Introduction
Malignant melanoma (MM) is a cutaneous and/or
extracutaneous tumor that arises from the embryological remnants of
neural crest cells/melanocytes, melanoma can happen in eyes and
internal organs but rare (1). The
number of recorded new cases in 2018 was 2,87,723, whereas the
number of deaths in 2018 in both sexes and across all ages was
60,712 (Globocan 2018, International Agency for Research on Cancer,
World Health Organization; https://gco.iarc.fr/today/data/factsheets/cancers/16-Melanoma-of-skin-fact-sheet.pdf).
According to the National Cancer Institute, the incidence of
melanoma has increased sharply over the last 30 years, and ~7,230
deaths were caused by melanoma in 2019(2). Cancer Research UK estimated that the
incidence rate of melanoma is 3.1/10,000, with a mortality rate of
0.8/10,000 worldwide (3). Despite
the advances in the treatment of metastatic melanoma, the 5-year
survival rate of melanoma with distant metastasis remains low at
~16% (4,5). Due to the high propensity for
metastasis and poor prognosis of MM, early diagnosis and timely
treatment are crucial. Amelanotic MM (AMM) is a rare clinical type
of melanoma that comprises 2-8% of all MM cases (6,7). AMM
is a clinically diverse entity and its clinical characteristics are
non-specific; hence, the diagnosis of AMM is difficult, and it can
easily be misdiagnosed as other cutaneous diseases and/or tumors,
such as benign ulcerations or squamous cell carcinoma (8,9). The
aim of the present study was to report a rare case of primary acral
AMM that was initially misdiagnosed as cutaneous squamous cell
carcinoma.
Case report
On 1 January 2019, a 61-year-old man presented to
the Dermatology Clinic at the Affiliated Hospital of Guangdong
Medical University with a painful pinkish tumor on the sole of his
left foot that had been enlarging over the last 3 months.
Approximately 1 year before the patient presented at the
Dermatology Clinic of the Affiliated Hospital of Guangdong Medical
University, he observed an ulcerated mass with exudate in the sole
of the left foot, sized ~2x3 cm, that developed after an injury.
The ulcerated wound was treated with local povidone iodine and oral
cefuroxime axetil for 2 months at a local clinic, but without
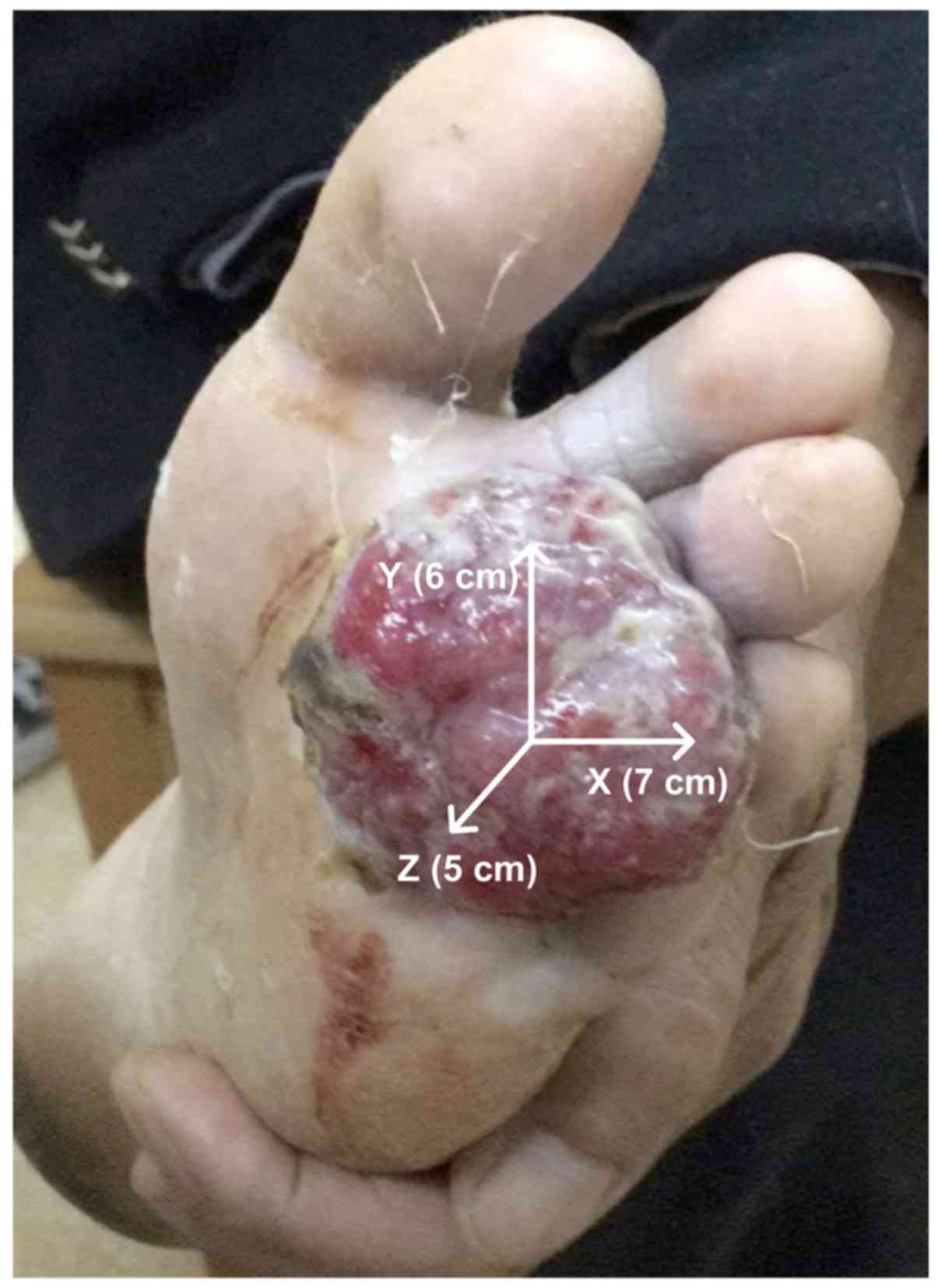
obvious improvement. After 3 months, the mass in the left foot
gradually increased in size to 7x6x5 cm (Fig. 1). Macroscopically, the mass was
cauliflower-like, with a pinkish color and no pigmentation. The
mass appeared to be locally infiltrative with poorly defined
boundaries, and was adherent to the subcutaneous tissue with poor
mobility (Fig. 1). The findings of
the chest X-ray, electrocardiogram and abdominal B-ultrasound were
normal. A biopsy was performed in the previous hospital and the
lesion was originally diagnosed as squamous cell carcinoma. The
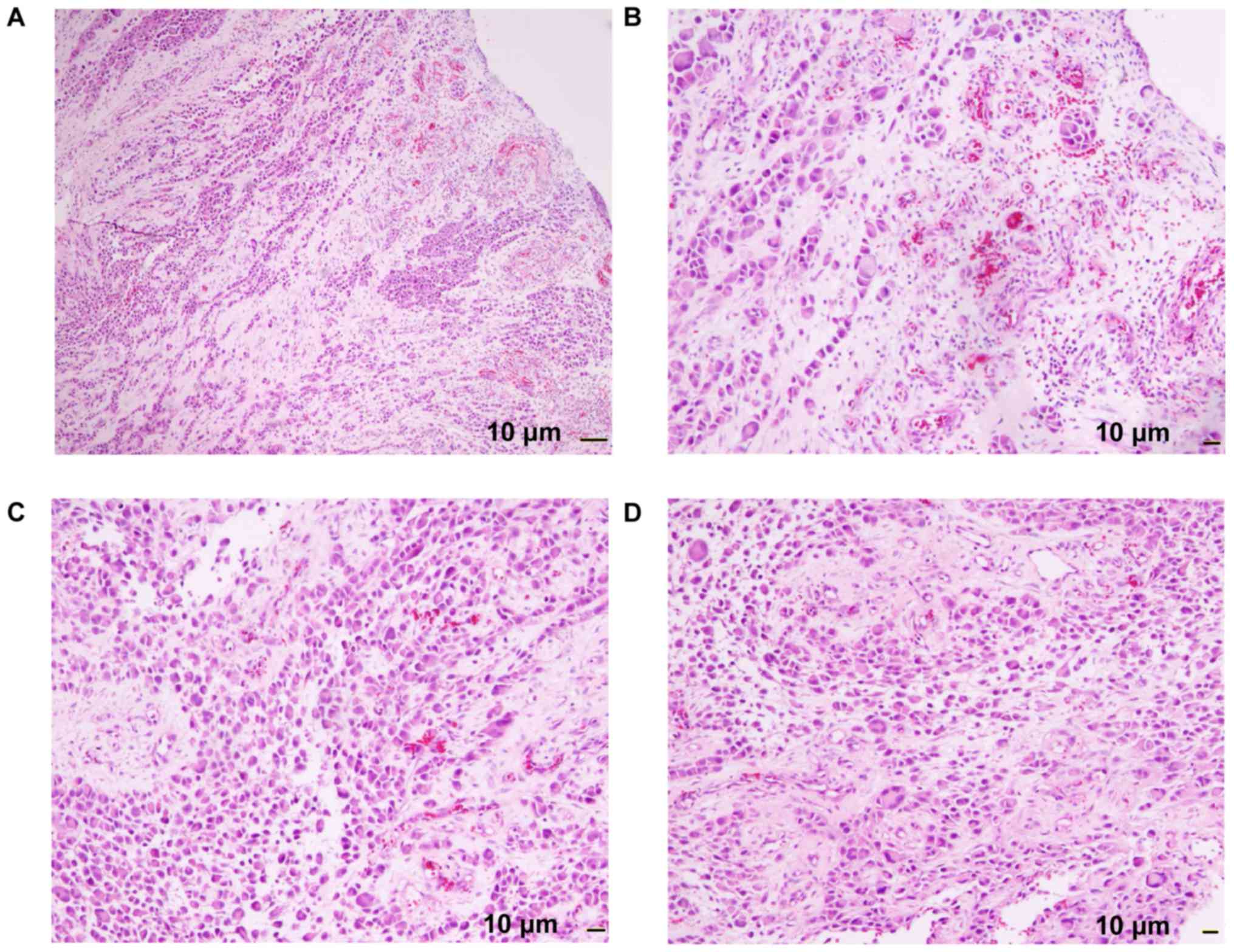
mass was surgically resected, and histopathological examination
revealed loss of epidermis with ulcer formation, abundant tumor
cells throughout the whole thickness of the dermis (characterized
by deeply stained nuclei, reddish cytoplasm and visible
multinucleated giant cells), heterogeneous nuclear division,
partial formation of nests and bundled distribution, and lack of
pigment particles (Fig. 2). The
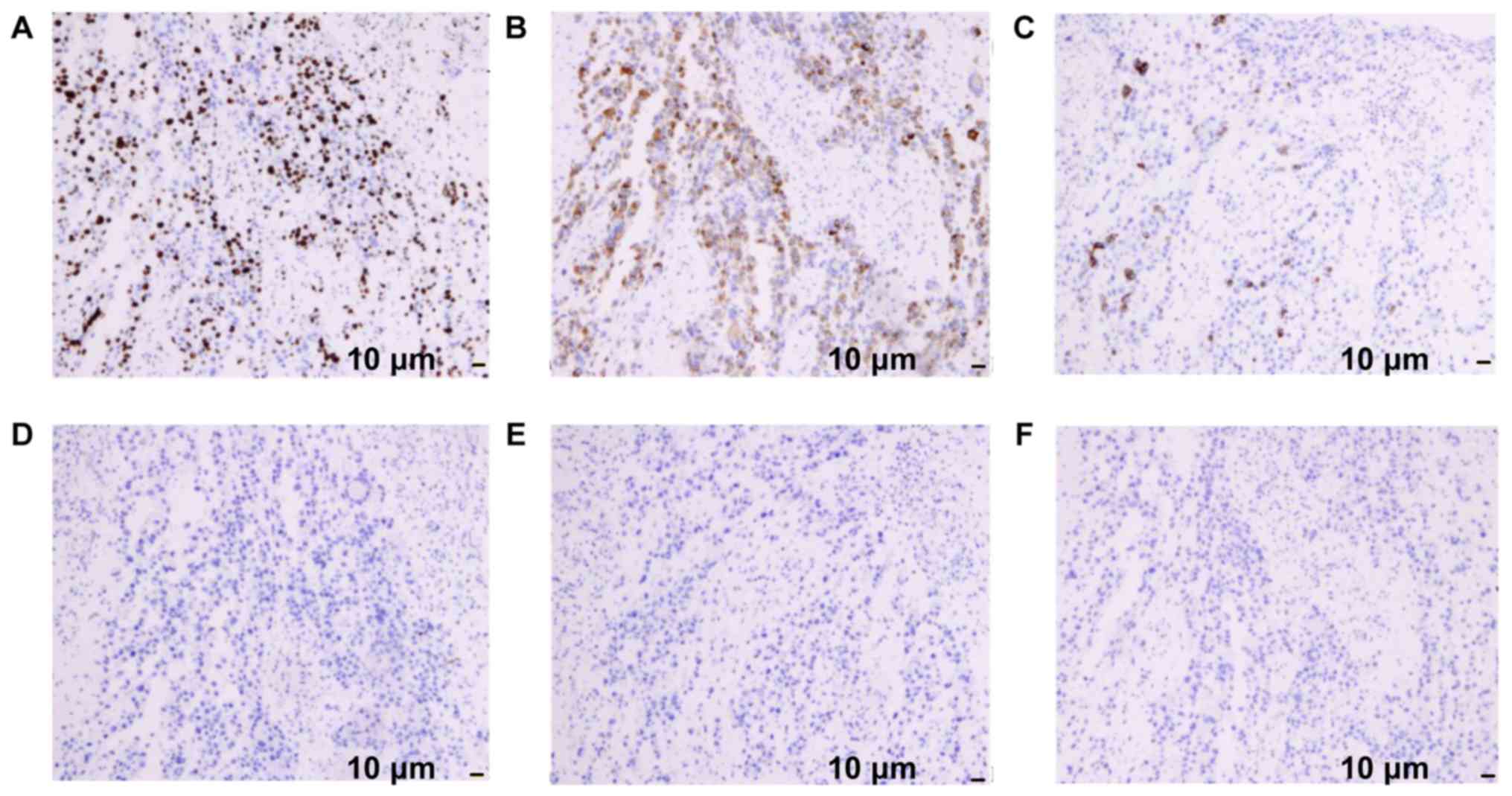
results of immunohistochemical examination were as follows: Ki67
(+++), Melan-A (+++), human melanoma black (HMB)45 (+), CD20 (-),
cytokeratin (CK)7 (-) and CK5/6 (-) (Fig. 3). The evidence mentioned above was
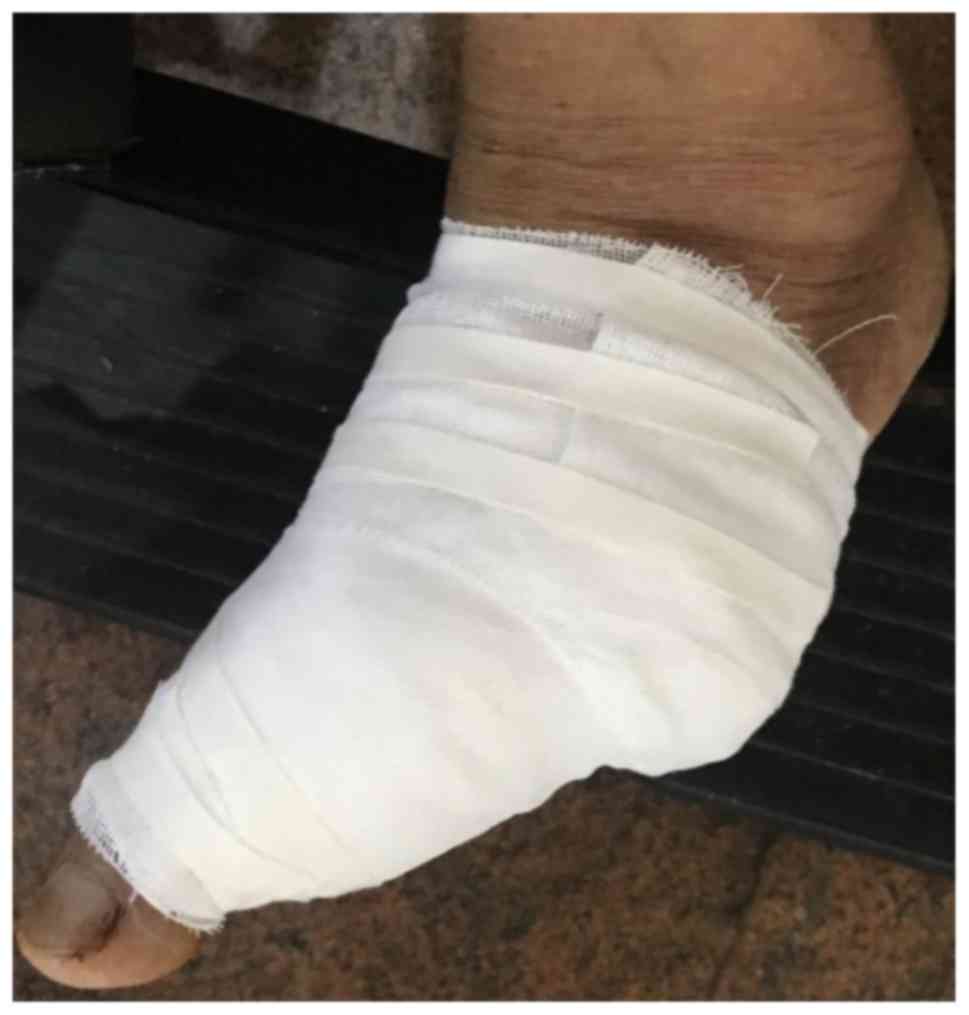
consistent with the diagnosis of AMM. The patient was subjected to
partial amputation of the left foot and plastic reconstructive
surgery on January 4, 2019, and the pathological examination of the
surgical specimen further confirmed the diagnosis of AMM (Fig. 4). The patient is currently followed
up and awaiting positron emission tomography-computed tomography
(PET-CT) examination to determine the presence of metastatic
disease. The last follow-up was on January 23, 2019.
Discussion
MM is a highly malignant tumor, accounting for
6.8-20% (third in incidence) of cutaneous malignant tumors, that
mostly occurs in the skin, although rare cases of non-cutaneous
melanoma may be encountered in the eyes and internal organs
(10). Primary acral AMM is an
aggressive rare neoplasm, with only few cases reported to date. AMM
does not produce melanin, therefore it may easily be misdiagnosed
as benign ulcer or/and squamous cell carcinoma (8,9). When
the patient was first referred to our hospital, he had stage T4b
disease. A PET-CT scan was suggested; however, there were no
readily identifiable metastases around the MM lesions, and the
patient refused. Without a PET-CT scan, the presence or absence of
visceral metastases could not be confirmed. The etiology of AMM
remains unclear, although it was previously hypothesized that
decreased tyrosinase activity and/or melanin transport disorders
may constitute possible reasons (11). AMM has been reported in the skin and
the mucosa of the digestive and/or genitourinary tracts (1,10). AMM
is a clinically diverse entity without specific characteristics;
early lesions are atypical and usually appear as pinkish papules,
either as a single lesion or in clusters (10,12).
Invasive growth during the later stages may manifest as red plaques
with granulomatous nodules or ulcers; therefore, it is important to
be aware of the diagnostic difficulties of primary acral AMM. In
the present case, the diagnosis of AMM was confirmed by
histopathology and immunohistochemistry. The immunohistochemistry
staining may help diagnose AMM using common immunological markers,
including HMB-45, Melan-A, S-100 and Ki-67(13). Ohnishi et al (13) have reported that Melan-A is the most
precise marker in terms of sensitivity and specificity; in the
present case, the immunohistochemical results revealed that the
tumor was positive for HMB-45, Melan-A and Ki-67, while it was
negative for CD20, CK7 and CK5/6.
The characteristics of AMM occurring in the limbs
and body differ from those of other tumors, such as squamous cell
carcinoma (13), basal cell
carcinoma and lymphoma. AMMs are characterized by a higher
proportion of nodular and acral lentiginous melanoma subtypes
compared with pigmented melanomas. AMMs are also characterized by a
greater Breslow thickness, higher mitotic rate, more frequent
ulceration, higher tumor stage at diagnosis, and lower survival
rates compared with pigmented melanomas (14). Early surgical resection is the first
choice of curative treatment and chemotherapy is the most commonly
used palliative treatment. Although there have been advances in MM
immunotherapy for patients with late-stage disease and metastasis,
such as PD-1/CTLA-4 antibodies and IL-2 targeted therapy, due to
the prognosis, early detection and treatment remain crucial for
prolonging survival.
Acknowledgements
The authors would like to thank Dr Wu Wei for
immunohistochemical examination and pathological diagnosis.
Funding
The present study was supported by Natural science
foundation of Guangdong Province of China (grant nos.
2016A030313682 and 2020A1515010281).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JZ and RC were responsible for writing the
manuscript. HY, JL and FZ performed data collection. JZ and JL
performed analysis. JZ was responsible for arranging the figures.
JS and RC were responsible for critically reviewing and editing the
manuscript. JZ, JS and RC were responsible for case design. All the
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent prior
to treatment.
Patient consent for publication
The patient provided written informed consent to the
publication of the case details and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garg R and Gupta N: Immunohistochemistry:
Sole tool in diagnosing a rare case of primary vaginal amelanotic
melanoma. Obstet Gynecol Sci. 61:698–701. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
American Cancer Society: Cancer Facts
& Figures 2019. Am Cancer Society 2019.
|
|
3
|
Cancer Research UK: Melanoma: Statistics
and Outlook, 2019. https://about-cancer.cancerresearchuk.org/about-cancer/melanoma/survival?_ga=2.246444610.753256537.1598053022-1991053779.1597413295%20%E2%80%91professional/cancer%E2%80%91%20statistics/statistics%E2%80%91by%E2%80%91cancer%E2%80%91type/melanoma%E2%80%91skin%E2%80%91cancer.
Accessed January 16, 2019.
|
|
4
|
Howlader N, Noone AM, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al:
SEER cancer statistics review, 1975-2010. National Cancer Institute
12: 2013.
|
|
5
|
Weinstein D, Leininger J, Hamby C and
Safai B: Diagnostic and prognostic biomarkers in melanoma. J Clin
Aesthet Dermatol. 7:13–24. 2014.PubMed/NCBI
|
|
6
|
Bono A, Maurichi A, Moglia D, Camerini T,
Tragni G, Lualdi M and Bartoli C: Clinical and dermatoscopic
diagnosis of early amelanotic melanoma. Melanoma Res. 11:491–494.
2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pizzichetta MA, Talamini R, Stanganelli I,
Puddu P, Bono R, Argenziano G, Veronesi A, Trevisan G, Rabinovitz H
and Soyer HP: Amelanotic/hypomelanotic melanoma: Clinical and
dermoscopic features. Br J Dermatol. 150:1117–1124. 2015.
|
|
8
|
Yeşil S, Demir T, Akinci B, Pabuccuoglu U,
Ilknur T and Saklamaz A: Amelanotic melanoma misdiagnosed as a
diabetic foot ulcer. J Diabetes Complications. 21:335–337.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hakamifard A, Mohaghegh F, Shabib S,
Larki-Harchegani A and Samani RE: The heel amelanotic melanoma, a
rare subtype of skin cancer misdiagnosed as foot ulcer: A case
report. Int Wound J. 17:819–822. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kobayashi J, Fujimoto D, Murakami M,
Hirono Y and Goi T: A report of amelanotic malignant melanoma of
the esophagus diagnosed appropriately with novel markers: A case
report. Oncol Lett. 15:9087–9092. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park HC, Kang HS and Kim JS: An amelanotic
malignant melanoma of the lip: Unusual shape and atypical location.
Cutis. 92:250–252. 2013.PubMed/NCBI
|
|
12
|
Srivastava P, Rath S, Hadi R and Husain N:
Primary amelanotic malignant melanoma of cervix masquerading as
squamous cell carcinoma presenting with extensive metastases. BMJ
Case Rep. 2018(bcr2018224723)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ohnishi Y, Watanabe M, Fujii T, Sunada N,
Yoshimoto H, Kubo H, Wato M and Kakudo K: A rare case of amelanotic
malignant melanoma in the oral region: Clinical investigation and
immunohistochemical study. Oncol Lett. 10:3761–3764.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gong HZ, Zheng HY and Li J: Amelanotic
melanoma. Melanoma Res. 29:221–230. 2019.PubMed/NCBI View Article : Google Scholar
|