Introduction
Oral cancer development is a multi-step process, in
which the malignancy is preceded by a susceptible epithelium
(1,2). In general, histological diagnosis of
oral epithelial dysplasia is considered as the most important
indicator for the risk of progression to oral cancer (3). Oral epithelial dysplasia refers to the
chronic and progressive histopathological alterations that result
in premalignant transformation of the oral mucosa. In the oral
cavity, dysplasia manifests as a series of clinical and
histological variations that may include leukoplakia,
erythroplakia, or the combination of the two (4). However, the literature also reports
that this is a subjective diagnosis, with both inter- and
intra-examiner variations in diagnostic criteria (5). The increase in the degree of dysplasia
(from mild to moderate to severe) has been associated with a high
rate of progression to cancer, with the rates ranging from 6 to 36%
(6). In addition, some dysplastic
lesions may remain clinically unchanged, or even exhibit complete
regression (3,6).
The identification of oral dysplastic lesions with a
high risk of transformation to oral cancer remains a clinical
challenge, which, if resolved, would allow patients to benefit from
early interventions. According to the cancer dictionary (https://www.cancer.gov/publications/dictionaries/cancer-terms/def/transformation),
‘transformation’ designates the changes that a normal cell
undergoes as it becomes malignant. When these changes become
visible (such as potentially malignant lesions and macroscopic
cancer), it indicates that the cell has undergone a long process
that includes subtle molecular alterations, which are the true and
most meaningful changes in terms of malignant transformation.
There are currently no biomarkers (defined as
molecules or characteristics used as indicators of a biological
state) that are routinely used in the clinical setting to predict
high-risk oral dysplastic lesions. Taking into account that several
biomarkers have been suggested as predictors of the malignant
transformation of oral dysplasia, a systematic review was
conducted, which is widely accepted as the ‘gold standard’ in
evidence-based medicine (7). The
objective of the review was to identify, evaluate and summarize the
currently available evidence on biomarkers of progression to oral
cancer in patients diagnosed with dysplasia.
Only articles that reported risk values from
multivariate analysis (binary logistic regression or Cox
proportional hazards models) were selected. After filtering the
results, high-grade epithelial dysplasia and three proteins, namely
retinal dehydrogenase 1 (ALDH1A1), prominin-1 (PROM1) and
podoplanin (PDPN), were determined as risk factors for malignant
transformation.
Materials and methods
Study design
A systematic review was conducted. The independent
variables were the prognostic biomarkers; the dependent variable
was malignant transformation from a dysplastic state to oral
cancer. A well-defined protocol was created. This protocol was
imported into the International Prospective Register of Systematic
Reviews (PROSPERO), which includes health records, under the code:
CRD42018086476. These steps were undertaken to minimize the risk of
bias.
Prognostic biomarker
A prognostic biomarker was defined as a molecule or
histological characteristic obtained from a study that involved a
binary logistic regression analysis or a Cox proportional hazards
model. To be included in the present review, articles must have
demonstrated a significant association between oral dysplasia
biomarkers and malignant transformation (8). The computed risk, odds ratio (OR) or
hazard ratio (HR), should have been reported as the risk of
progression to oral cancer from the biomarker group vs. the
reference group, with OR/HR >1 indicating increased risk and
OR/HR <1 indicating decreased risk (9).
Search strategy
A systematic search was conducted through
MEDLINE/PubMed and Scopus databases for all literature published in
English up to January 18, 2018. The search was conducted using the
following keyword combinations: Oral dysplasia [Title/Abstract] or
leukoplakia [Title/Abstract] or erythroplakia [Title/Abstract] AND
biomarkers [MeSH Terms] AND risk [Title/Abstract] or risk ratio
[Title/ Abstract] or relative risk [Title/Abstract] or odds ratio
[Title/Abstract] AND human [MeSH Terms] AND English [Language]. All
selected studies were original researches evaluating biomarkers of
progression to oral cancer in patients diagnosed with epithelial
dysplasia.
Inclusion and exclusion criteria
Articles were included based on previously published
protocols (9,10). Briefly, we selected studies that
investigated biomarkers with an impact on malignant transformation,
which were subjected to multivariate analysis and presented the
possibility of constructing the study groups. Articles that did not
include oral dysplasia, leukoplakia or erythroplakia and risk terms
in their titles, abstracts or keywords, studies not carried out on
humans and non-primary researches, were excluded. Additionally,
articles that did not report risk values, those with unclear
definition criteria for groups and variables, and those with errors
in statistical information, were also excluded.
Data extraction
Titles and abstracts were imported into Rayyan
online application (https://rayyan.qcri.org) (11) and they were analyzed independently
by two trained reviewers. Discrepancies were resolved by consensus.
Biomarker names, experimental design, statistical method, sample
size, risk values, P-values and confidence intervals were extracted
from the selected articles.
Quality assessment
Quality assessment was performed in duplicate using
Reporting Recommendations for Tumor Marker Prognostic Studies
(REMARK) (12). The evaluators'
agreement level was assessed by Kappa analysis.
Scientific output trends
To determine the most extensively studied
biomarkers, SciCurve Open was used. SciCurve Open is a search
engine that transforms a systematic review into a comprehensible
environment (9).
Results
Most biomarkers are proteins evaluated
by immunohistochemistry
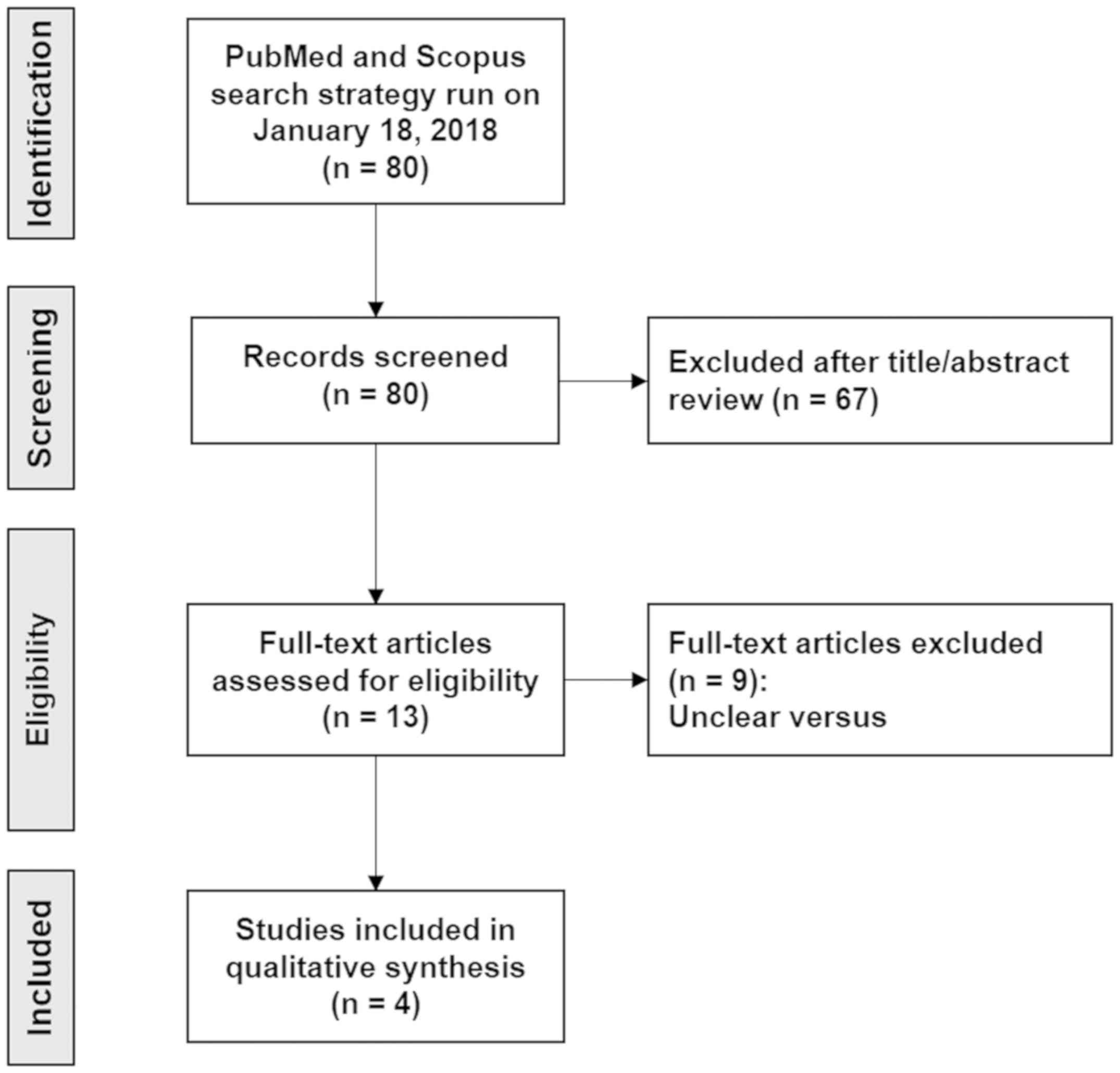
A total of 80 articles (Data S1, https://doi.org/10.5281/zenodo.2574148)
were identified, of which a duplicate and 67 that did not meet the
eligibility criteria were excluded. A total of 13 studies were
subjected to full-text review, of which 9 articles were excluded
due to the impossibility of constructing groups of interest, i.e.,
cases and controls expressing different levels of the biomarker
associated with the history of malignant transformation. Finally, 4
articles that met the inclusion criteria were retrieved (6,13-15).
The PRISMA flow chart is shown in Fig.
1.
The descriptive aspects of each study were
extracted. This information is summarized in Table I. Four articles evaluated 4
biomarkers in convenience samples collected between 1978 and 2010.
All studies had a retrospective design, mostly evaluating proteins
by immunohistochemistry. We herein present the main conclusions of
each investigation and the number of citations it received (up to
November 2018).
 | Table IAll included studies are
retrospective. |
Table I
All included studies are
retrospective.
| Study, year | Biomarker | Change | Design | Research remarks | Citations | (Refs.) |
|---|
| Insensitivity to
anti-growth signals | | | | | | |
|
Feng et
al, 2013 |
ALDH1A1a | (+) | Retrospective
(1993-2009) IHC China | ALDH1A1 expression
was found to be significantly associated with increased risk of
transformation | 32 | (13) |
|
Liu et
al, 2013 |
ALDH1A1a
PROM1a | (+) (+) | Retrospective
(1978-2008) IHC China | ALDH1A1 and PROM1
were correlated with malignant transformation in patients with
premalignant oral leukoplakia | 50 | (15) |
| Tissue invasion and
metastasis | | | | | | |
|
de Vicente
et al, 2013 | PDPNa | (+) | Retrospective
(2000-2005) IHC Spain | May be a valuable
biomarker for risk assessment of malignant transformation in
patients with oral leukoplakia along with histological
assessment | 50 | (14) |
| Histopathological
characteristics | | | | | | |
|
Kaur et
al, 2014 | Dysplasia
(grade) | (+) | Retrospective
(2000-2010) Histopathology Canada | Degree of dysplasia
emerged as an independent factor for identi fying high-risk
dysplasia | 38 | (6) |
|
Liu et
al, 2013 | Dysplasia
(grade) | (+) | Retrospective
(1978-2008) Histopathology China | Grade of dysplasia
was significantly associated with increased risk of malignant
transformation | 50 | (15) |
Malignant transformation appears to be
the result of a high biomarker expression
Oral lesions with high expression of biomarkers
presented a higher risk for malignant transformation (Table II). Variable cohort sizes were
used, ranging from 34 to 141 patients. A total of 10 covariables
were incorporated into 4 multivariate analyses. The variables most
frequently used in adjustments were proteins (3 models) and smoking
habit (2 models). However, none were found to be statistically
significantly associated with malignant transformation. Therefore,
the reported biomarkers may be considered as independent prognostic
markers. Of these, degree of dysplasia, ALDH1A1 and PROM1 stand
out. These markers were evaluated in investigations that included a
greater number of subjects and presented smaller confidence
intervals. Due to the heterogeneity of the studies, a meta-analysis
was not performed.
 | Table IIHigh expression of biomarkers
constitutes a risk for malignant transformation (dysplasia to oral
cancer). |
Table II
High expression of biomarkers
constitutes a risk for malignant transformation (dysplasia to oral
cancer).
| Study, year |
Biomarkera | Clinical
diagnosisb | N | Cases vs. reference
(events/group) | HR | CI | P-value | (Refs.) |
|---|
| Insensitivity to
anti-growth signals | | | | | | | | |
|
Feng et
al, 2013 | ALDH1A1 | Oral
erythroplakia | 34 | Positive (14/19)
vs. negative (3/15) | 8.9c | 1.7-47.4 | 0.011 | (13) |
|
Liu et
al, 2013 | ALDH1A1 PROM1 | Oral
leukoplakia | 141 141 | Positive (26/54)
vs. negative (11/76) Positive (19/32) vs. negative (18/109) | 4.2 2.9 | 2.0-8.9
1.5-5.6 | <0.001
0.002 | (15) |
| Tissue invasion and
metastasis | | | | | | | | |
|
de Vicente
et al, 2013 | PDPN | Oral
leukoplakia | 58 | Score 2-3 (11/22)
vs. 0-1 (2/36) | 8.7 | 1.8-41.6 | 0.007 | (14) |
| Histopathological
characteristics | | | | | | | | |
|
Kaur et
al, 2014 | Dysplasia
Dysplasia | Oral lesions with
dysplasia | 97 71 | Moderate (18/39)
vs. mild (12/58) Severe (9/13) vs. mild (12/58) | 2.5 5.4 | 1.6-10.8
2.6-23.2 | 0.013
<0.001 | (6) |
|
Liu et
al, 2013 | Dysplasia | Oral
leukoplakia | 141 | High-grade (13/32)
vs. low-grade (24/109) | 2.4 | 1.2-4.8 | 0.018 | (15) |
Studies do not report how the sample
size was determined
The researchers' agreement level was 0.86, which is
classified as almost optimal. Differences were resolved by
consensus. According to the REMARK analysis, the studies are of
high quality (they met >15 criteria). However, none of the
studies reported how sample size and biological effect were
established, or how missing data were handled. These aspects are
relevant for biomarker validation. More details may be found in
Data S1, (https://doi.org/10.5281/zenodo.2574148).
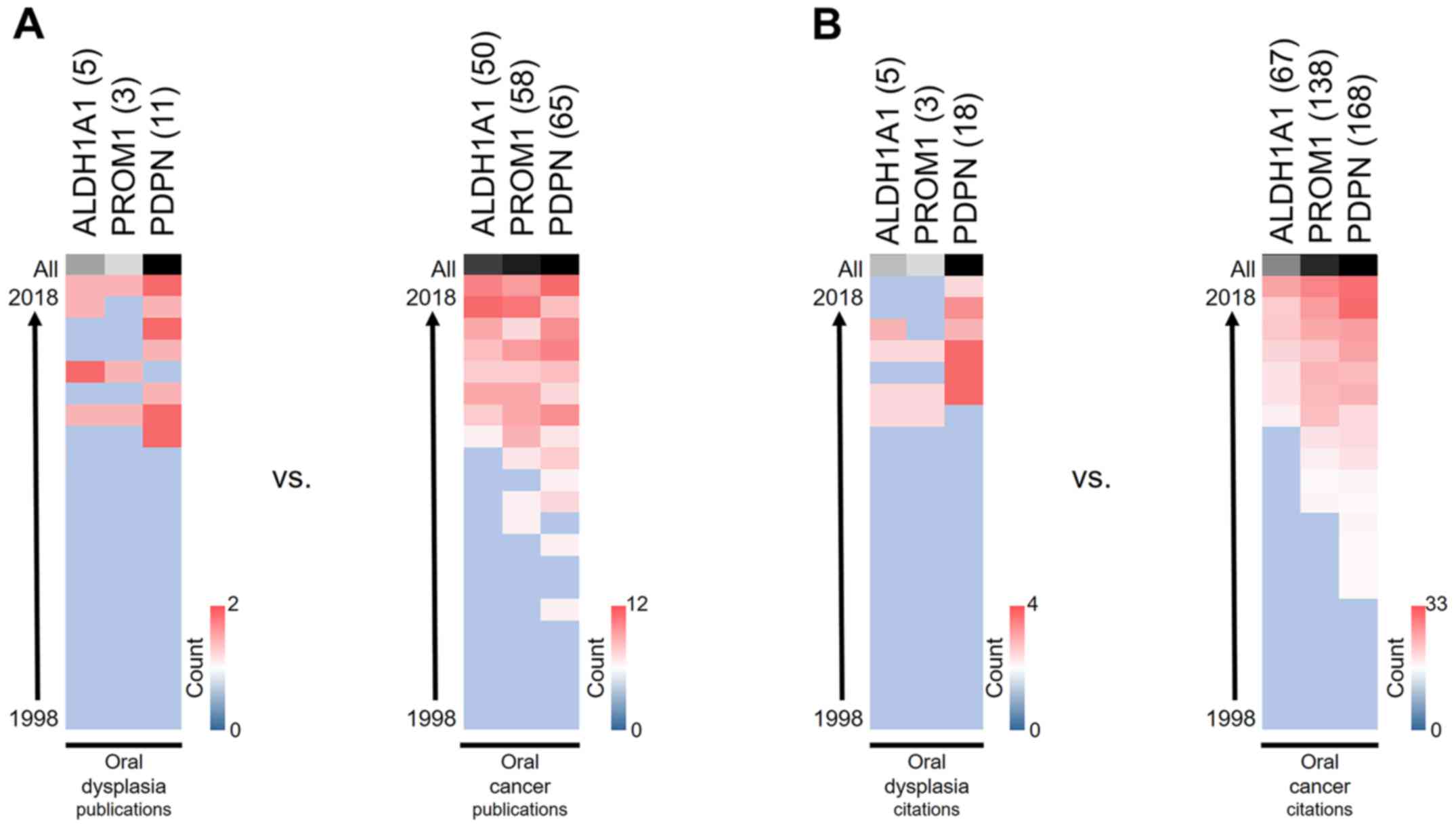
PDPN is the most extensively
researched protein
To identify the research trends relating to proposed
protein biomarkers, biomedical information in the SciCurve Open
online tool was explored. SciCurve uses PubMed to generate graphs
and curves that help to reveal trends in the literature (16), which allows for the identification
of publications, citations, authors and the most prolific countries
conducting research in a given area, among factors. As shown in
Fig. 2, PDPN is the most
extensively investigated biomarker in oral epithelial dysplasia, as
well as in oral cancer.
Discussion
Biomarkers are currently a field of particular
interest, as they may prove helpful in resolving diagnostic
challenges. In the future, they may enable personalized diagnoses
and guide early therapeutic interventions. Oral epithelial
dysplasia is considered as the most important prognostic indicator
for determining the risk of malignant transformation of lesions
that have this potential (17).
However, histopathology is limited in its ability to predict the
cancerization of these epithelial lesions (18). In this systematic review, only 4
potential biomarkers that could support more precise determinations
of clinical risk were identified: The degree of dysplasia and 3
proteins recognized as cancer stem cell markers.
The ‘natural history’ of oral cancer allows for the
study of different phases in malignant progression (19). It is well understood that a
susceptible oral epithelium may be represented by the typical
architectural changes of oral epithelial dysplasia. This
transformation starts and progresses through several steps, from
hyperplasia and dysplasia (mild, moderate and severe) to carcinoma
in situ and invasive cancer.
Two of the studies included in the present review
demonstrated that patients with advanced or high-risk dysplasia are
2-5 times more likely to develop oral cancer (6,15).
This finding has also been reported in other types of cancer. For
example, the presence of dysplasia is the gold standard biomarker
for cancer risk in Barrett's esophagus (20). In the context of cervical cancer,
women with high-grade cervical dysplasia (referred to as
intraepithelial neoplasia) have a higher risk of malignancy
(21,22). In terms of therapy, a systematic
review concluded that the surgical removal of lesions displaying
severe epithelial dysplasia significantly reduced the progression
to cancer. Untreated lesions had a risk of malignant transformation
(~39%) that was 6 times higher compared with that of treated
lesions (~8%) (23). These results
emphasize that the degree of epithelial dysplasia is useful for
determining the potential for progression to oral cancer,
justifying surgical removal of high-grade lesions and continuous
monitoring. Similarly, we believe that the degree of oral
epithelial dysplasia should be included in multivariate models that
study any lesion aspect.
Studies have demonstrated that an early diagnosis
(24) and a short interval from
diagnosis to treatment are associated with high survival rates
(25). As carcinogenesis is
progressive, studying the beginning may provide more clinical
opportunities (2). The onset may be
represented by an epithelium displaying mild dysplasia (since
severe lesions are a precursor to cancer). Identifying the
alterations occurring in the epithelium beyond architectural
changes may enable us to recognize which lesion will evolve. It is
likely that, in this context, oral carcinogenesis starts with the
transformation of a limited number of keratinocytes (1). A large number of proteins with diverse
normal functions are involved in human cancer, and identifying them
may help elucidate the clinical course of oral epithelial
dysplasia. In the context of this research, these proteins are
referred to as ‘biomarkers’. We selected a total of 4 studies that
described multivariate analyses for 3 proteins analyzed by
immunohistochemistry: ALDH1A1, PROM1 and PDPN. The high expression
of these proteins was associated with malignant transformation of
dysplastic lesions.
ALDH1A1 activity can define normal tissue stem cells
and cancer stem cell populations, where it is involved in
self-renewal, differentiation and self-protection (26). High ALDH1A1 activity and
overexpression are associated with poor prognosis of lung (27), esophageal (28) and breast cancers (29). Accumulating evidence suggests that
ALDH1A1 may represent a useful therapeutic cancer stem cell target
in tissues that do not normally express high levels of
ALDH1A1(26). Considering that this
protein is not present at high levels in the oral mucosa (30), ALDH1A1 may represent a therapeutic
opportunity for preventing the progression to oral cancer in
patients with dysplasia.
The precise physiological function of PROM1 is
unclear, but its ubiquitous presence indicates its relevance
(31). PROM1, a membrane
glycoprotein, is widely used for identifying stem cells in various
normal tissues and cancer stem cells. This protein is a key
regulator ensuring appropriate response of stem cells to
extracellular signals (32). Its
usefulness for cancer stem cells appears to be extremely important,
as by inhibiting PROM1, the signaling pathways that are involved in
angiogenesis and cell proliferation will also be inhibited
(31).
PDPN is a transmembrane glycoprotein considered to
be a specific marker for lymphatic endothelial cells (33). However, PDPN is not restricted to
endothelial cells, its expression also being detected in epithelial
basal cells of the oral mucosa (34). Populations of cancer stem cells
expressing PDPN has high clonal expansion rates, which helps
establish squamous cell carcinomas (35). PDPN has also been shown to promote
cancer cell clonal capacity, migration, epithelial-to-mesenchymal
transition, invasion, metastasis and inflammation (33). According to our results, PDPN has
been the most widely investigated biomarker over the last 20 years,
in both oral dysplasias and oral cancer. However, publication and
citation numbers reveal that research has tended to focus more on
the end of progression, which is cancer. We believe that this trend
should be reversed, and that analyses should be focused on lesions
that have not yet progressed to oral cancer, as this would give a
more preventive character to biomedical efforts.
All reported protein biomarkers are associated with
populations of stem cells in cancer. Cumulatively, the studies we
have considered support the role of cancer stem cells in promoting
tumor progression from a dysplastic state. Two recent articles
highlight the fact that that potentially malignant disorders of the
oral mucosa expressing markers of cancer stem cells are at high
risk of evolving into oral cancer (36,37).
We previously performed a systematic review to
identify published prognostic oral cancer biomarkers (9). In that investigation, we evaluated
cancer biomarkers associated with common clinical endpoints:
Overall survival, disease-free survival and cause-specific
survival. In that context, ALDH1A1, PROM1 and PDPN were identified
as potential prognostic biomarkers for disease-free survival,
indicating that these proteins are important for malignant
transformation as well as for the absence of signs of disease after
treatment.
Oral cancer is generally considered as a preventable
disease, as smoking and drinking habits are reported in the
majority of the patients, and exerting a synergistic effect
(38). However, there is an
increasing number of non-smoking, non-drinking patients, both male
and female, who develop oral cancers that are currently not
considered preventable (39).
Surprisingly, none of the selected articles reported a statistical
association of these and other factors (such as age, sex, oral
subsite and previous oral cancer) with oral malignant
transformation. Recognizing the importance of these clinical
variables, we believe that the results of multivariate models
should be interpreted with caution.
The selected articles were critically analyzed
according to the REMARK guidelines. None of the studies included
information on sample size determination or data loss management.
This is a clear limitation of the present study, since an
appropriate sample enables more efficient and reliable
investigations. The absence of sample calculations also limits
result interpretations (40) The
increasing availability and use of predictive models to facilitate
clinical decision-making highlights the need for a careful
evaluation of the validity of these models (41). The development of biomarkers
involves multiple processes, linking initial discovery in basic
studies, validation, and clinical implementation (42). Accordingly, the reported biomarkers
must be placed in the discovery phase, since they must now undergo
a necessary validation process to determine their true value in the
clinical setting.
In the present review, few biomarkers that may
explain the progression from epithelial dysplasia to oral cancer
were identified. The currently available literature states that
advanced or high-grade epithelial dysplasia determines the need for
lesion removal. To the best of our knowledge, the main contribution
of a set of immunohistochemical biomarkers may be at earlier
dysplastic stages. The use of a complete panel that reveals the
presence of cancer stem cells may prove fundamental to the early
recognition of oral cancer. The present systematic review used
strict statistical criteria for article inclusion. The findings
were based on 4 studies, which is rather insufficient to draw any
definitive conclusions, and these findings must be validated
through further research. Studies on protein variability in a large
number of patients and tissues are also recommended.
Supplementary Material
Supplementary Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
CR conceived the study and was in charge of overall
direction and planning. CR, RG and CF conducted the systematic
review, including the design and drafting of the manuscript. CR
verified the data extracted following the literature search. CR, RG
and CF were responsible for proofreading and critically revising
the review for intellectual content. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
2
|
Rivera C: The challenge of the state of
susceptibility to oral cancer. J Oral Res. 4:8–9. 2015.
|
|
3
|
van der Waal I: Potentially malignant
disorders of the oral and oropharyngeal mucosa; terminology,
classification and present concepts of management. Oral Oncol.
45:317–323. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mohajertehran F and Sahebkar A: The
promise of stem cell markers in the diagnosis and therapy of
epithelial dysplasia and oral squamous cell carcinoma. J Cell
Physiol. 233:8499–8507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ranganathan K and Kavitha L: Oral
epithelial dysplasia: Classifications and clinical relevance in
risk assessment of oral potentially malignant disorders. J Oral
Maxillofac Pathol. 23:19–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kaur J, Matta A, Kak I, Srivastava G, Assi
J, Leong I, Witterick I, Colgan TJ, Macmillan C, Siu KW, et al:
S100A7 overexpression is a predictive marker for high risk of
malignant transformation in oral dysplasia. Int J Cancer.
134:1379–1388. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stern C, Munn Z, Porritt K, Lockwood C,
Peters MDJ, Bellman S, Stephenson M and Jordan Z: An international
educational training course for conducting systematic reviews in
health care: The Joanna Briggs Institute's Comprehensive Systematic
Review Training Program. Worldviews Evid Based Nurs. 15:401–408.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ballman KV: Biomarker: Predictive or
prognostic? J Clin Oncol. 33:3968–3971. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rivera C, Oliveira AK, Costa RAP, De Rossi
T and Paes Leme AF: Prognostic biomarkers in oral squamous cell
carcinoma: A systematic review. Oral Oncol. 72:38–47.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mallett S, Timmer A, Sauerbrei W and
Altman DG: Reporting of prognostic studies of tumour markers: A
review of published articles in relation to REMARK guidelines. Br J
Cancer. 102:173–180. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ouzzani M, Hammady H, Fedorowicz Z and
Elmagarmid A: Rayyan-a web and mobile app for systematic reviews.
Syst Rev. 5(210)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Altman DG, McShane LM, Sauerbrei W and
Taube SE: Reporting recommendations for tumor marker prognostic
studies (REMARK): Explanation and elaboration. PLoS Med.
9(e1001216)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feng JQ, Xu ZY, Shi LJ, Wu L, Liu W and
Zhou ZT: Expression of cancer stem cell markers ALDH1 and Bmi1 in
oral erythroplakia and the risk of oral cancer. J Oral Pathol Med.
42:148–153. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Vicente JC, Rodrigo JP,
Rodriguez-Santamarta T, Lequerica-Fernandez P, Allonca E and
Garcia-Pedrero JM: Podoplanin expression in oral leukoplakia:
Tumorigenic role. Oral Oncol. 49:598–603. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu W, Wu L, Shen XM, Shi LJ, Zhang CP, Xu
LQ and Zhou ZT: Expression patterns of cancer stem cell markers
ALDH1 and CD133 correlate with a high risk of malignant
transformation of oral leukoplakia. Int J Cancer. 132:868–874.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Connected Researchers. SciCurve: Revealing
life science's curves, 2014. Available from: http://connectedresearchers.com/tag/scicurve/.
Accessed November 16, 2018.
|
|
17
|
Liu W, Wang YF, Zhou HW, Shi P, Zhou ZT
and Tang GY: Malignant transformation of oral leukoplakia: A
retrospective cohort study of 218 Chinese patients. BMC Cancer.
10(685)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taxy JB: Pathology of head and neck
neoplasms. UptoDate (online). Waltham, MA: UpToDate Inc. 2018
[cited 2018 10/25]. Available from: https://www.uptodate.com/contents/pathology-of-head-and-neck-neoplasms.
|
|
19
|
Rivera C: Opportunities for biomarkers
with potential clinical use in oral cancer. Medwave.
15(e6186)2015.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
20
|
Dunbar KB and Souza RF: Beyond dysplasia
grade: The role of biomarkers in stratifying risk. Gastrointest
Endosc Clin N Am. 27:447–459. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rebolj M, Helmerhorst T, Habbema D, Looman
C, Boer R, van Rosmalen J and van Ballegooijen M: Risk of cervical
cancer after completed post-treatment follow-up of cervical
intraepithelial neoplasia: Population based cohort study. BMJ.
345(e6855)2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
McCredie MR, Sharples KJ, Paul C, Baranyai
J, Medley G, Jones RW and Skegg DC: Natural history of cervical
neoplasia and risk of invasive cancer in women with cervical
intraepithelial neoplasia 3: A retrospective cohort study. Lancet
Oncol. 9:425–434. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang L, Lubpairee T, Laronde DM and Rosin
MP: Should severe epithelial dysplasia be treated? Oral Oncol.
60:125–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dissanayaka WL, Pitiyage G, Kumarasiri PV,
Liyanage RL, Dias KD and Tilakaratne WM: Clinical and
histopathologic parameters in survival of oral squamous cell
carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 113:518–525.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tsai WC, Kung PT, Wang YH, Huang KH and
Liu SA: Influence of time interval from diagnosis to treatment on
survival for oral cavity cancer: A nationwide cohort study. PLoS
One. 12(e0175148)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tomita H, Tanaka K, Tanaka T and Hara A:
Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget.
7:11018–11032. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li X, Wan L, Geng J, Wu CL and Bai X:
Aldehyde dehydrogenase 1A1 possesses stem-like properties and
predicts lung cancer patient outcome. J Thorac Oncol. 7:1235–1245.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang L, Ren Y, Yu X, Qian F, Bian BS, Xiao
HL, Wang WG, Xu SL, Yang J, Cui W, et al: ALDH1A1 defines invasive
cancer stem-like cells and predicts poor prognosis in patients with
esophageal squamous cell carcinoma. Mod Pathol. 27:775–783.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Morimoto K, Kim SJ, Tanei T, Shimazu K,
Tanji Y, Taguchi T, Tamaki Y, Terada N and Noguchi S: Stem cell
marker aldehyde dehydrogenase 1-positive breast cancers are
characterized by negative estrogen receptor, positive human
epidermal growth factor receptor type 2, and high Ki67 expression.
Cancer Sci. 100:1062–1068. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
The Human Protein Atlas. ALDH1A1: Protein
expression overview [cited 2019 accessed 11 January 2019].
Available from: https://www.proteinatlas.org/ENSG00000165092-ALDH1A1/tissue.
|
|
31
|
Barzegar Behrooz A, Syahir A and Ahmad S:
CD133: Beyond a cancer stem cell biomarker. J Drug Target.
27:257–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Singer D, Thamm K, Zhuang H, Karbanová J,
Gao Y, Walker JV, Jin H, Wu X, Coveney CR, Marangoni P, et al:
Prominin-1 controls stem cell activation by orchestrating ciliary
dynamics. EMBO J. 38(e99845)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Krishnan H, Rayes J, Miyashita T, Ishii G,
Retzbach EP, Sheehan SA, Takemoto A, Chang YW, Yoneda K, Asai J, et
al: Podoplanin: An emerging cancer biomarker and therapeutic
target. Cancer Sci. 109:1292–1299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cirligeriu L, Cimpean AM, Raica M and
Doros CI: Dual role of podoplanin in oral cancer development. In
Vivo. 28:341–347. 2014.PubMed/NCBI
|
|
35
|
Miyashita T, Higuchi Y, Kojima M, Ochiai A
and Ishii G: Single cell time-lapse analysis reveals that
podoplanin enhances cell survival and colony formation capacity of
squamous cell carcinoma cells. Sci Rep. 7(39971)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Saluja TS, Ali M, Mishra P, Kumar V and
Singh SK: Prognostic value of cancer stem cell markers in
potentially malignant disorders of oral mucosa: A meta-analysis.
Cancer Epidemiol Biomarkers Prev. 28:144–153. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Surendran S, Siddappa G, Mohan A, Hicks W
Jr, Jayaprakash V, Mimikos C, Mahri M, Almarzouki F, Morrell K,
Ravi R, et al: Cancer stem cell and its niche in malignant
progression of oral potentially malignant disorders. Oral Oncol.
75:140–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Koontongkaew S: The tumor microenvironment
contribution to development, growth, invasion and metastasis of
head and neck squamous cell carcinomas. J Cancer. 4:66–83.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Vargas-Ferreira F, Nedel F, Etges A, Gomes
AP, Furuse C and Tarquinio SB: Etiologic factors associated with
oral squamous cell carcinoma in non-smokers and non-alcoholic
drinkers: A brief approach. Braz Dent J. 23:586–590.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Faber J and Fonseca LM: How sample size
influences research outcomes. Dental Press J Orthod. 19:27–29.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Taylor JM, Ankerst DP and Andridge RR:
Validation of biomarker-based risk prediction models. Clin Cancer
Res. 14:5977–5983. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Goossens N, Nakagawa S, Sun X and Hoshida
Y: Cancer biomarker discovery and validation. Transl Cancer Res.
4:256–269. 2015.PubMed/NCBI View Article : Google Scholar
|