Introduction
Chemoradiotherapy (CRT) with concurrent high-dose
(100 mg/m2 every 3 weeks) cisplatin (CDDP) is a standard
treatment for patients with locally advanced squamous cell
carcinoma of the head and neck (LA-SCCHN) (1-4).
However, high-dose CDDP is often unfeasible for patients with
advanced age, and renal, cardiac, or neurogenic dysfunction.
Therefore, clinical studies regarding CRT with high-dose CDDP have
targeted limited patients with normal organ function and good
general condition (1,2).
Because no statistically significant improvement in
survival has been shown in several phase 3 trials comparing
induction chemotherapy (ICT) followed by CRT versus CRT alone
(5-8),
the benefit of ICT for all patients with LA-SCCHN is controversial.
However, ICT prior to CRT is still an option for cases classified
as clinical nodal stage N2c, N3, which have a high risk of distant
metastasis in our clinical practice. According to randomized phase
3 trials comparing ICT regimens, the standard regimen of ICT is
docetaxel plus CDDP and fluorouracil (TPF) (9-11).
Notably, high-dose CDDP-based CRT after TPF-ICT is often associated
with nephrotoxicity, neurotoxicity, and ototoxicity, partly because
of the cumulative CDDP dosage (12,13).
Thus, it has been an unmet need that subsequent CRT regimens after
TPF-ICT should be optimized. The purpose of this study was to
evaluate the efficacy and safety of TPF-ICT followed by concurrent
CRT with split-dose CDDP as an alternative regimen of high-dose
CDDP in our clinical practice.
Patients and methods
Patients
This study involved patients with LA-SCCHN stage III
or IV (Union for International Cancer Control Tumor, Node,
Metastasis classification, 7th Edition) who were treated with
TPF-ICT followed by split-dose CDDP plus RT at Shizuoka Cancer
Center between January 2011 and December 2017. Patients were
selected based on the following criteria: (1) Age ≥20 years; (2) Eastern Cooperative Oncology Group
(ECOG) Organization performance status (PS) ≤2; and (3) histologically confirmed diagnosis of
squamous cell carcinoma of head and neck. For oropharyngeal cancer,
HPV positivity was examined, which was defined as positive for p16
on immunohistochemistry. Approval for this study was obtained from
the Institutional Review Board of Shizuoka Cancer Center and met
the standards set forth in the Declaration of Helsinki. Written
informed consent was obtained from all patients in this study.
Treatment
The TPF-ICT regimen consisted of DOC (70
mg/m2) and CDDP (70 mg/m2) given IV on day 1
and a continuous infusion of 5-FU (750 mg/m2/day) for 5
days. TPF was repeated every 3 weeks for three cycles. Patients
were administered prophylactic antibiotics, ciprofloxacin or
levofloxacin, at every cycle to prevent bacterial infections.
Granulocyte colony-stimulating factor was used in patients with
grade 4 or febrile neutropenia. Subsequently, patients received
concurrent CRT. Selection of a concurrent CRT regimen with
split-dose CDDP regimen was at the physician's discretion. The
administration of radiation therapy was performed at a total dose
of 70 Gy given in single, daily, 2-Gy fractions. Single-agent CDDP
at 20 mg/m2 was administered intravenously on days 1-4,
22-25, and 43-46. Three-dimensional conformal RT or
intensity-modulated RT was applied.
Evaluation
All clinical data were retrospectively obtained from
electronic medical records. We evaluated pre-treatment information,
including demographic features, physical examination results,
laboratory tests, computed tomography (CT), magnetic resonance
imaging (MRI), and 18F-fluorodeoxyglucose
positron-emission tomography/CT fusion imaging (PET-CT) findings.
Tumor response was assessed by CT or MRI at 6-8 weeks after
completing RT or when clinical signs suggested progressive disease.
Adverse events were evaluated according to the Common Terminology
Criteria for Adverse Events (CTCAE) v4.0.
Statistical analysis
The overall survival (OS) was measured from the
first day of CRT until the day of death from any cause or censored
at the last follow-up visit. Progression-free survival (PFS) was
calculated from the first day of CRT until disease relapse by
imaging, or censored at the last confirmation of survival. The
survival curves were generated using the Kaplan-Meier method. We
used the Mann-Whitney U test for comparisons of continuous
variables and Fisher's exact test for comparisons of categorical
variables between the groups. P-values <0.05 were considered
statistically significant. All statistical analyses were performed
using EZR (Saitama Medical Center, Jichi Medical University,
Saitama, Japan), a graphical user interface for R version 2.13.0
(The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
During the study period, 38 LA-SCCHN patients had
undergone concurrent CRT with split-dose CDDP. Of these, 17
patients were excluded, including 3 patients with nasopharyngeal
carcinoma, 6 patients who had undergone surgery before the
administration of CRT, and 8 patients who had not undergone ICT. In
total, 21 patients were included in this study. The patients'
characteristics before starting TPF-ICT are shown in Table I. Median age was 66 years (48-75
years). All patients were male. Eighteen patients (86%) were at
clinical stage IV. All patients had an ECOG performance status (PS)
of 0 to 1. Of eight patients with oropharyngeal cancer, 2 patients
had evidence of p16-positive squamous-cell carcinoma on tissue
specimen.
 | Table IPatient characteristics (n=21). |
Table I
Patient characteristics (n=21).
| Characteristics | Number of
patients |
|---|
| Median age, years
(range) | 66 (48-75) |
| Sex | |
|
Male | 21 |
|
Female | 0 |
| PS (ECOG) | |
|
0 | 14 |
|
1 | 7 |
| Primary site | |
|
Larynx | 4 |
|
Hypopharynx | 8 |
|
Oropharynx | 8 |
|
Oral
cavity | 1 |
| T stage | |
|
T1 | 2 |
|
T2 | 10 |
|
T3 | 3 |
|
T4 | 6 |
| N stage | |
|
N0 | 2 |
|
N1 | 1 |
|
N2 | 18 |
|
N3 | 0 |
| Disease stage | |
|
III | 3 |
|
IV | 18 |
| Resectability | |
|
Resectable | 10 |
|
Unresectable | 11 |
| Smoking
history | |
|
≥10
pack-years | 20 |
|
<10
pack-years | 1 |
| Habitual alcohol
use | |
|
Yes | 15 |
|
No | 6 |
| Histological
differentiation | |
|
Poorly | 5 |
|
Moderate | 10 |
|
Well | 1 |
|
Unknown | 5 |
| HPV status
(Oropharyngeal cancer) | |
|
Positive | 2 |
|
Negative | 6 |
Treatment compliance
The treatment compliance of TPF-ICT and CRT with
split-dose CDDP is shown in Table
II. Sixteen patients (76%) completed three cycles of TPF-ICT.
The median cumulative dose of CDDP in TPF-ICT was 180.0
mg/m2 (57.1-206.0 mg/m2). All patients
completed 70 Gy RT. RT pause was required in 3 patients (14.3%).
The rate of completion of CDDP, which is designated as cases with
no dose reduction of CDDP and no delay in CDDP administration, was
61.9%. Three cycles of CDDP administration were performed in 14
patients (66.7%). The median cumulative dose of CDDP in concurrent
CRT with split-dose CDDP was 206.7 mg/m2 (65.1-233.0
mg/m2). Thirteen patients (61.9%) received a cumulative
dose of CDDP of more than 200 mg/m2 during CRT. The
median duration of CRT was 50 days.
 | Table IITreatment compliance. |
Table II
Treatment compliance.
| A, TPF-ICT |
|---|
| Treatment | Number of patients
(%) |
|---|
| Number of
cycles | |
|
1 | 2 (9.5) |
|
2 | 3 (14.3) |
|
3 | 16 (76.2) |
| Median cumulative
dose of CDDP (mg/m2) (range) | 180.0
(57.1-206.0) |
| B, CRT |
| RT (70 Gy)
completion rate | 21(100) |
| RT pause rate | 3 (14.3) |
| CDDP completion
ratea | 13 (61.9) |
| Number of CDDP
cycles | |
|
1 | 2 (9.5) |
|
2 | 5 (23.8) |
|
3 | 14 (66.7) |
| Median cumulative
dose of | 206.7
(65.1-233.0) |
| CDDP
(mg/m2) (range) | |
| CRT duration
(day) | 50 (48-52) |
Treatment outcome
The objective response rate (ORR) after TPF-ICT was
documented in 17 patients (81.0%), including no cases with complete
response (CR) and 17 (81.0%) with partial response (PR). After the
completion of CRT, CR was documented in 16 patients (76.2%) and PR
in 3 patients (14.3%). The median length of follow-up for censored
cases was 51.5 months (range, 30.7-94.4). Median PFS was not
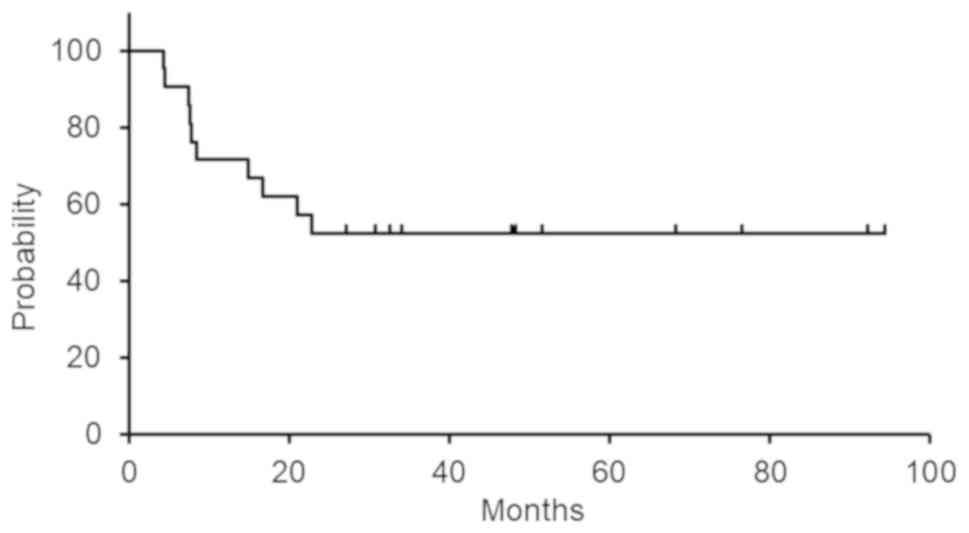
reached and 5-year PFS was 52.4% (Fig.
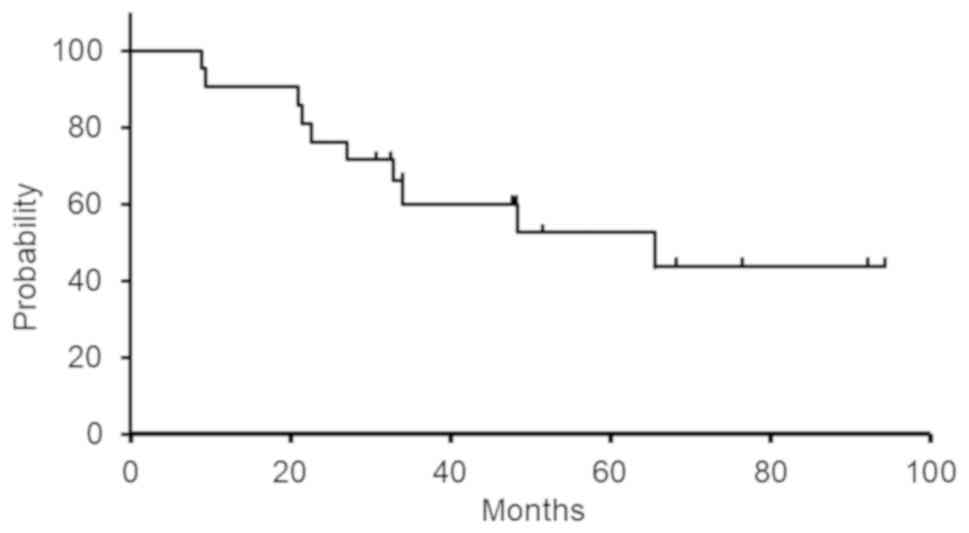
1). Median OS was 65.5 months. 3-year and 5-year OS was 60.4
and 52.9%, respectively (Fig.
2).
Adverse events associated with
CRT
The grade of toxicities observed during CRT is
demonstrated in Table III. The
most common grade 3 or worse toxicities were stomatitis (48%),
dysphagia (21%), anorexia (17%), and leukopenia (14%). However,
there was no grade 2 or worse nephrotoxicity, neurotoxicity, or
ototoxicity. We further compared creatinine clearance (Cockcroft
and Gault equation), ototoxicity, and neurotoxicity before CRT
(after TPF-ICT) with those after CRT. There was no deterioration of
renal, auditory and neural function even after CRT (Table IV). No treatment-related deaths
were observed.
 | Table IIIAdverse events associated with
CRT. |
Table III
Adverse events associated with
CRT.
| Adversity | Any Gr, n (%) | ≥Gr3, n (%) |
|---|
| Stomatitis | 21(100) | 14(48) |
| Dysphagia | 20(95) | 6(21) |
| Anorexia | 21(100) | 5(17) |
| Leukopenia | 17(81) | 4(14) |
| Infection | 2(7) | 2(7) |
| Febrile
neutropenia | 2(7) | 2(7) |
| Anemia | 20(95) | 1(3) |
|
Thrombocytopenia | 18(86) | 1(3) |
| Nausea | 15(71) | 1(3) |
| Radiation
dermatitis | 21(100) | 1(3) |
| Dry mouth | 21(100) | 1(3) |
|
Ototoxicitya | 3(10) | 0 (0) |
|
Neuropathya | 1(3) | 0 (0) |
| AST/ALT
elevation | 5(17) | 0 (0) |
| Creatinine
elevationa | 11(52) | 0 (0) |
 | Table IVComparison of renal function,
ototoxicity, and neurotoxicity before and after CRT. |
Table IV
Comparison of renal function,
ototoxicity, and neurotoxicity before and after CRT.
| Renal function
factor | Pre CRT | Post CRT | P-value |
|---|
| Median creatinine
clearance (ml/min) (range) | 85.6
(46.9-115.1) | 85.1
(49.6-98.7) | 0.94 |
|
Ototoxicitya | 1 | 3 | 0.606 |
|
Neuropathya | 1 | 1 | 1.000 |
Discussion
The present study retrospectively investigated the
efficacy and feasibility of TPF-ICT followed by concurrent CRT with
split-dose CDDP. Complete response was achieved in 76.2% after CRT,
and long-term follow-up of survival confirmed that the median OS
was 65.5 months. Acute toxicities as well as late ones were
manageable and acceptable. These findings suggest that split-dose
CDDP is an effective regimen for patients with LA-SCCHN after
TPF-ICT using high-dose CDDP.
It is recommended that the total cumulative dose of
CDDP used in combination with radiation be more than 200
mg/m2 because reducing the total CDDP dose influences
the treatment outcome (14,15). However, the National Comprehensive
Cancer Network (NCCN) guidelines do not recommend high-dose CDDP
(100 mg/m2 every 21 days, three times) after CDDP-based
ICT because of toxicity concerns (16). Indeed, one patient experienced
decreased creatinine clearance (46.9 ml/min) and in another patient
PS deteriorated (PS2) after TPF-ICT in our study. These findings
suggest that, in some patients receiving TPF-ICT, high-dose CDDP in
the following CRT phase is not feasible. Furthermore, the TREMPLIN
randomized phase II study, in which ICT followed by CRT using
high-dose CDDP was compared with ICT followed by cetuximab plus
radiotherapy for larynx preservation, revealed that residual renal
dysfunction (grade 1) and grade 3-4 neuropathy were observed in
22.4 and 3.4% of cases after CRT, respectively (17). Late toxicities induced by high-dose
CDDP included nephrotoxicity, neurotoxicity, and ototoxicity
(17-20),
which are irreversible and likely to reduce quality of life in
patients treated with CRT.
CDDP-induced nephrotoxicity is dose-dependent and
involves necrosis, apoptosis, and necroptosis of renal cells
(21-24).
It is also well known that CDDP-induced neuropathy is
dose-dependent, with symptoms typically occurring at cumulative
doses of greater than 350 mg/m2 (25-29).
Notably, the risk of neurotoxicity was also reported to increase
with higher single doses of CDDP (26,28-31).
Furthermore, it has been reported that symptoms continued to
progress even after the cessation of treatment in more than half of
patients treated with high-dose CDDP (29).
To reduce the high-dose CDDP-associated toxicities
after TPF-ICT, alternative methods of CDDP administration without
reducing the total dosage should be optimized. Split-dose CDDP has
been conventionally used for head and neck cancers as an
alternative to high-dose CDDP. A prospective study of TPF-ICT
followed by concurrent CRT with split-dose CDDP regimen was
performed (32). This trial
targeted unresectable LA-SCCHN alone, whereas ours targeted both
resectable and unresectable cases. The treatment compliance and
efficacy of TPF and subsequent CRT in our study are consistent with
those in this previous study. However, no data are available
regarding late toxicities. We also followed up late toxicities,
focusing on nephrotoxicity, neurotoxicity, and ototoxicity. Our
safety profile revealed no grade 2 or worse nephrotoxicity,
neurotoxicity, or ototoxicity, whereas overall grade 3 or worse
toxicities occurred in 76.2% of cases. These results suggest that
the method of split-dose administration without reducing the total
dosage could decrease CDDP-related toxicities without compromising
treatment outcomes.
Instead of high-dose CDDP, alternative CRT regimens
after TPF have been suggested. A randomized phase 3 trial comparing
TPF-ICT followed by CRT or by cetuximab-RT in patients with
unresectable LA-SCCHN is ongoing (33). Although the definitive results are
yet to be released, this trial aims to evaluate the non-inferiority
of cetuximab-RT versus CRT in terms of overall survival.
Furthermore, the combination of carboplatin with RT was
conventionally utilized after ICT in several large clinical trials,
such as the TAX324(9) and PARADIGM
trials (8). Against this
background, carboplatin or cetuximab used concurrently with
radiation could be options after TPF.
Our study has a number of limitations. First, this
study is statistically underpowered due to its small sample size.
The retrospective study design at a single center may have led to
selection bias. There were also no definitive criteria for the
concurrent CRT regimen with split-dose CDDP. The selection of
concurrent CRT regimen was at the physician's discretion.
In conclusion, the sequential strategy of TPF-ICT
followed by concurrent CRT with split-dose CDDP may be tolerable
and efficacious in the treatment of LA-SCCHN. However, the most
appropriate CRT regimens after TPF-ICT, in terms of late toxicities
and survival outcome, need to be investigated in a future
randomized study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from Shizuoka Cancer Center but restrictions apply to the
availability of these data, which were used under license for the
current study, and so are not publicly available. Data are however
available from the authors upon reasonable request and with
permission of Shizuoka Cancer Center.
Authors' contributions
TY, MS, SH, HS, YO, HO, TOno, TK, MF, HI, KF, and
TOni participated in the research design. Acquisition of the data
was performed by MS and TY. Interpretation of the data was
conducted by all authors. The manuscript was prepared and written
by TY and MS. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Approval for the current study was obtained from the
Institutional Review Board of Shizuoka Cancer Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC collaborative group. Meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955.
2000.PubMed/NCBI
|
|
4
|
Pignon JP, le Maître A, Maillard E and
Bourhis J: MACH-NC Collaborative Group. Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang L, Jiang N, Shi Y, Li S, Wang P and
Zhao Y: Induction chemotherapy with concurrent chemoradiotherapy
versus concurrent chemoradiotherapy for locally advanced squamous
cell carcinoma of head and neck: A meta-analysis. Sci Rep.
5(10798)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Haddad RI, Posner M, Hitt R, Cohen EEW,
Schulten J, Lefebvre JL and Vermorken JB: Induction chemotherapy in
locally advanced squamous cell carcinoma of the head and neck:
Role, controversy, and future directions. Ann Oncol. 29:1130–1140.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Budach W, Bölke E, Kammers K, Gerber PA,
Orth K, Gripp S and Matuschek C: Induction chemotherapy followed by
concurrent radio-chemotherapy versus concurrent radio-chemotherapy
alone as treatment of locally advanced squamous cell carcinoma of
the head and neck (HNSCC): A meta-analysis of randomized trials.
Radiother Oncol. 118:238–243. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Blanchard P, Bourhis J, Lacas B, Posner
MR, Vermorken JB, Cruz Hernandez JJ, Bourredjem A, Calais G,
Paccagnella A, Hitt R, et al: Taxane-cisplatin-fluorouracil as
induction chemotherapy in locally advanced head and neck cancers:
An individual patient data meta-analysis of the meta-analysis of
chemotherapy in head and neck cancer group. J Clin Oncol.
31:2854–2860. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Caballero M, Mackers P, Reig O, Buxo E,
Navarrete P, Blanch JL and Grau JJ: The role of audiometry prior to
high-dose cisplatin in patients with head and neck cancer.
Oncology. 93:75–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mizuno T, Ishikawa K, Sato W, Koike T,
Kushida M, Miyagawa Y, Yamada K, Hirata S, Imai E and Noda Y: The
risk factors of severe acute kidney injury induced by cisplatin.
Oncology. 85:364–369. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS,
Rosenthal DI, Soulieres D, Kim H, Silverman C, Raben A, Galloway
TJ, et al: Randomized phase III trial to test accelerated versus
standard fractionation in combination with concurrent cisplatin for
head and neck carcinomas in the radiation therapy oncology group
0129 trial: Long-term report of efficacy and toxicity. J Clin
Oncol. 32:3858–3866. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Loong HH, Ma BB, Leung SF, Mo F, Hui EP,
Kam MK, Chan SL, Yu BK and Chan AT: Prognostic significance of the
total dose of cisplatin administered during concurrent
chemoradiotherapy in patients with locoregionally advanced
nasopharyngeal carcinoma. Radiother Oncol. 104:300–304.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
NCCN guidelines with NCCN evidence
blocks™-head and neck cancers version 1, 2020.
|
|
17
|
Lefebvre JL, Pointreau Y, Rolland F,
Alfonsi M, Baudoux A, Sire C, de Raucourt D, Malard O, Degardin M,
Tuchais C, et al: Induction chemotherapy followed by either
chemoradiotherapy or bioradiotherapy for larynx preservation: The
TREMPLIN randomized phase II study. J Clin Oncol. 31:853–859.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hitt R, Grau JJ, López-Pousa A, Berrocal
A, García-Girón C, Irigoyen A, Sastre J, Martínez-Trufero J,
Brandariz Castelo JA, Verger E, et al: A randomized phase III trial
comparing induction chemotherapy followed by chemoradiotherapy
versus chemoradiotherapy alone as treatment of unresectable head
and neck cancer. Ann Oncol. 25:216–225. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adelstein DJ, Moon J, Hanna E, Giri PG,
Mills GM, Wolf GT and Urba SG: Docetaxel, cisplatin, and
fluorouracil induction chemotherapy followed by accelerated
fractionation/concomitant boost radiation and concurrent cisplatin
in patients with advanced squamous cell head and neck cancer. A
Southwest oncology group phase II trial (S0216). Head Neck.
32:221–228. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Driessen CML, Leijendeckers J, Snik A, van
der Graaf WTA, de Boer JP, Gelderblom H, Kaanders JHAM, Takes R and
van Herpen CML: Ototoxicity in locally advanced head and neck
cancer patients treated with induction chemotherapy followed by
intermediate or high-dose cisplatin-based chemoradiotherapy. Head
Neck. 41:488–494. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lieberthal W, Triaca V and Levine J:
Mechanisms of death induced by cisplatin in proximal tubular
epithelial cells: Apoptosis vs. necrosis. Am J Phys. 270:F700–F708.
1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee RH, Song JM, Park MY, Kang SK, Kim YK
and Jung JS: Cisplatin-induced apoptosis by translocation of
endogenous Bax in mouse collecting duct cells. Biochem Pharmacol.
62:1013–1023. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ozkok A and Edelstein CL: Pathophysiology
of cisplatin-induced acute kidney injury. Biomed Res Int.
2014(967826)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Volarevic V, Djokovic B, Jankovic MG,
Harrell CR, Fellabaum C, Djonov V and Arsenijevic N: Molecular
mechanisms of cisplatin-induced nephrotoxicity: A balance on the
knife edge between renoprotection and tumor toxicity. J Biomed Sci.
26(25)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park SB, Goldstein D, Krishnan AV, Lin CS,
Friedlander ML, Cassidy J, Koltzenburg M and Kiernan MC:
Chemotherapy-induced peripheral neurotoxicity: a critical analysis.
CA Cancer J Clin. 63:419–437. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Oldenburg J, Fosså S and Dahl A: Scale for
chemotherapy-induced long-term neurotoxicity (SCIN): Psychometrics,
validation, and findings in a large sample of testicular cancer
survivors. Qual Life Res. 15:791–800. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
LoMonaco M, Milone M, Batocchi AP, Padua
L, Restuccia D and Tonali P: Cisplatin neuropathy: Clinical course
and neurophysiological findings. J Neurol. 239:199–204.
1992.PubMed/NCBI View Article : Google Scholar
|
|
28
|
van den Bent MJ, van Putten WL, Hilkens
PH, de Wit R and van der Burg ME: Retreatment with dose-dense
weekly cisplatin after previous cisplatin chemotherapy is not
complicated by significant neuro-toxicity. Eur J Cancer.
38:387–391. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
von Schlippe M, Fowler CJ and Harland SJ:
Cisplatin neurotoxicity in the treatment of metastatic germ cell
tumour: Time course and prognosis. Br J Cancer. 85:823–826.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cavaletti G, Marzorati L, Bogliun G,
Colombo N, Marzola M, Pittelli MR and Tredici G: Cisplatin-induced
peripheral neurotoxicity is dependent on total-dose intensity and
single-dose intensity. Cancer. 69:203–207. 1992.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Brydøy M, Oldenburg J, Klepp O, Bremnes
RM, Wist EA, Wentzel-Larsen T, Hauge ER, Dahl O and Fosså SD:
Observational study of prevalence of long-term Raynaud-like
phenomena and neurological side effects in testicular cancer
survivors. J Natl Cancer Inst. 101:1682–1695. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Okano S, Enokida T, Onoe T, Ota Y, Motegi
A, Zenda S, Akimoto T and Tahara M: Induction TPF chemotherapy
followed by CRT with fractionated administration of cisplatin in
patients with unresectable locally advanced head and neck cancer.
Int J Clin Oncol. 24:789–797. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hitt R, Mesia R, Grau JJ, Iglesias L, Del
Barco E, Lozano A, Trufero JM, Giron CG, Martin AL and Hernandez
JJC: Randomized phase III trial of induction chemotherapy (ICT)
with docetaxel-cisplatin-5fluorouracil (DCF) followed by
cisplatin-radiotherapy (CRT) or cetuximab-radiotherapy (CetRT) in
patients (pts) with locally advanced unresectable head and neck
cancer (LAUHNC). J Clin Oncol. 34: (15 Suppl)(S6001)2016.
|