mutations in patients
with non-small cell lung cancer. Oncol Lett. 18:3887–3895.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akamatsu H, Katakami N, Okamoto I, kato T,
Kim YH, Imamura F, Shinkai M, Hodge RA, Uchida H and Hida T:
Osimertinib in Japanese patientswith EGFR T790M mutation-positive
advanced non-small-cell lung cancer: AURA3 trial. Cancer Sci.
109:1930–1938. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shukuya T, Mori K, Amann JM, Bertino EM,
Otterson GA, Shields PG, Morita S and Carbone DP: Relationship
between overall survival and response or progression free survival
in advanced non-small cell lung cancer patients treated with
anti-PD-1/PD-L1 antibodies. J Thorac Oncol. 11:1927–1939.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Passiglia F, Galvano A, Rizzo S, Incorvaia
L, Listi A, Bazan V and Russo A: Looking for the best
immune-checkpoint inhibitor in pre-treated NSCLC patients: An
indirect comparison between nivolumab, pembrolizumab and
atezolizumab. Int J Cancer. 142:1277–1284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sakurada T, Kakiuchi S, Tajima S,
Horinouchi Y, Okada N, Nishisako H, Nakamura T, Teraoka K, Kawazoe
K, Yanagawa H, et al: Characteristics of and risk factors for
interstitial lung disease induced by chemotherapy for lung cancer.
Ann Pharmacother. 49:398–404. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kalisz KR, Ramaiya NH, Laukamp KR and
Gupta A: Immune checkpoint inhibitor therapy-related pneumonitis:
Pattern and management. Radiographics. 39:1923–1937.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Michot JM, Bigenwald C, Champiat S,
Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A,
Bahleda R, Hollebecque A, et al: Immune-related adverse events with
immune checkpoint blockade: A comprehensive review. Eur J Cancer.
54:139–148. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
Society of Clinical Oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mori S, Usami N, Fukumoto K, Mizuno T,
Kuroda H, Sakakura N, Yokoi K and Sakao Y: The significance of the
prognostic nutritional index in patients with completely resected
non-small cell lung cancer. PLoS One. 10(e0136897)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shimizu K, Okita R, Saisho S, Maeda A,
Nojima Y and Nakata M: Preoperative neutrophil/lymphocyte ratio and
prognostic nutritional index predict survival in patients with
non-small cell lung cancer. World J Surg Oncol.
13(291)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shoji F, Morodomi Y, Akamine T, Takamori
S, Katsura M, Takada K, Suzuki Y, Fujishita T, Okamoto T and
Maehara Y: Predictive impact for postoperative recurrence using the
preoperative prognostic nutritional index in pathological stage I
non-small cell lung cancer. Lung Cancer. 98:15–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sheng J, Yang YP, Ma YX, Qin T, Hu ZH,
Hong SD, Zhou T, Huang Y, Zhao HY and Zhang L: Low prognostic
nutritional index correlates with worse survival in patients with
advanced NSCLC following EGFR-TKIs. PLoS One.
11(e0147226)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Okada S, Shimada J, Kato D, Tsunezuka H,
Teramukai S and Inoue M: Clinical significance of prognostic
nutritional index after surgical treatment in lung cancer. Ann
Thorac Surg. 104:296–302. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hu Y, Shen J, Liu R, Feng Z, Zhang C, Ling
L and Chen L: Prognostic value of pretreatment prognostic
nutritional index in non-small cell lung cancer: A systematic
review and meta-analysis. Int J Biol Markers. 33:372–378.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ramos R, Nadal E, Peiró I, Masuet-Aumatell
C, Macia I, Rivas F, Rosado G, Rodriguez P, Ureña A, Padrones
, et al: Preoperative nutritional status assessment predicts
postoperative outcomes in patients with surgically resected
non-small cell lung cancer. Eur J Surg Oncol. 44:1419–1424.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kawai H and Ota H: Low perioperative
prealbumin predicts early recurrence after curative pulmonary
resection for non-small-cell lung cancer. World J Surg.
26:2853–2857. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nakagawa T, Toyozaki T, Chiba N, Ueda Y
and Gotoh M: Prognostic value of body mass index and change in body
weight in postoperative outcomes of lung cancer surgery. Interact
CardioVasc Thorac Surg. 23:560–566. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kawai H, Saito Y and Suzuki Y: Gender
differences in the correlation between prognosis and postoperative
weight loss in patients with non-small cell lung cancer. Interact
CardioVasc Thorac Surg. 25:272–277. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Braga M, Gianotti L, Vignali A and Carlo
VD: Preoperative oral arginine and n-3 fatty acid supplementation
improves the imminometabolic host response and outcome after
colorectal resection for cancer. Surgery. 132:805–814.
2002.PubMed/NCBI View Article : Google Scholar
|