Introduction
Feline nasal lymphoma is the most common nasal
tumour diagnosed in cats (1,2). It is
a relatively treatable tumour, with remission rates of 65-75%
observed when various chemotherapy protocols are used (3). The median survival time (MST) of
feline nasal lymphoma is 473 days with combined radiation therapy
and chemotherapy, and 320 days with chemotherapy alone (4). Chemotherapy and radiation therapy are
effective for the treatment of feline nasal lymphoma, and surgery
is not required (4). Despite the
effectiveness in treating feline nasal lymphoma, chemotherapy and
radiation have significant limitations; both have severe side
effects, including bone marrow suppression and skin ulcers
(5-8).
Moreover, treatment is restricted by a repeated requirement for
anaesthesia and high cost. The development of less burdensome and
low-cost treatments would be beneficial in clinical settings.
Photohyperthermal therapy (PHT), combining
photodynamic therapy (PDT) and photothermal therapy, has been
investigated as safe and low-cost treatments for cancer (9-11).
PHT is used in combination with near-infrared light (NIR) and a
photosensitizer, such as indocyanine green (ICG) or aminolevulinic
acid. The resulting activated oxygen has an anti-tumour effect
(10,11). ICG has a peak spectral absorption of
~780 nm and peak fluorescence emission at ~820 nm. It induces heat
and singlet oxygen formation in response to NIR light with a
wavelength of 800 nm and is characterized by low general toxicity
(10). The principal disadvantage
of ICG is its rapid clearance from the body (plasmatic half-life of
2-4 min), limiting its accumulation within tumours (12). Several studies have reported the use
of liposomally formulated ICG for optical imaging and cancer
therapy, which improves its tumour-accumulating ability and
stability (12-14).
Liposomal drug delivery systems have enhanced permeability
retention effects, which can increase the stability and
accumulation within tumours of ICG (15,16).
Suganami et al (13),
designed and synthesised a novel NIR photoactivating probe, which
is more hydrophobic compared with conventional ICG to promote
liposome formation. Toyota et al (17), reported that a liposomally
formulated ICG derivative (ICG-Lipo) yielded strong fluorescence
images under an NIR-fluorescence imaging system. PDT using ICG-Lipo
was reported to induce antitumour effects in vitro and in
vivo (18,19). The present study describes a case of
feline lymphoma that was treated with the combination therapy of
PHT with ICG-Lipo.
Case report
A 10-year-old male cat (weight, 4.1 kg) presented
with primary symptoms of sneezing, and nasal mucus and conjunctival
injection in the right eye. The cat was initially diagnosed with an
upper respiratory infection and prescribed an antibiotic. All the
symptoms disappeared, with the exception of conjunctival
hyperaemia. After 1 month, sneezing, nasal haemorrhage, protrusion
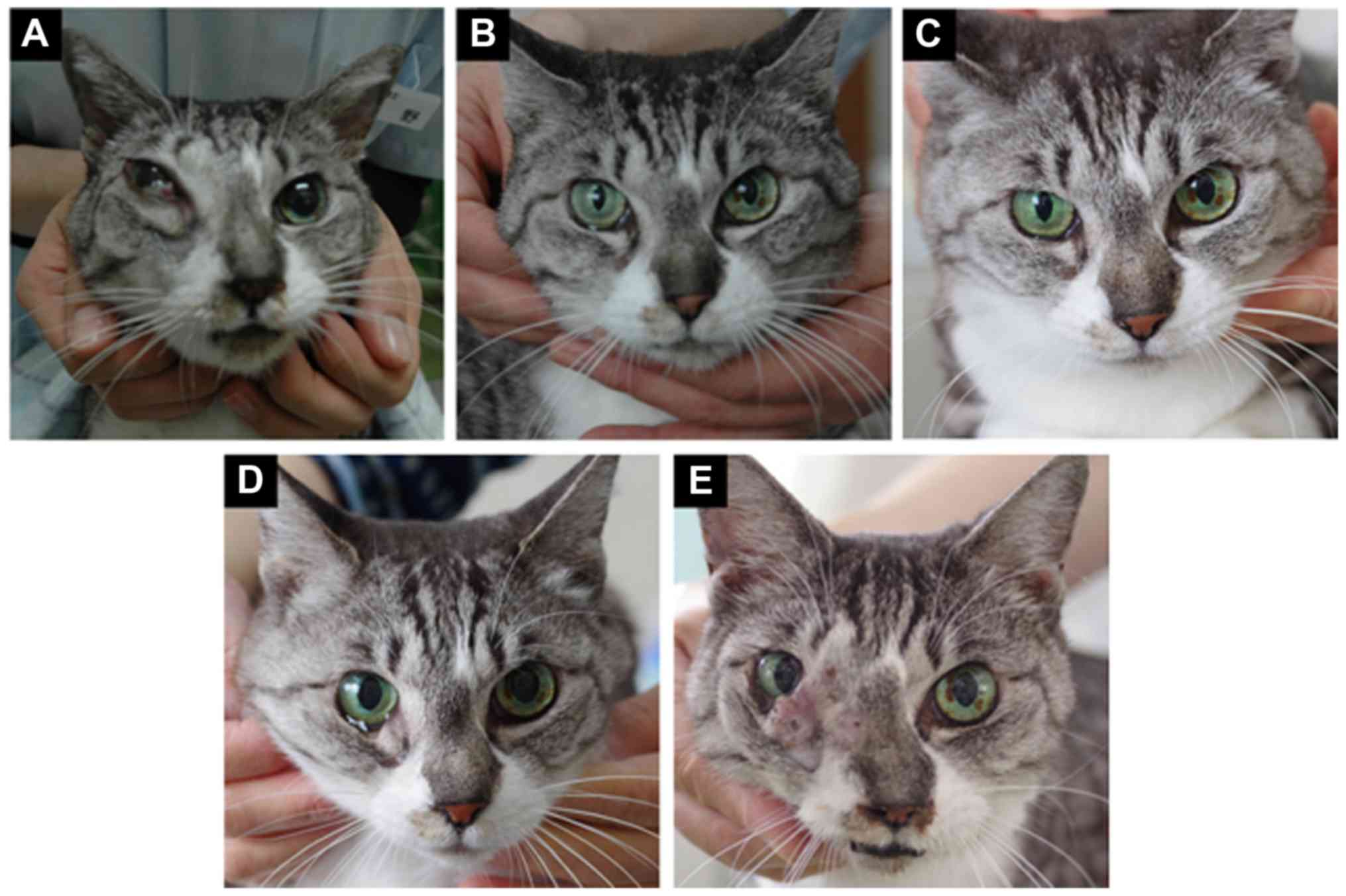
of the right eye and facial swelling were observed (Fig. 1A). Gingivitis was observed around
the right upper premolar, which led to the diagnosis of a root
abscess. Tooth extraction was performed with a routine course of
post-operative antibiotics. However, the symptoms did not improve,
and the facial swelling deteriorated. Cancer was suspected, and the
owner was advised to seek treatment at a secondary hospital. The
owner selected to have the cat treated at the primary hospital,
where the combination therapy of PHT with ICG-Lipo was performed.
This procedure was approved by the Organization for Research
Initiative and Promotion in Tottori University (ethical approval
no. H28-007).
ICG-Lipo, comprised of 2.25 mg ICG-C18 (ICG
derivative in which ICG is tagged with an octadeca-alkyl chain), 10
mg carboplatin (Nichi-Iko Pharmaceutical Co., Ltd.) and 0.6 mg
paclitaxel (Bristol-Myers Squibb), was diluted with 50 ml PBS (pH
7.4) at room temperature and administered intravenously at a rate
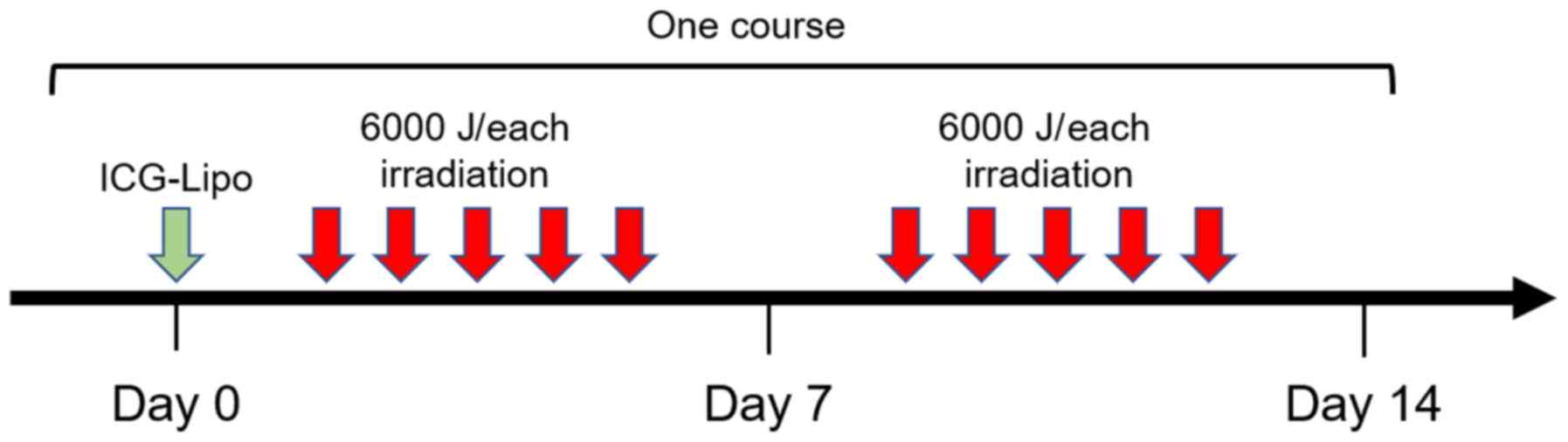
of 50 ml/h. Light irradiation was performed using a basic
semiconductor laser (DVL-15; Asuka Medical, Inc.) five times a week
for 2 weeks (Fig. 2). The total
dose of light that the cat was subjected to per irradiation was
6,000 J; light was irradiated at a dose rate of 5-10 W. Adjuvant
therapy for the tumour was not administered. Fluid infusion was
administered only when the cat exhibited a poor appetite. Five
courses of combination therapy of PHT with ICG-Lipo were
administered at 0, 25, 43, 60 and 74 days. Progressive improvement
and deterioration of symptoms was observed between the first and
fourth course of treatment. After the fifth course, facial swelling
and nasal congestion showed dramatic improvement (Fig. 1B). Remission was confirmed and
combination therapy was completed at the end of the fifth course.
The tumour remained in remission for 151 days (Fig. 1C). The cat experienced skin burns
and showed bleeding from the irradiated hard palate. These
complications were treated by haemostasis under anaesthesia. No
side effects of the antitumour drugs were observed, which was
supported by complete blood counts between treatments. The symptoms
reappeared after 168 days (95 days after the end of PHT; Fig. 1D) and the facial swelling increased
(Fig. 1E). Six additional courses
of combination therapy were administered at 173, 202, 224, 239,
252, 273 and 359 days. The effects of the treatment were reduced
compared with those observed during the first course of treatment.
The cat was diagnosed with renal insufficiency on day 359. The
cat's general condition progressively worsened, and the cat was
euthanised on day 401. The facial tumour was diagnosed as lymphoma
and metastases were found in the kidney during necropsy.
Immunohistochemical examination was not performed, as the owner did
not desire it for economic reasons.
Discussion
The survival time (401 days) in the present case was
longer than the MST (320 days) observed with chemotherapy alone
(4). The increased survival time
compared with the MST suggested that the combination therapy of PHT
with ICG-Lipo is potentially effective for treating feline nasal
lymphoma. The effects of the combination therapy were not observed
until 2 months after the commencement of treatment, at the fifth
course, when there was a dramatic decrease in the tumour. The
effect of the treatment continued, and the remission phase lasted
for ~3 months. It is proposed that continuous treatment is required
for long-term remission.
It was recently reported that the antitumour effect
of the PHT/ICG-Lipo combination therapy was associated with an
immunological mechanism (20).
According to a report into using immunotherapy with programmed
death (PD)-ligand 1 to treat a tumour, the tumour size temporarily
increased and subsequently decreased (21). The tumour volume may have increased
due to an inflammatory response. Alternatively, there may be a
time-lag between the initiation of treatment and observation of the
effect. In the present case, improvement and deterioration of the
symptoms were observed throughout the course of treatment. The
patient's response may have been a result of an increased immune
response induced by PHT with ICG-Lipo.
However, significant treatment effects were not
observed after relapse. The tumour cells may have become
drug-resistant. If the antitumour effect was related to an
immunological mechanism, the tumour may have expressed immune
checkpoint molecules (such as PD-1 or cytotoxic T-lymphocyte
associated protein 4). However, a biopsy could not be performed,
thus it was not possible to compare molecular expressions between
the primary and recurrent tumour. The immune effects of PHT with
ICG-Lipo may be demonstrated via immunohistochemical examination
for immune checkpoint molecules.
This is the first report of PHT with ICG-Lipo
containing an antitumour drug. In this case, ICG-Lipo contained
carboplatin and paclitaxel, and their dose was ~10% of the standard
administration protocol (22-24).
Notable therapeutic effects were observed without the usual side
effects of antitumour drugs. The encapsulation capacities of
carboplatin and paclitaxel in liposomal pharmaceuticals were
reported as 12 and 28%, respectively (25). Antitumour effects were demonstrated
in a previous study (25); however,
the compound ratio of ICG-Lipo was not validated in this study. It
is proposed that the compound ratio is similar to that utilised in
the previous report (25), thus an
antitumour effect was predicted.
In human medicine, nasal lymphoma is known as
extranodal natural killer/T-cell lymphoma, nasal type (26). The lymphoma originates from either
natural killer cells or γδ T-cells, both of which express
CD56(26). Conversely, a B-cell
phenotype comprises 40% of feline nasal lymphomas, and a T-cell
phenotype comprises 47% (27).
Moreover, the pathological features of human and feline nasal
lymphomas are different. Thus, the findings from the present study
may not contribute directly to human medicine. However, the
treatment strategies for feline lymphoma are relatively similar to
that for human lymphoma. It is proposed that PHT with ICG-Lipo has
the potential to be effective in treating human lymphomas.
In conclusion, the present case report described the
first treatment of nasal feline lymphoma using combination therapy
of PHT with ICG-Lipo. The present case showed a satisfactory
outcome compared with chemotherapy alone. PHT with ICG-Lipo is easy
to perform and the side effects are less severe. However, a single
case is insufficient to prove the exact treatment effects of PHT
with ICG-Lipo. A clinical trial with a large study population is
required to prove the efficacy of PHT with ICG-Lipo for treating
nasal feline lymphoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MY was involved in designing the treatment protocol
of PHT with ICG-Lipo, evaluating the treatment effect and preparing
the manuscript draft. MM oversaw all aspects of treatment and
drafted a report on the case. AS and YT synthesised ICG-Lipo. KA
and TT were involved in designing the fluid therapy and reviewing
the manuscript. NI and TI were involved in analysing haematological
data and reviewing the manuscript. YT and YO designed the treatment
plan, and reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The treatment protocol was approved by the
Organization for Research Initiative and Promotion in Tottori
University (ethical approval no. H28-007).
Patient consent for publication
The owner of the pet that was the subject of this
case report provided written consent for the publication of the
case report.
Competing interests
The author declare that they have no competing
interests.
References
|
1
|
Henderson SM, Bradley K, Day MJ, Tasker S,
Caney SM, Hotston Moore A and Gruffydd-Jones TJ: Investigation of
nasal disease in the cat-a retrospective study of 77 cases. J
Feline Med Surg. 6:245–257. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mukaratirwa S, van der Linde-Sipman JS and
Gruys E: Feline nasal and paranasal sinus tumours:
Clinicopathological study, histomorphological description and
diagnostic immunohistochemistry of 123 cases. J Feline Med Surg.
3:235–245. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Couto CG: Chapter 80: Lymphoma in the cat
and dog. In: Nelson RW Couto CG (eds.). Small animal internal
medicine, 4th edition. St. Louis, Elsevier. pp1174–1186. 2009.
|
|
4
|
Haney SM, Beaver L, Turrel J, Clifford CA,
Klein MK, Crawford S, Poulson JM and Azuma C: Survival analysis of
97 cats with nasal lymphoma: A multi-institutional retrospective
study (1986-2006). J Vet Intern Med. 23:287–294. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pinard CL, Mutsaers AJ, Mayer MN and Woods
JP: Retrospective study and review of ocular radiation side effects
following external-beam Cobalt-60 radiation therapy in 37 dogs and
12 cats. Can Vet J. 53:1301–1307. 2012.PubMed/NCBI
|
|
6
|
Knapp DW, Richardson RC, Bonney PL and
Hahn K: Cisplatin therapy in 41 dogs with malignant tumors. J Vet
Intern Med. 2:41–46. 1988.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Machado MC, da Costa-Neto JM, Portela RD,
D'Assis MJMH, Martins-Filho OA, Barrouin-Melo SM, Borges NF, Silva
FL and Estrela-Lima A: The effect of naltrexone as a carboplatin
chemotherapy-associated drug on the immune response, quality of
life and survival of dogs with mammary carcinoma. PLos One.
13(e0204830)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oun R, Moussa YE and Wheate NJ: The side
effects of platinum-based chemotherapy drugs: A review for
chemists. Dalton Trans. 47:6645–6653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
O'Reilly S, Rowinsky E, Slichenmyer W,
Donehower RC, Forastiere A, Ettinger D, Chen TL, Sartorius S,
Bowling K, Smith J, et al: Phase I and pharmacologic studies of
topotecan in patients with impaired hepatic function. J Natl Cancer
Inst. 88:817–824. 1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Radzi R, Osaki T, Tsuka T, Imagawa T,
Minami S, Nakayama Y and Okamoto Y: Photodynamic hyperthermal
therapy with indocyanine green (ICG) induces apoptosis and cell
cycle arrest in B16F10 murine melanoma cells. J Vet Med Sci.
74:545–551. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Urbanska K, Romanowska-Dixon B, Matuszak
Z, Oszajca J, Nowak-Sliwinska P and Stochel G: Indocyanine green as
a prospective sensitizer for photodynamic therapy of melanomas.
Acta Biochim Pol. 49:387–391. 2002.PubMed/NCBI
|
|
12
|
Porcu EP, Salis A, Gavini E, Rassu G,
Maestri M and Giunchedi P: Indocyanine green delivery systems for
tumour detection and treatments. Biotechnol Adv. 34:768–789.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suganami A, Toyota T, Okazaki S, Saito K,
Miyamoto K, Akutsu Y, Kawahira H, Aoki A, Muraki Y, Madono T, et
al: Preparation and characterization of phospholipid-conjugated
indocyanine green as a near-infrared probe. Bioorg Med Chem Lett.
22:7481–7485. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xue X, Fang T, Yin L, Jiang J, He Y, Dai Y
and Wang D: Multistage delivery of CDs-DOX/ICG-loaded liposome for
highly penetration and effective chemo-photothermal combination
therapy. Drug Deliv. 25:1826–1839. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fang J, Nakamura H and Maeda H: The EPR
effect: Unique features of tumor blood vessels for drug delivery,
factors involved, and limitations and augmentation of the effect.
Adv Drug Deliv Rev. 63:136–151. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
17
|
Toyota T, Fujito H, Suganami A, Ouchi T,
Ooishi A, Aoki A, Onoue K, Muraki Y, Madono T, Fujinami M, et al:
Near-infrared-fluorescence imaging of lymph nodes by using
liposomally formulated indocyanine green derivatives. Bioorg Med
Chem. 22:721–727. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Suganami A, Iwadate Y, Shibata S,
Yamashita M, Tanaka T, Shinozaki N, Aoki I, Saeki N, Shirasawa H,
Okamoto Y and Tamura Y: Liposomally formulated
phospholipid-conjugated indocyanine green for intra-operative brain
tumor detection and resection. Int J Pharm. 496:401–406.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maruyama T, Akutsu Y, Suganami A, Tamura
Y, Fujito H, Ouchi T, Akanuma N, Isozaki Y, Takeshita N, Hoshino I,
et al: Treatment of near-infrared photodynamic therapy using a
liposomally formulated indocyanine green derivative for squamous
cell carcinoma. PLoS One. 10(e0122849)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shibata S, Shinozaki N, Suganami A,
Ikegami S, Kinoshita Y, Hasegawa R, Kentaro H, Okamoto Y, Aoki I,
Tamura Y and Iwadate Y: Photo-immune therapy with liposomally
formulated phospholipid-conjugated indocyanine green induces
specific antitumor responses with heat shock protein-70 expression
in a glioblastoma model. Oncotarget. 10:175–183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gustafson DL and Page RL: Cancer
Chemotherapy. In: Withrow SJ Vail DM and Page RL (eds.). Small
animal clinical oncology, 5th edition. St. Louis, Elsevier.
pp157–179. 2012.
|
|
23
|
Kisseberth WC, Vail DM, Yaissle J, Jeglum
KA, Couto CG, Ward H, Khanna C and Obradovich JE: Phase I clinical
evaluation of carboplatin in tumor-bearing cats: A veterinary
cooperative oncology group study. J Vet Intern Med. 22:83–88.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Poirier VJ, Hershey AE, Burgess KE,
Phillips B, Turek MM, Forrest LJ, Beaver L and Vail DM: Efficacy
and toxicity of paclitaxel (Taxol) for the treatment of canine
malignant tumors. J Vet Intern Med. 18:219–222. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang X, Liu Y, Kim YJ, Mac J, Zhuang R
and Wang P: Co-delivery of carboplatin and paclitaxel via
cross-linked multilamellar liposomes for ovarian cancer treatment.
RSC Adv. 7:19685–19693. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Harabuchi Y, Takahara M, Kishibe K, Nagato
T and Kumai T: Extranodal natural Killer/T-cell lymphoma, nasal
type: Basic science and clinical progress. Front Pediatr.
7(141)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nagata K, Lamb M, Goldschmidt MH, Duda L
and Walton RM: The usefulness of immunohistochemistry to
differentiate between nasal carcinoma and lymphoma in cats: 140
Cases (1986-2000). Vet Comp Oncol. 12:52–57. 2014.PubMed/NCBI View Article : Google Scholar
|