Introduction
Despite the developments in the chemo- and
radiotherapy, and treatment with biological agents of patients with
pulmonary metastases, surgery is still an important option in this
situation. Thomford et al (1), presented the criteria for pulmonary
metastasectomy, which included adequate cardiopulmonary reserves of
the patient, the control of the primary tumor site, no evidence of
metastatic disease elsewhere in the body and that the pulmonary
metastases being limited to one lung. However, these criteria have
been continuously extended and modified over time. Today, extended
pulmonary metastasectomy has become feasible in patients with
distant metastases such as a solitary liver metastasis or multiple
and bilateral pulmonary metastases. Therefore, it is time to
analyze the survival of patients undergoing extended pulmonary
metastasectomy.
Peritoneal dissemination originating from
gastrointestinal tract organs, pseudomyxoma peritonei, or ovarian
cancer has for long been considered as a lethal disease with poor
prognosis. Recently, however, new treatment methods have emerged,
and nowadays even cure can be achieved with cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy in these patients
(2). Consequently, we also need to
analyze the prognosis of patients with peritoneal dissemination
controlled by the treatments.
The purpose of this study was to analyze the current
data for overall survival after extended pulmonary metastasectomy,
including in patients with treated peritoneal dissemination at our
institution and to clarify whether above described prognostic
factors affect survival.
Patients and methods
We obtained institutional review board approval for
this study (approval no. 2019-015). The requirement for informed
patient consent was waived because of the retrospective nature of
the study.
The present study was a retrospective analysis of
the medical records of 80 patients who underwent complete resection
for lung metastases at our hospital between September 2007 and
February 2019. The record of each patient was reviewed for age,
sex, primary tumor, disease-free interval, number of pulmonary
nodules, side of pulmonary nodules, extrapulmonary metastases, the
maximum diameter of the pulmonary nodules, peritoneal dissemination
of the primary tumor and surgical procedure for metastasectomy. In
case multiple metastases were present, the diameter of the largest
nodule was recorded.
The eligibility criteria for this study were as
follows: i) the primary tumor was controlled; ii) if extrapulmonary
metastases (including peritoneal dissemination) existed, these had
been controlled by local treatment or such treatment was planned;
iii) the one to three months follow-up computed tomography (CT)
after the first assessment revealed no increase of pulmonary
metastatic disease and iv) pulmonary metastases could be resected
completely.
We excluded patients whose performance status was
over 2, or whose forced expiratory volume in one second during the
preoperative pulmonary function test was lower than 1,000 ml. We
included any types of primary tumors and did not exclude patients
with multiple or bilateral pulmonary metastases. All patients with
peritoneal dissemination received cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy prior to pulmonary
resection of metastases. We judged extrapulmonary metastases as
‘controlled’ when one to six months follow-up imaging examinations
revealed no evidence of relapse after local treatment. If a more
effective treatment modality than surgery was available, the
patients received the optimal treatment.
Our standard surgical method includes the complete
resection of nodules after palpation, while mediastinal lymph node
dissection is not routinely performed. The surgical approach is
performed by thoracoscopically assisted or lateral thoracotomy. We
usually attempt to remove pulmonary nodules through a wedge
resection whereas hilar nodules require lobectomy or pneumonectomy.
We defined ‘complete resection’ as no obvious residual cancer on
the resection stump of the lung. Patients are followed-up for up to
10 years after metastasectomy.
Statistical analysis was performed using SPSS
Statistics for Windows, v22 (SPSS Inc.). The Kaplan-Meier method
was used to determine overall, relapse-free, and median survival
from the time of metastasectomy to the last follow-up, death or
relapse. The log-rank test was used to compare survival differences
for each variable. P<0.05 was considered to indicate a
statistically significant difference.
Results
During the study period, 80 patients underwent
pulmonary resection for lung metastases in our hospital. We
extracted the data of these 80 patients. Their clinical, tumor and
surgical characteristics are shown in Table I. The mean age at the time of
thoracic surgery was 63.1 years, range 29-84 years. There were 46
male and 34 female patients. The mean period between primary tumor
surgery and pulmonary resection was 39.7 months. The primary tumor
was colorectal cancer in 43 patients, pseudomyxoma peritonei in 9
patients, head and neck cancer in 8 patients and other cancers in
20 patients. Twenty-two patients were treated for peritoneal
dissemination and were recurrence-free based on computed tomography
or magnetic resonance imaging during a follow-up of at least eight
months after the intervention. The primary tumor of peritoneal
dissemination was colorectal cancer in 11 patients, pseudomyxoma
peritonei in 9 patients and uterine leiomyosarcoma in 2 patients.
Fifty-five patients received wedge resection, 13 patients received
segmentectomy, 10 patients received lobectomy, and 2 patients
received pneumonectomy. Post-operative complications occurred in 13
patients (16.3%), which consisted of pneumonia in 3 patients,
empyema in 3 patients, intrathoracic bleeding in 1 patient,
gastrointestinal bleeding in 1 patient, respiratory failure
requiring oxygen inhalation in 1 patient, heart failure in 1
patient, ileus in 1 patient, deterioration in liver function in 1
patient and recurrent nerve paralysis in 1 patient.
 | Table ICharacteristics of 80 patients
undergoing pulmonary resection for lung metastases. |
Table I
Characteristics of 80 patients
undergoing pulmonary resection for lung metastases.
| | Number of
patients | |
|---|
| Characteristics | n=80 | % |
|---|
| Age (years) | 63.1 (29-84) | - |
| Sex | | |
|
Male | 46 | 58 |
|
Female | 34 | 42 |
| Primary tumor | | |
|
Colorectal | 43 | 54 |
|
Pseudomyxoma
peritonei | 9 | 11 |
|
Head and
neck cancer | 8 | 10 |
|
Other
cancers | 20 | 25 |
| Disease-free interval
(Mo) | 26.2 (0-156) | - |
| Pulmonary metastases
(No) | 1.7 (1-13) | |
|
1 | 58 | 73 |
|
2 | 11 | 14 |
|
≥3 | 11 | 14 |
| Side | | |
|
Unilateral | 66 | 82 |
|
Bilateral | 14 | 18 |
| Extrapulmonary
metastasis | | |
|
Yes | 13 | 16 |
|
No | 67 | 84 |
| Maximum tumor
size | 20.8 (7-146) | |
|
≤20 mm | 55 | 69 |
|
>20
mm | 25 | 31 |
| Peritoneal
dissemination | | |
|
Yes | 22 | 28 |
|
No | 58 | 72 |
| Surgical
procedure | | |
|
Wedge
resection | 55 | 69 |
|
Segmentectomy | 13 | 16 |
|
Lobectomy | 10 | 13 |
|
Pneumonectomy | 2 | 3 |
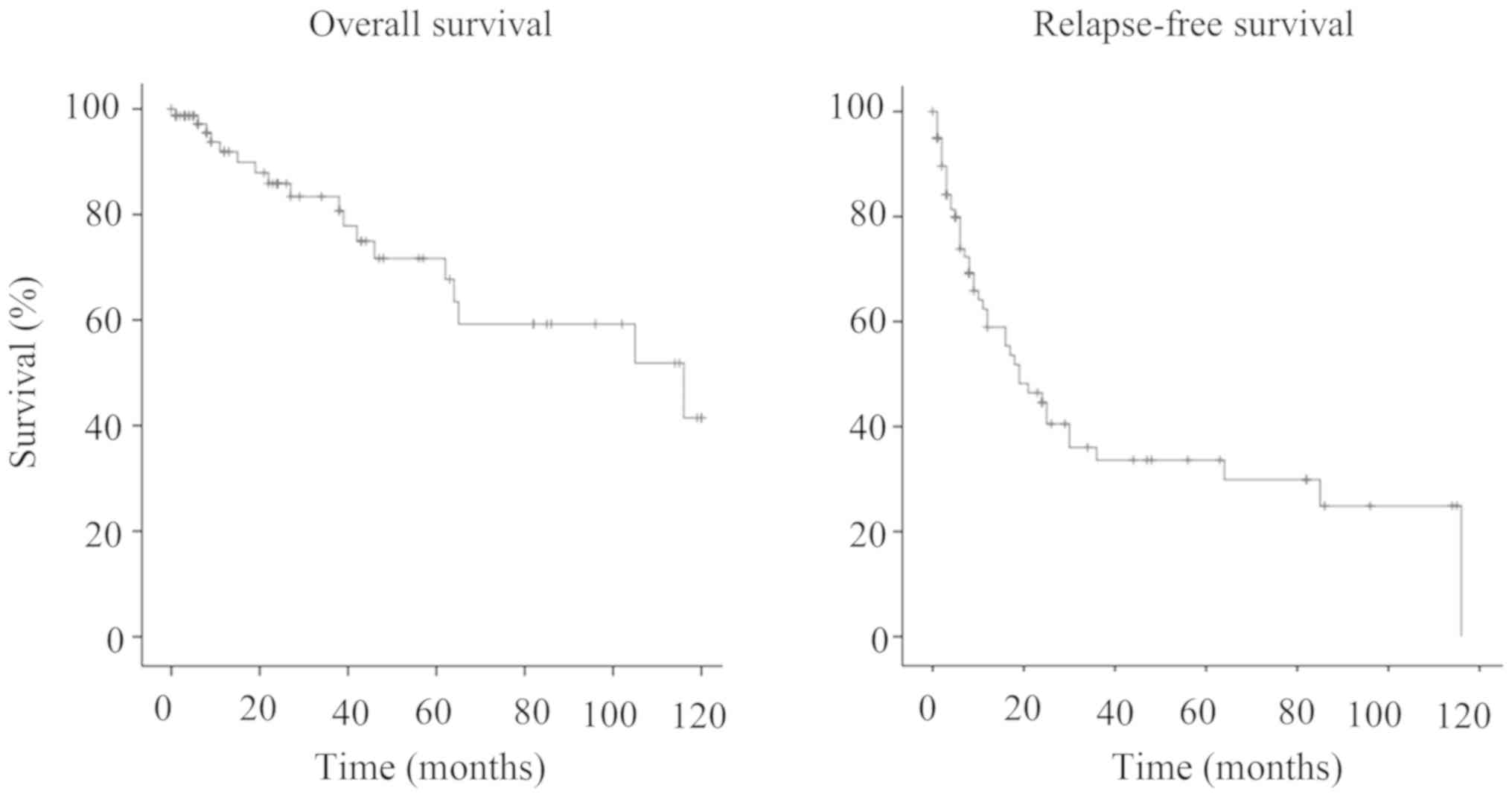
The mean follow-up after thoracic surgery was 36
months, range 1-120 months. The 5-year and 10-year survival rates
after metastasectomy were 71.7 and 41.5%, respectively (Fig. 1). Relapse-free survival at five
years after metastasectomy was 33.6% (Fig. 1) and median survival time 84.7
months.
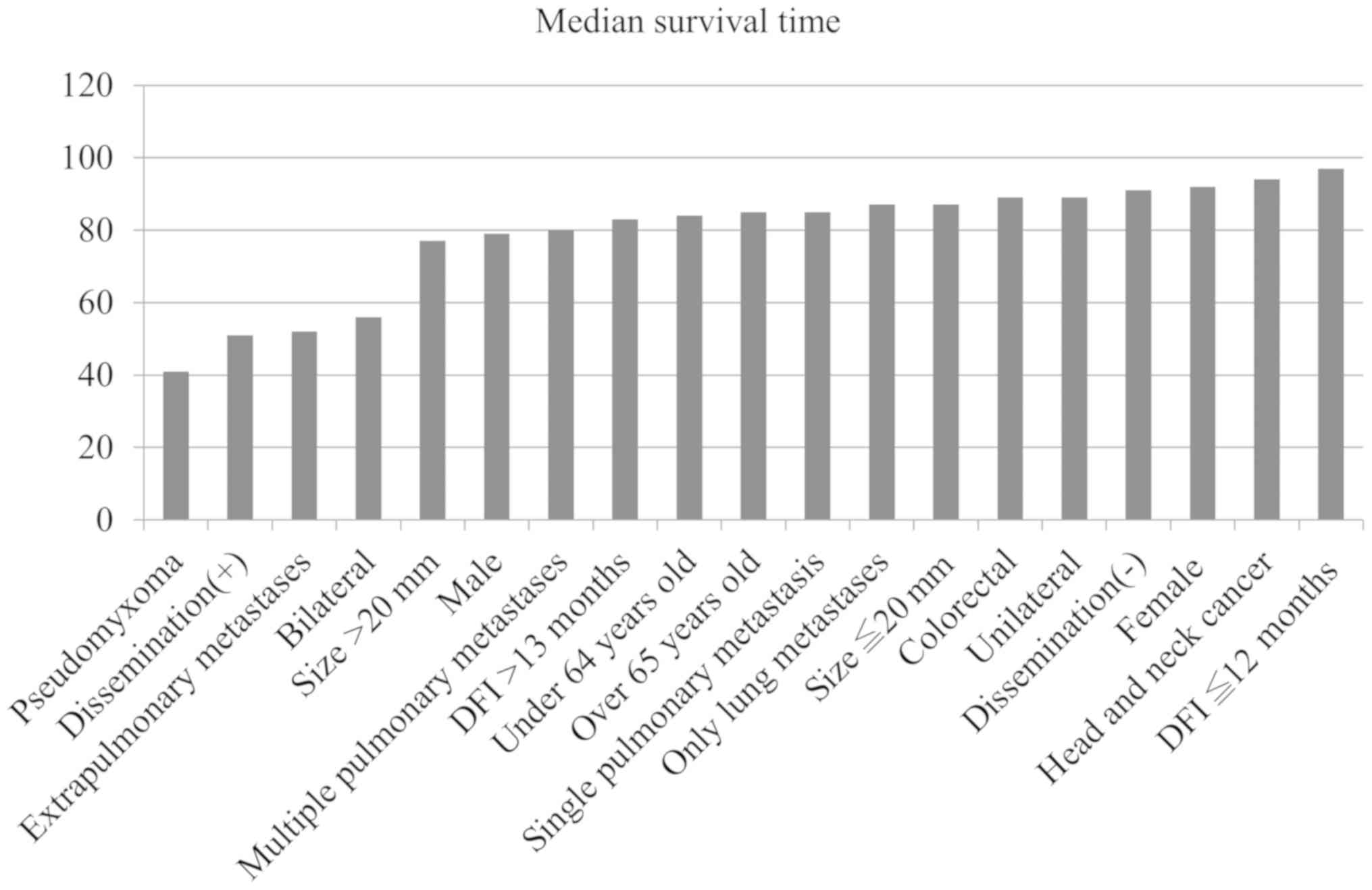
We compared the median survival times with the
clinical factors of age, gender, primary tumor, disease-free
interval, number of pulmonary metastases, laterality of the
metastases, maximum tumor size, extrapulmonary metastases, and
peritoneal dissemination. In the univariate analysis, none of these
factors showed a significant association with the median survival
time (Table II). However,
bilateral metastases, extrapulmonary metastases, treated peritoneal
dissemination, and histology of pseudomyxoma peritonei tended to be
associated with lower survival (Fig.
2).
 | Table IIMedian survival time in relation to
clinical factors in 80 patients undergoing pulmonary resection for
lung metastases. |
Table II
Median survival time in relation to
clinical factors in 80 patients undergoing pulmonary resection for
lung metastases.
| Variables | N | Mean survival time
(months) | P-value |
|---|
| Age (years) | | | 0.700 |
|
<65 | 38 | 84.7 | |
|
≥65 | 42 | 84.1 | |
| Sex | | | 0.379 |
|
Male | 46 | 79.1 | |
|
Female | 34 | 92.5 | |
| Primary tumor | | | |
|
Colorectal | 43 | 81.1 | 0.686 |
|
Pseudomyxoma
peritonei | 9 | 41.1 | 0.296 |
|
Head and
neck cancer | 8 | 94.4 | 0.277 |
| Disease-free interval
(Months) | | | 0.821 |
|
0-12 | 30 | 96.6 | |
|
13- | 48 | 83.0 | |
| Pulmonary metastases
(No) | | | 0.891 |
|
1 | 58 | 84.6 | |
|
≥2 | 22 | 80.0 | |
| Laterality of the
metastases | | | 0.124 |
|
Unilateral | 66 | 88.9 | |
|
Bilateral | 14 | 56.4 | |
| Maximum tumor
size | | | 0.513 |
|
≤20 mm | 55 | 87.0 | |
|
>20
mm | 25 | 77.1 | |
| Extrapulmonary
metastases | | | 0.261 |
|
Yes | 13 | 52.0 | |
|
No | 67 | 87.3 | |
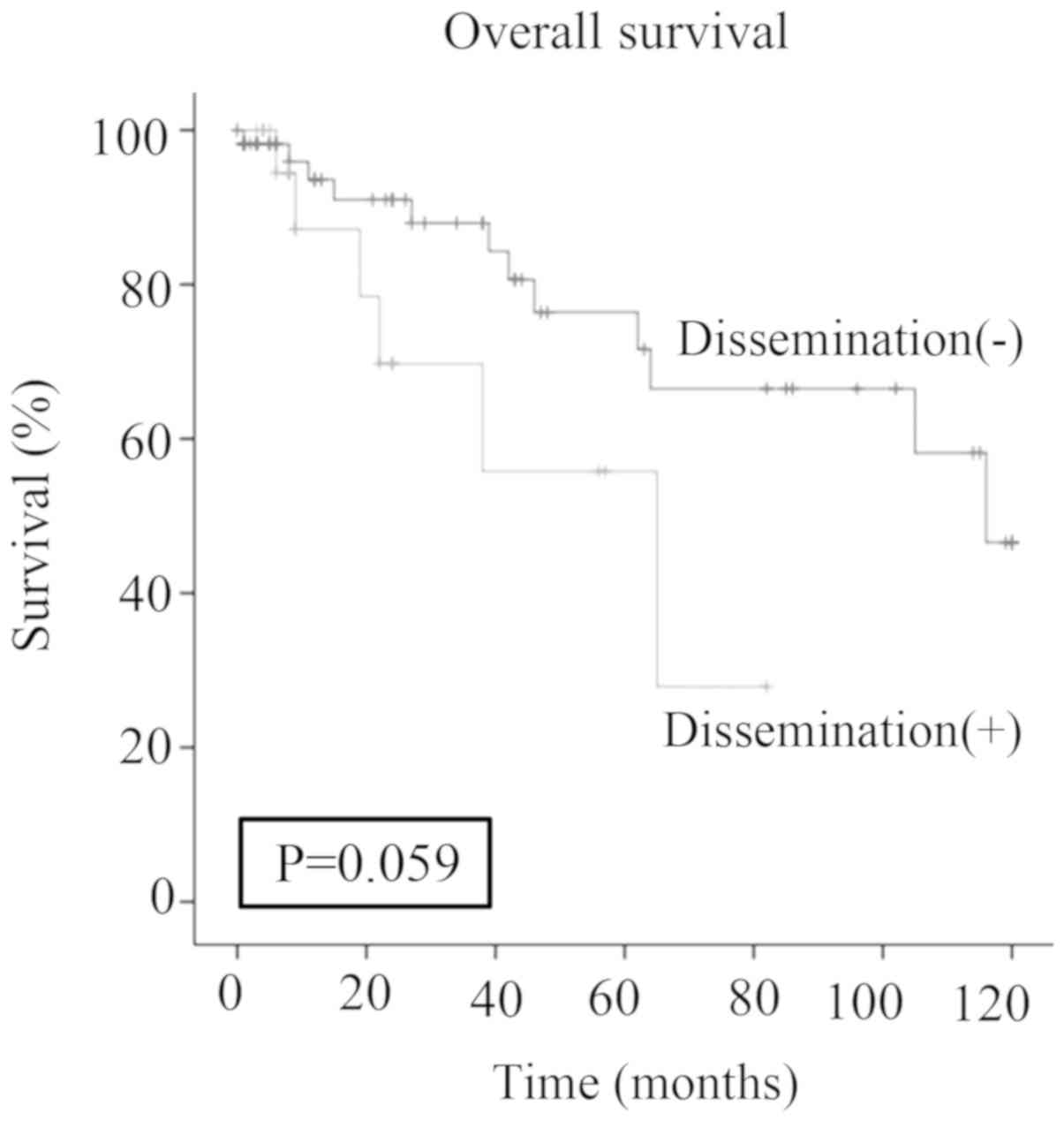
| Peritoneal
dissemination | | | 0.059 |
|
Yes | 22 | 50.9 | |
|
No | 58 | 90.9 | |
There was no significant difference (P=0.059) in the
5-year survival of patients with peritoneal dissemination (55.8%)
and patients without history of peritoneal dissemination (76.4%)
(Fig. 3).
Discussion
The indication for pulmonary metastasectomy has been
based on Thomford's criteria (1)
for a long time, but recently, it was increasingly extended to
patients with multiple lesions, bilateral lesions, and solitary
liver metastasis. Furthermore, the development of cytoreductive
surgery and hyperthermic intraperitoneal chemotherapy appears to
contribute to better prospects of cure for patients with peritoneal
dissemination (2). In this study,
we included survival in patients who had been or were foreseen to
be treated for peritoneal dissemination.
At our institution, the extended criteria for
pulmonary resection include multiple lesions, extrapulmonary
metastases, bilateral metastases, and peritoneal dissemination. A
previous article reported that multiple pulmonary metastases were
not a prognostic factor for overall survival (3) whereas another article reported them to
be a significant factor (4). In our
study, the prognosis of multiple pulmonary metastases was no
different from a solitary metastasis. Although patients with
bilateral metastases, extrapulmonary metastases, and peritoneal
dissemination tended to obtain less benefit from surgery, the
differences between their mean survival time and those of other
patients were not statistically significant. Several studies have
investigated the difference between unilateral and bilateral
metastases in terms of survival. Only two articles reported that
unilateral pulmonary metastases were a positive prognostic factor
for overall survival (3,5). There have been several reports about
metastasectomy in patients with extrapulmonary metastases. McAfee
et al (6) and Saito et
al (7), both showed that the
presence of resectable or controllable extrapulmonary metastases
before or at the time of thoracotomy was not associated with
decreased survival. These results suggest that metastasectomy might
be beneficial when extrapulmonary metastases are controllable, for
example, in the case of a solitary liver metastasis.
The original aspect of our study was to include
patients with previously treated peritoneal dissemination. There
have been no reports on pulmonary metastasectomy in these patients.
Peritoneal dissemination originating from the gastrointestinal
organs, pseudomyxoma peritonei or ovarian cancer has often been
considered as a systemic and lethal disease with poor prognosis. As
a consequence, supportive care and systemic chemotherapy were the
main treatment strategy for long, even though systemic chemotherapy
is not sufficient to control peritoneal dissemination. However,
more recently, specialized hospitals have reported that even cure
could be achieved by the combination of cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy, with 5-year survival
rates of 45% in colorectal cancer, 10.7% in gastric cancer, 58% in
ovarian cancer and a 10-year survival rate of 63% in pseudomyxoma
peritonei (2) after cytoreductive
surgery. Furthermore, we showed for the first time a 5-year
survival rate of 55.8% and a mean survival time of 50.9 months
after pulmonary metastasectomy in these patients. Considering that
there was long period between primary tumor surgery and pulmonary
metastasectomy (39.7 months) in our study, our survival rate after
pulmonary metastasectomy might be sufficiently acceptable. With
increased survival in patients with peritoneal dissemination, the
opportunity of pulmonary metastasectomy will also increase.
With regard to the different primary tumors,
colorectal and head and neck cancer achieved a mean survival time
of 81.1 and 94.4 months, respectively, whereas pseudomyxoma
peritonei showed a poor prognosis. Pseudomyxoma peritonei is
divided into a low-grade and high-grade form. The 5-year overall
survival rate was found to be 63% for low grade but only 23% for
high grade disease after surgical resection (8,9).
Metastases tend to occur in high-grade disease, which might
contribute to the poor prognosis of pulmonary metastases of
pseudomyxoma peritonei in our study.
The overall 5-year and 10-year survival rate in our
study was 71.7 and 41.5%, respectively, and the mean survival time
was 84.7 months. Thomford et al reported a 5-year survival
rate of 30.3%. Another more recent report in 2012 showed a 5-year
survival rate of 43.7% and a 10-year survival rate of about 30%
(4). Despite our extended criteria
for pulmonary resection of metastases, we achieved better survival
rates. The reason for the better survival might be threefold.
First, the new modes of non-surgical treatment such as systemic
chemotherapy, hyperthermic intraperitoneal chemotherapy, biological
agents and radiotherapy might prolong the overall survival in
patients with advanced cancer. Second, we excluded all cases with
increasing pulmonary metastases during one to three months. We
hypothesized that when metastasic disease increased in the short
term, systemic chemotherapy was likely to be more effective than
local treatment such as surgery. Third, we extracted data only on
cases with complete resection of pulmonary metastases. Predictors
of good prognosis usually include long disease-free interval, the
histology of the primary tumor, a small number of metastases,
unilateral metastases, and complete resection. Among these, the
most consistent predictor is complete resection (10).
The main limitation of this study is its
retrospective nature. The final decision to select a patient for
surgery may vary among surgeons. Therefore, our study does not
reflect the entire population of patients with pulmonary
metastases. A second limitation is the small number of cases. This
might result in insufficient power to detect significant
differences, even in cases that potentially might show statistical
differences. The final limitation is the heterogeneity of the
histology of the primary tumor. Tumor characteristics such as
growth speed, metastatic potential, and anticancer drug sensitivity
differ depending on the histology of the primary lesion. We also
recognize that non-surgical treatment such as chemotherapy,
thermotherapy, biological agents or radiotherapy may substantially
affect the prognosis of patients in our study. Based on this pilot
study, we plan to perform a prospectively designed study with
sufficiently large patient numbers and segregation by
histology.
In conclusion, our extended pulmonary metastasectomy
achieved 5-year and 10-year survival rates of 71.7 and 41.5%,
respectively. Extended criteria in our study including multiple
lung lesions, bilateral metastases, controlled extrapulmonary
metastases and controlled peritoneal dissemination might be
acceptable for pulmonary metastasectomy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK has made substantial contributions to conception,
design, acquisition of data, analysis and interpretation of data,
and been involved in drafting the manuscript and revising it
critically for important intellectual content. JH, YO, KO, RK, KH,
TS and MY made substantial contributions to analysis and
interpretation of data. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
Institutional review board approval was obtained for
the current study (approval no. 2019-015). The requirement for
informed patient consent was waived because of the retrospective
nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests.
References
|
1
|
Thomford NR, Woolner LB and Clagett OT:
The surgical treatment of metastatic tumors in the lungs. J Thorac
Cardiovasc Surg. 49:357–363. 1965.PubMed/NCBI
|
|
2
|
Canbay E, Torun BC, Torun ES and Yonemura
Y: Evolution of management in peritoneal surface malignancies. Ulus
Cerrahi Derg. 32:203–207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Younes RN, Abrao F and Gross J: Pulmonary
metastasectomy for colorectal cancer: Long-Term survival and
prognostic factors. Int J Surg. 11:244–248. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Younes RN, Fares AL and Gross JL:
Pulmonary metastasectomy: A multivariate analysis of 440 patients
undergoing complete resection. Interact Cardiovasc Thorac Surg.
14:156–161. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen F, Hanaoka N, Sato K, Fujinaga T,
Sonobe M, Shoji T, Sakai H, Miyahara R, Bando T, Okubo K, et al:
Prognostic factors of pulmonary metastasectomy for colorectal
carcinomas. World J Surg. 33:505–511. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McAfee MK, Allen MS, Trastek VF, Ilstrup
DM, Deschamps C and Pairolero PC: Colorectal lung metastases:
Results of surgical excision. Ann Thorac Surg. 53:780–785.
1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Saito Y, Omiya H, Kohno K, Kobayashi T,
Itoi K, Teramachi M, Sasaki M, Suzuki H, Takao H and Nakade M:
Pulmonary metastasectomy for 165 patients with colorectal
carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg.
124:1007–1013. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Carr NJ, Finch J, Ilesley IC,
Chandrakumaran K, Mohamed F, Mirnezami A, Cecil T and Moran B:
Pathology and prognosis in pseudomyxoma peritonei: A review of 274
cases. J Clin Pathol. 65:919–923. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawaguchi Y, Hanaoka J, Ohshio Y, Okamoto
K, Kaku R, Hayashi K, Shiratori T and Yoden M: Patient survival
after surgical management in intrathoracic pseudomyxoma peritonei.
Ann Surg Oncol. 26:238–243. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pastorino U, Buyse M, Friedel G, Ginsberg
RJ, Girard P, Goldstraw P, Johnston M, McCormack P, Pass H and
Putnam JB Jr: International Registry of Lung Metastases. Long-Term
results of lung metastasectomy: Prognostic analyses based on 5206
cases. J Thorac Cardiovasc Surg. 113:37–49. 1997.PubMed/NCBI View Article : Google Scholar
|