Introduction
Regorafenib is an oral a multi-target tyrosine
kinase inhibitor blocking several protein kinases and targeting
tumor angiogenesis, as well as the oncogenic kinases KIT, RET and
B-RAF (1). The antitumor effect of
regorafenib has been demonstrated in colorectal cancer,
gastrointestinal stromal tumors (GIST) and hepatocellular cancer
(2-4).
Since imatinib was one of the first tyrosine kinase inhibitors to
be approved for the treatment of GIST in 2002, the therapeutic
landscape has changed markedly. The efficacy of tyrosine kinase
inhibitors has been demonstrated for a number of other diseases,
but several common side effects have also been reported, including
hypertension, palmar-plantar erythrodysesthesia syndrome, diarrhea
and fatigue (5).
Hepatic toxicity (HT) has been observed in patients
under treatment with various tyrosine kinase inhibitors. The
incidence of severe HT was estimated to be 5% in a systematic
review of patients treated with various tyrosine kinase inhibitors
(3,6-9).
In a pivotal phase III trial of regorafenib in patients with GIST
(n=132), apart from 1 case of drug-related fatal hepatic failure,
no HT or biliary toxicity were reported (2); however, our data indicate that almost
one-quarter of patients treated with regorafenib experience
laboratory and/or clinical signs of HT (2).
The incidence of HT among GIST patients treated with
regorafenib may be underreported. The aim of the present study was
to conduct a retrospective analysis of a real-world cohort, treated
at three German tertiary hospitals, in order to investigate the
incidence of HT among GIST patients treated with regorafenib, as
timely management is crucial for allowing continuation of
regorafenib treatment with a durable palliative result, while
severe HT may require treatment discontinuation and compromise the
outcome of heavily pretreated patients.
Patients and methods
Patients and ethics statement
The present study included patients with GIST who
were treated with regorafenib between September 2012 and May 2014
at three German University Hospitals (Tübingen, Heidelberg and
Hannover). The last follow-up was documented in August 2017.
Patient data were anonymized and assessed retrospectively. All
analyses were performed in concordance with the recommendations of
the local ethics committees, and after obtaining approval from the
Ethics Committee of Hannover Medical School (Hannover, Germany,
reference no. 3167-2016). The study protocol conformed to the
principles outlined in the latest amendment of the Declaration of
Helsinki (10).
Regorafenib treatment
Regorafenib was administered after obtaining written
informed consent from the patients. Dosing was adjusted according
to investigator's judgement following the product information. All
patients received computed tomography or magnetic resonance imaging
examinations at baseline and subsequently every 3 months, according
to local standards. Clinical chemistry and blood count measurements
were performed according to local standards.
Definition of HT
HT was defined as any alteration in the serum values
of aspartate aminotransferase (AST), alanine aminotransferase
(ALT), γ-glutamyltransferase (γ-GT), alkaline phosphatase (AP) and
bilirubin, corresponding to an adverse event of grade ≥3 according
to the Common Terminology Criteria of Adverse Events (CTCAE),
version 4.0, and/or by corresponding clinical signs (e.g., icterus,
pruritus and exanthema) (11). The
values were documented at the start of therapy, at maximum peak and
at the end of therapy, or last value recorded (resolution). HT was
considered as regorafenib-associated only in patients without
evidence of hepatic tumor progression and no other plausible
cause.
The institutional cut-off for pathological values
was applied, and CTCAE grading was performed by the multiplication
of the upper limit of normal.
Statistical analysis
Statistical analysis was performed using SPSS 24.0
(IBM Corp.). Descriptive patient and treatment characteristics were
evaluated. Clinical progression-free survival (cPFS) was defined as
the time from initiation of regorafenib treatment until the first
evidence of either clinical or radiological progression, and it was
calculated by Kaplan-Meier curves. Overall survival (OS) was
defined as the time from initiation of regorafenib treatment until
death from any cause, and it was calculated using Kaplan-Meier
curves.
Results
Patient characteristics
A total of 21 patients treated with regorafenib for
GIST were identified within the observation period. The median
follow-up was 14.8 months (range, 2-42 months) and the median age
at diagnosis was 67.2 years (range, 31-87 years). The primary
origin of GIST was mostly in the small intestine (n=8, 38.1%),
followed by the stomach (n=6, 28.6%). Synchronous metastases at
initial diagnosis were present in 12 patients (57.1%). A total of 7
patients (33.3%) suffered from liver metastases at initial
diagnosis, while overall hepatic metastases developed in 19
patients (90.5%) (Table I). The
patterns of metastatic spread are shown in supplementary Table SI.
 | Table IBaseline patient and tumor
characteristics at diagnosis. |
Table I
Baseline patient and tumor
characteristics at diagnosis.
| Baseline
parameters | No. (%)a |
|---|
| Patients | 21 (100.0) |
| Median age at
diagnosis, years (range) | 67.2 (31.4-87) |
| Sex |
|
Male | 16 (76.2) |
|
Female | 5 (23.8) |
| Primary GIST
location |
|
Esophagus | 3 (14.3) |
|
Stomach | 6 (28.6) |
|
Small
intestine | 8 (38.1) |
|
Colon | 2 (9.5) |
|
Peritoneum | 2 (9.5) |
| Median time to
metastasis, months (range) | 0 (0-64) |
| Synchronous
metastasis at initial diagnosis of GIST | 12 (57.1) |
| Synchronous liver
metastasis at initial diagnosis of GIST | 7 (33.3) |
| Primary molecular
pathology |
|
c-KIT exon
11 mutation | 8 (38.1) |
|
c-KIT exon 9
mutation | 4 (19.0) |
|
c-KIT
wild-type | 1 (4.8) |
|
Not
available | 8 (38.1) |
Treatment
Patients received 3-8 therapeutic lines prior to the
initiation of regorafenib, while the median duration of regorafenib
treatment was 5.15 months (range, 2-20 months). Dose reduction of
regorafenib was necessary in 15 patients (71.4%). The subsequent
therapeutic lines ranged from 0 to 5 (Table II).
 | Table IITreatment parameters of 21 patients
treated with regorafenib. |
Table II
Treatment parameters of 21 patients
treated with regorafenib.
| Treatment
parameters | No. (%)a |
|---|
| Median age at
regorafenib initiation, years (range) | 70 (35-86) |
| Median number of
therapeutic lines before regorafenib (range) | 4 (3-8) |
| Median duration of
regorafenib treatment, months (range) | 5.15 (2-20) |
| Regorafenib dose
reduction (due to any reason) | 15 (71.4) |
| Reason for
discontinuation of regorafenib | |
|
Disease
progression | 15 (71.4) |
|
Toxicity
other than HT | 1 (4.8) |
|
Death | 1 (4.8) |
|
Not
evaluable | 4 (19.0) |
| Median number of
systemic treatments after regorafenib failure (range) | 1 (0-5) |
Incidence of HT
A total of 5 patients with clinical and/or
laboratory HT were identified (23.5%). The median time to HT was
1.7 months (range, 0.9-11.2 months). Of these 5 patients, 1
exhibited liver progression of GIST at time of HT, while the
remaining 4 patients were able to continue regorafenib treatment
without reoccurrence of HT. Only 1 patient discontinued treatment
with regorafenib permanently due to severe toxicities other than HT
(Table III).
 | Table IIICharacteristics of HT in patients
treated with regorafenib. |
Table III
Characteristics of HT in patients
treated with regorafenib.
| Hepatic toxicity | No. (%)a |
|---|
| Patients with liver
metastasis prior to regorafenib treatment | 19 (90.5) |
| Number of patients
with HT | 5 (23.5) |
|
HT
laboratory value alterations | 4 (80.0) |
|
Clinical
signs only of HT | 1 (20.0) |
| Median time to HT,
months (range) | 1.7 (0.9-11.2) |
| HT patients on
hepatotoxic comedication | 3 (14.3) |
| Liver progression at
the time of HTb | 1 (4.8) |
| Regorafenib
continuation after HT | 4 (19.0) |
| Reoccurrence of
HT | 0 (0.0) |
Laboratory changes in patients with
HT
The levels of AST, ALT, γ-GT, AP and bilirubin were
elevated in patients with HT (Fig.
1A-F). The median maximum level of ALT in patients with HT was
200 U/l (range, 12-992 U/l) compared with 30 U/l (range, 22-72 U/l)
in non-HT patients; AST, HT 118 U/l (range, 38-1,104 U/l) vs.
non-HT 38 U/l (range, 26-94 U/l); AP, HT 398 U/l (range, 208-571
U/l) vs. non-HT 108 U/l (range, 32-772 U/l); γ-GT, HT 499 U/l
(range, 205-771 U/l) vs. non-HT 98 U/l (range, 13-1,400 U/l);
bilirubin, HT 79 µmol/l (range, 36-127 µmol/l) vs. non-HT 20 µmol/l
(range, 1-47 µmol/l); and lactate dehydrogenase (LDH), HT 527 U/l
(range, 356-610 U/l) vs. non-HT 278 U/l (range, 181-412 U/l). The
last available values, or those measured at the end of treatment,
did not differ significantly from baseline levels. The median ALT
at resolution in patients with HT was 29 U/l (range, 21-37 U/l),
the AST was 30 U/l (range, 27-32 U/l), the AP was 90 U/l (range,
72-107 U/l), the γ-GT was 48 U/l (range, 46-50 U/l), the bilirubin
was 9 µmol/l (range, 4-13 µmol/l) and the LDH was 275 U/l (range,
268-281 U/l) (Fig. 1). The patterns
of laboratory changes in patients with HT are summarized in
supplementary Table SII.
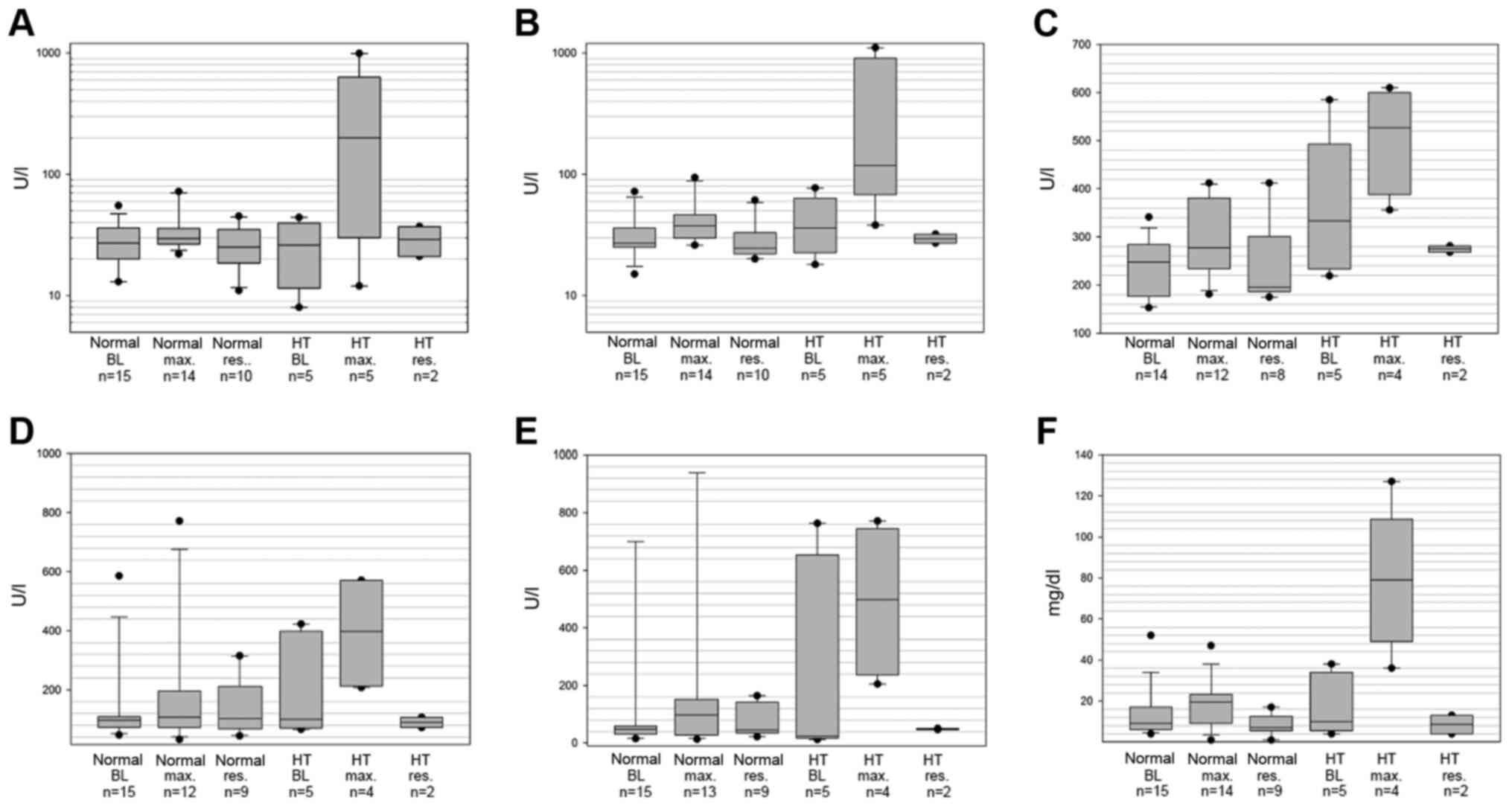 | Figure 1Box plot displaying laboratory values
of (A) ALT, (B) AST, (C) LDH, (D) AP, (E) γ-GT and (F) bilirubin.
γ-GT was elevated and grade >3 at BL for 2 patients with HT, of
whom 1 patient exhibited a significant increase in AST and ALT from
normal to grade 3 and 4 values, without further alteration of γ-GT;
the other patient exhibited a minor increase in AST, ALT, AP and
γ-GT levels. Median, interquartile range, 95% confidence interval,
maximum and minimum levels measured at BL, levels at maximum
elevation (max.) and resolution of toxicity (res.) are shown for
patients with HT and those without HT (normal). HT, hepatic
toxicity; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; LDH, lactate dehydrogenase; AP, alkaline
phosphatase; γ-GT, γ-glutamyltransferase; BL, baseline. |
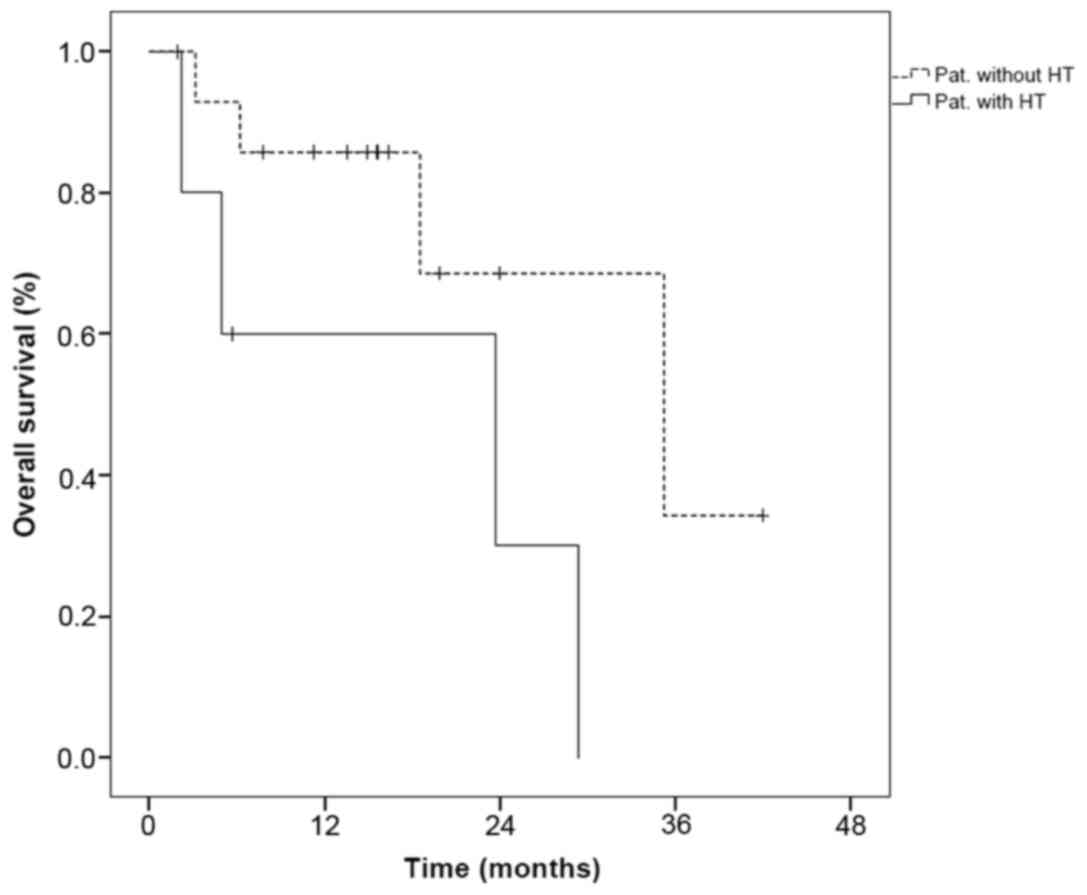
Survival
The median cPFS under treatment with regorafenib was
8.6 months [95% confidence interval (CI): 0-17.8 months], while the
median OS from initiation of regorafenib treatment was 29.3 months
(95% CI: 17.1-41.5 months). When comparing HT with non-HT patients,
no significant differences in cPFS or OS were observed (cPFS
log-rank P=0.9; OS log-rank P=0.07; Fig. 2).
Discussion
Tyrosine kinase inhibitor-induced HT has been
previously described, but its relevance in GIST patients receiving
regorafenib treatment has not been clearly defined by previous
studies (9). While the pivotal
phase III trial of regorafenib in advanced GIST initially did not
report any severe HT, unlike the phase III trial of regorafenib in
colorectal cancer, our data demonstrated that almost one-quarter of
patients treated with regorafenib (5/21; 23.5%) exhibited
laboratory and/or clinical signs of HT (2,3).
The pivotal phase III trial of regorafenib vs.
placebo in patients with refractory GIST did not initially report
relevant HT, apart from 1 case of drug-related fatal hepatic
failure (2). Only elevation of AST
levels was observed in 8% of the cases in a subsequent publication
(12). Similarly, a phase III trial
of regorafenib monotherapy for metastatic colorectal cancer only
reported hyperbilirubinemia in 65/500 (11%) of the patients,
whereas no other signs of HT were reported (3). The phase III trial of regorafenib
monotherapy in patients with hepatocellular carcinoma revealed an
increase in bilirubin, ALT and AST as a drug related adverse event.
Elevated drug related bilirubin was found in ~25% (95/374) of
patients treated with regorafenib, while 1 patient experienced
grade 4 toxicity. Drug-related elevation in AST was observed in
~18% of patients treated with regorafenib, while 3 patients
experienced grade 4 toxicity (4).
HT as predefined toxicity has been most sufficiently reported in
two retrospective studies of regorafenib in GIST from the UK.
First, a report of 20 patients treated within the UK Managed Access
Program revealed hepatobiliary toxicity in 10% (severe in 5%) of
the patients; more importantly, a recent single-center study of 50
patients reported HT in only 2% of the patients (13,14).
Hence, previous studies of regorafenib in GIST have reported an HT
incidence of only up to 10%, while a review of HT in patients with
solid neoplasias treated with tyrosine kinase inhibitors reported
elevated AST, ALT or bilirubin levels in 21-47.3% of the treated
patients, ultimately underlining the need for awareness of HT
during tyrosine kinase inhibitor treatment (9).
Reasonable explanations for differences in HT
between study populations and individuals treated with regorafenib
outside the trials remain elusive. Due to the small sample size,
retrospective design of the analysis and lack of liver biopsies, a
convincing explanation is not possible. Of note, a selection bias
is possible, since all patients in the present study have been
treated at German tertiary care centers. However, dose reductions
of regorafenib in our cohort were necessary in 71.4% due to any
adverse event, which is very similar to the 72.0% dose reductions
in the phase III study of regorafenib in GIST (2). Therefore, differences in dosing and
therapeutic management cannot explain the discrepancy in reported
HT. Furthermore, a clear definition of HT as an integrative term
for an adverse event that may be defined by complex changes in a
number of laboratory values is not available, and may explain HT
being previously less recognized and/or underreported.
Interestingly, hepatic metastases prior to initiation of
regorafenib treatment were reported in 85.7% (n=24/28) of the
patients in an analysis of GIST by Schvartsman et al, which
is comparable to our reported incidence of liver metastasis in
90.5% of regorafenib-treated patients (15). However, whether this associated with
the occurrence of HT remains elusive. Furthermore, at least in our
cohort, no association with hepatotoxic medication or intrahepatic
tumor progression was apparent as a possible etiology for the
observed HT. Other possible explanations for HT, such as UGT1A1
polymorphisms, suggested for pazopanib-associated HT, remain
speculative (16). Differences in
metastatic spreading patterns within the liver, as well as
supportive liver treatment or previous liver interventions, may
also affect the course of disease. Effect size and multifactorial
reasoning do not permit any definitive conclusions on these
issues.
In conclusion, taking the present data as well as
previous studies of regorafenib treatment in other tumor patients
into consideration, it may be suggested that HT in GIST patients
treated with regorafenib has been underreported thus far. However,
clinical monitoring and adequate therapy management allows
continuation of regorafenib treatment. Regorafenib administration
may achieve a durable palliative result, while even significant HT,
once adequately monitored, may not lead to a compromised outcome in
these heavily pretreated patients.
Supplementary Material
Location of metastases at the onset of
regorafenib treatment.
Adverse event characteristics in
patients with regorafenib.induced hepatic toxicity.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Raw data were generated at Hannover Medical School.
Derived data supporting the findings of the present study are
available from the corresponding author on reasonable request.
Authors' contributions
VG, PI, BK, KH and HGK designed the project. MH, BK,
KH, HE, and HGK were responsible for data collection. PI and HE
performed statistical analysis and wrote the manuscript, with
support from AG and VG. All authors discussed the results and
contributed to the final manuscript.
Ethics approval and consent to
participate
The present study was performed with approval from
the Ethics Committee of Hannover Medical School (no. 3167-2016). No
further patient consent was deemed mandatory.
Patient consent for publication
Written consent is not applicable. Patient data were
anonymized.
Competing interests
PI has an advisory role for Novartis, Bayer and
Pfizer and has received travel grants from Novartis and Bayer. HE
has received travel grants from Ipsen. BK has an advisory role for
Bayer. AG has received advisory board honoraria from Novartis and
Celgene. VG has an advisory role for Novartis, Pfizer, GSK and
Bayer and has received honoraria for lectures from Novartis, Pfizer
and GSK. MH, KH and HGK declare that they have no competing
interests to disclose.
References
|
1
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73-4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Demetri GD, Reichardt P, Kang YK, Blay JY,
Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M,
Joensuu H, et al: Efficacy and safety of regorafenib for advanced
gastrointestinal stromal tumours after failure of imatinib and
sunitinib (GRID): An international, multicentre, randomised,
placebo-controlled, phase 3 trial. Lancet. 381:295–302.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Roderburg C, Özdirik B, Wree A, Demir M
and Tacke F: Systemic treatment of hepatocellular carcinoma: From
sorafenib to combination therapies. Hepat Oncol.
7(HEP20)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kudo M, Imanaka K, Chida N, Nakachi K, Tak
WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al: Phase
III study of sorafenib after transarterial chemoembolisation in
Japanese and Korean patients with unresectable hepatocellular
carcinoma. Eur J Cancer. 47:2117–2127. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iacovelli R, Palazzo A, Procopio G,
Santoni M, Trenta P, De Benedetto A, Mezi S and Cortesi E:
Incidence and relative risk of hepatic toxicity in patients treated
with anti-angiogenic tyrosine kinase inhibitors for malignancy. Br
J Clin Pharmacol. 77:929–938. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
World Medical Association. World medical
association declaration of helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cancer Therapy Evaluation Programm D NCI,
NIH, DHHS: Common terminology criteria for adverse events v4.0
(CTCAE), 2009.
|
|
12
|
Komatsu Y, Doi T, Sawaki A, Kanda T,
Yamada Y, Kuss I, Demetri GD and Nishida T: Regorafenib for
advanced gastrointestinal stromal tumors following imatinib and
sunitinib treatment: A subgroup analysis evaluating Japanese
patients in the phase III GRID trial. Int J Clin Oncol. 20:905–912.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kollàr A, Maruzzo M, Messiou C, Cartwright
E, Miah A, Martin-Liberal J, Thway K, McGrath E, Dunlop A, Khabra
K, et al: Regorafenib treatment for advanced, refractory
gastrointestinal stromal tumor: A report of the UK managed access
program. Clin Sarcoma Res. 4(17)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chamberlain F, Farag S, Williams-Sharkey
C, Collingwood C, Chen L, Mansukhani S, Engelmann B, Al-Muderis O,
Chauhan D, Thway K, et al: Toxicity management of regorafenib in
patients with gastro-intestinal stromal tumour (GIST) in a tertiary
cancer centre. Clin Sarcoma Res. 10(1)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schvartsman G, Wagner MJ, Amini B, Zobniw
CM, Trinh VA, Barbo AG, Lin HY, Wang WL, Conley AP, Ravi V, et al:
Treatment patterns, efficacy and toxicity of regorafenib in
gastrointestinal stromal tumour patients. Sci Rep.
7(9519)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu CF, Reck BH, Xue Z, Huang L, Baker KL,
Chen M, Chen EP, Ellens HE, Mooser VE, Cardon LR, et al:
Pazopanib-induced hyperbilirubinemia is associated with Gilbert's
syndrome UGT1A1 polymorphism. Br J Cancer. 102:1371–1377.
2010.PubMed/NCBI View Article : Google Scholar
|