Introduction
Colorectal cancer (CRC) is the third most common
malignancy worldwide (1) being a
frequent cause of cancer related death as close to 50% of patients
diagnosed with CRC eventually are diagnosed with liver metastases
(CRCLM) (2). Appropriately selected
patients with CRCLM can be treated in a potential curative setting
including both surgery and non-operable metastasis directed
therapies with reported 5 years survival rates of around 40%
(3-5).
Hepatic arterial infusion (HAI) of chemotherapy for CRCLM has been
explored since the 1970s (6), yet,
unable to be an established standard of care in current guidelines
(7). The theoretical advantage of
HAI is the direct delivery of cytotoxic agents into the metastases,
with oxaliplatin showing a favorable pharmacokinetic profile for
this administration (8). As only
10-15% of CRCLM are upfront resectable, there is an unmet need for
non-operative therapies and corresponding prognostic and/or
predictive biomarkers to individualize treatment.
Cell free DNA (cfDNA) is present in the blood stream
as a mixture of healthy and mutated tumor specific DNA (9). Although known for decades (10), cfDNA has in recent years attracted
increasing attention as a strong prognostic marker for patients
with metastatic colorectal cancer (mCRC) (11). The methodology for cfDNA analysis is
complex and heterogeneous, thus we have optimized and validated a
direct fluorescent assay (DFA) for the total cfDNA analysis. This
allows for a rapid quantification of cfDNA using only low volume
(40 µl) of plasma and no DNA preparation.
Cell free DNA can emerge as an essential future
biomarker, with possible applications in both systemic and
localized cancer treatment. Tumor specific cfDNA might display
unique mutational status regarding RAS, BRAF, hypermethylation and
microsatellite instability, while the total level of cfDNA can
serve as a more universal biomarker (11,12).
Here, we investigate the level of cfDNA, assessed by DFA, as a
prognostic and predictive marker for patients with CRCLM undergoing
HAI with oxaliplatin together with oral capecitabine.
Materials and methods
Patients and materials
Patients were treated according to a single arm
phase II study including patients with liver limited mCRC from
November 2004 to May 2010, who were not eligible for any other
standard local treatment. Therapy comprised intrahepatic infusion
of oxaliplatin 100 mg/m2 every second week with
concomitant oral capecitabine 3,500 mg/m2 every second
week for up to 12 cycles. The clinical data and outcome of the
study has been presented separately (13), thus only translational aspects are
addressed here. For comparison, we had blood samples available from
94 healthy individuals from a Danish biobank, as previously
described (14).
After HAI treatment patients underwent standard of
care surveillance with regular Computed Tomography (CT) scans. The
first occurrence of radiologically progression according to the
RESICT criteria was defined as the first progression.
Laboratory investigations
Blood samples for translational use were drawn at
baseline prior to the administration of the first intrahepatic dose
of oxaliplatin and at a fixed schedule during follow up. Analysis
of cfDNA was done using a direct fluorescent assay for cfDNA
analysis, not requiring any prior processing, as preliminary
reported by Douvdevani and colleagues (15,16)
and further modified by our group, as previously published
(14).
In short, 40 µl plasma, not requiring any prior
processing, were used and adding SYBR® Gold Nucleic Acid
Gel Stain (1:8,000). The quantification of fluorescence was
performed with a 96-well fluorometer (Infinite F200 PRO, Tecan) at
an emission wavelength of 535 nm and an excitation wavelength of
485 nm in a black 96-well plate (Bio-Plex Pro Flat Bottom Plates,
Bio-Rad). Using dilution with a 10% bovine serum albumin (BSA;
Sigma® Life Science) solution, DNA standards were
prepared from Human Control Genomic DNA (Life Technologies). From a
standard curve the concentration of the samples were calculated.
For each sample, we determined the concentration of cfDNA by
calculating the median value of four measurements, removing
outliers following Dixons q test if the standard deviation exceeded
10%. Carcino-Embryonic Antigen (CEA) was measured with routine
analysis with an upper normal limit (UNL) of 5 µg/l.
Statistics
The level of plasma cfDNA is expressed as median
value with 95% confidence interval (95% CI). Survival was
calculated from time of inclusion until death of any cause or
censoring at end of follow-up. We analyzed survival by the
Kaplan-Meier method and comparison between groups by log rank test.
The comparison of cfDNA levels between groups was done by the
Mann-Whitney U test and comparison of categorical variables between
groups by contingency tables and χ2 test or Fischer
exact test when appropriate. In Cox proportional hazard model, we
computed Hazard Ratios (HR) for mortality and included age, gender,
site of primary tumor, WHO Performance Status (PS), cfDNA, CEA and
KRAS mutational in archival tissue in the multivariate model.
Results
Baseline cfDNA and comparison with
healthy controls
Baseline blood samples for plasma cfDNA measurement
were available for 62 patients who all completed at least one HAI
treatment. The gender distribution was 61% male and the median age
was 61.3 years (range 40.8-74.8 years). Colon cancer was the site
of primary for 68%. The baseline clinical characteristics and
corresponding plasma cfDNA levels are presented in Table I. The only significant difference in
cfDNA levels among baseline characteristics, were patients with a
WHO PS of 1 or 2 having a higher level than patients with a WHO PS
of 0 (P<0.001). The baseline median level of CEA was 53 ng/l
(95% CI 28-97) (n=60).
 | Table IPatient characteristics and
corresponding level of plasma cfDNA. |
Table I
Patient characteristics and
corresponding level of plasma cfDNA.
| Characteristic | Number (%) N=62 | cfDNA, ng/ml
(95%CI) | P-value |
|---|
| Sex | | | 0.3 |
|
Male | 38(61) | 0.91 (0.75-0.99) | |
|
Female | 24(39) | 0.97 (0.86-1.33) | |
| Age | | | 0.4 |
| Median | 61.3 | | |
| Range | 40.8-74.8 | | |
|
≤65 | | 0.91 (0.76-0.99) | |
|
>65 | | 0.98 (0.77-1.44) | |
| Performance
status | | | <0.001 |
|
0 | 54(87) | 0.90 (0.75-0.94) | |
|
1 | 6(10) | 1.97 (1.07-2.74) | |
|
2 | 2(3) | 3.02 (2.51-3.52) | |
| Site of primary | | | 0.4 |
|
Colon | 42(68) | 1.08 (0.91-1.68) | |
|
Ascending | 11(18) | | |
|
Transverse | 1 (1.5) | | |
|
Descending | 3 (4.5) | | |
|
Sigmoid | 27(44) | | |
|
Rectal | 20(32) | 0.96 (0.86-1.28) | |
| Debut of
metastases | | | 0.6 |
|
Synchronous | 33(53) | 1.16 (0.98-1.84) | |
|
Metachronous | 29(47) | 1.50 (0.94-2.05) | |
| Response | | | 0.02 |
|
CR+PR | 56(90) | 0.91 (0.76-0.98) | |
|
SD+PD | 4(7) | 1.79
(0.99-2.57) | |
|
Not
RECIST | 2(3) | | |
| KRAS status | | | 0.21 |
|
Wt | 36(58) | 0.87
(0.75-0.98) | |
|
mut | 26(42) | 1.01
(0.89-1.29) | |
The median plasma cfDNA level for healthy controls
(n=94) was 0.52 ng/µl (95%CI 0.48-0.57) significantly lower than
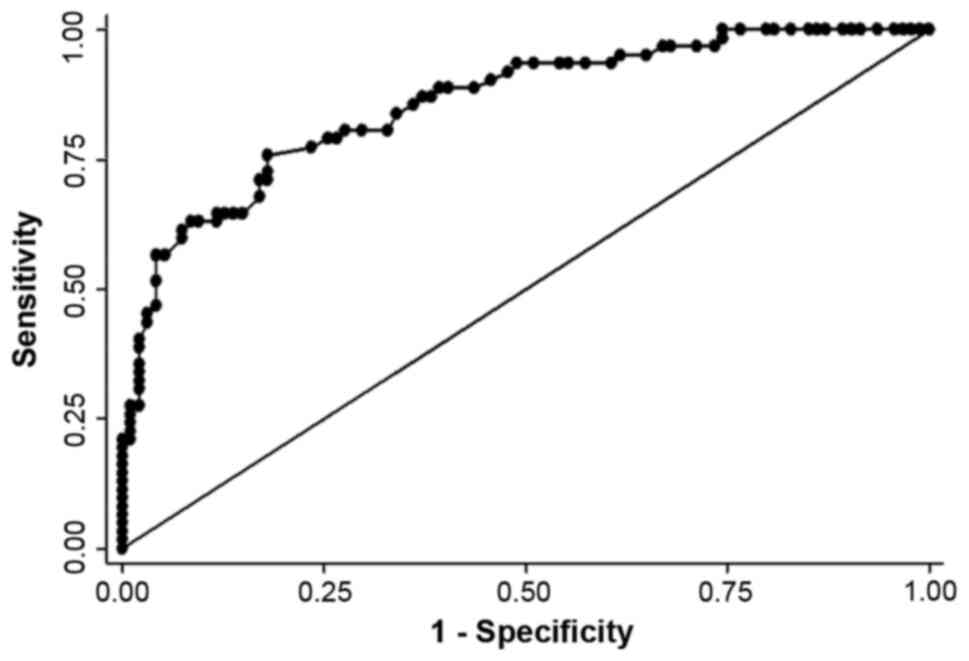
the HAI cohort (P<0.01). The discriminatory power of cfDNA
between healthy individuals and patients with CRCLM receiving HAI
was high with a ROC curve (Fig. 1)
with an AUC value of 0.86.
Sequential samples
The median baseline level of plasma cfDNA was 0.92
ng/µl (95%CI 0.84-1.00) (n=62). At the end of HAI treatment blood
samples were available for 56 patients with a median plasma cfDNA
level of 0.82 ng/µl (95% CI 0.73-0.89) (P=0.06 for comparison with
baseline). At the first time of progression, plasma samples
available from 32 patients at with a median level of 0.80 ng/µl
(95% CI 0.66-0.94) (P=0.01; compared with baseline). The last data
point was at 3 years follow-up were only 9 patients contributed
with plasma samples with a median cfDNA level of 0.82 µg/µl (95% CI
0.61-0.91) (P=0.5). At 3 years the estimated overall survival was
48% (95% CI 35-59).
During the HAI treatment 20 patients had an
increasing level of cfDNA while 32 patients had a decreasing value.
The median value of value of change in cfDNA level was a decline of
0.14 ng/µl (95%CI 0.00-0.21).
Clinical correlation of cfDNA
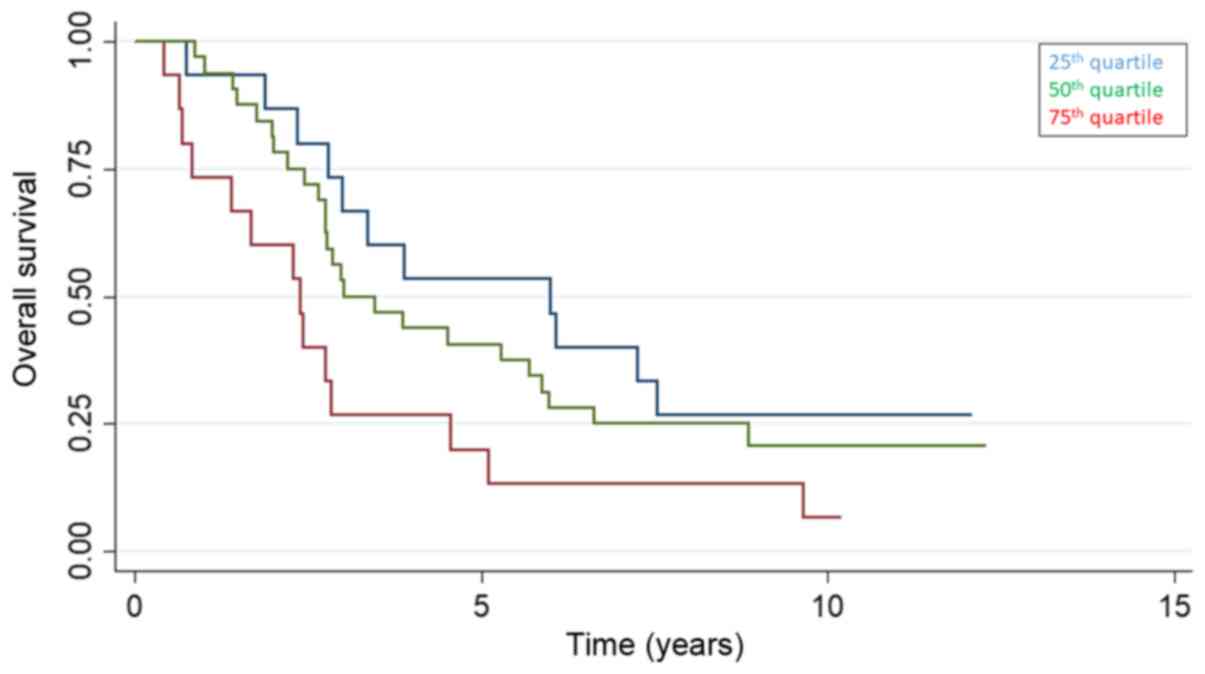
The 25, 50 and 75th quartile of baseline cfDNA were
0.71 (95% CI 0.61-0.81), 0.92 (95% CI 0.84-1.00) and 1.30 ng/µl
(1.00-1.67) and the overall survival stratified by baseline cfDNA
quartiles is presented in Fig. 2.
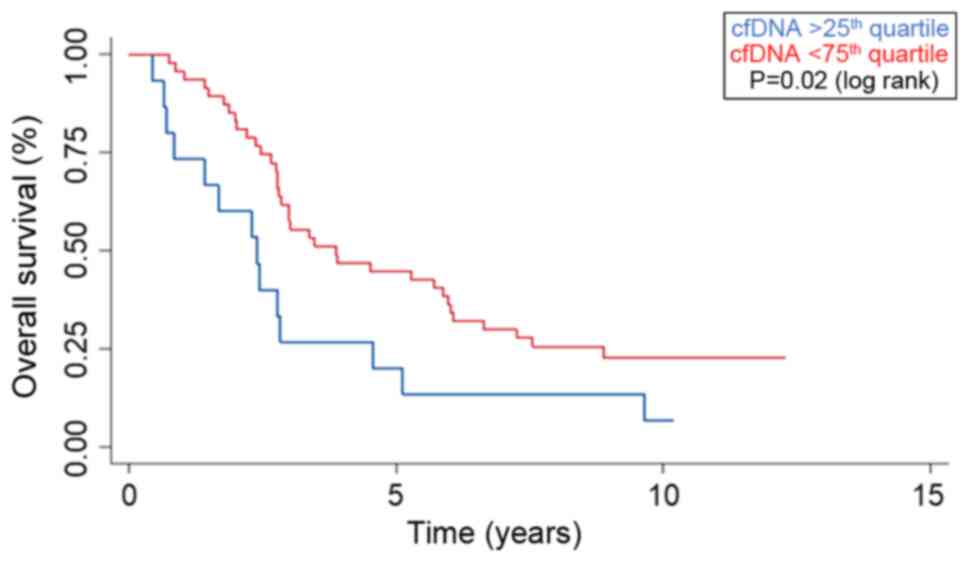
Patients with a baseline value of cfDNA above the 75th quartile had
a median overall survival (OS) of 2.4 years (95% CI 0.7-2.8),
compared to 3.9 years (95% CI 2.8-5.9) for patients below the 75th
quartile (P=0.02) (Fig. 3). Plasma
samples at the end of HAI (n=56) showed a tendency for a longer
survival for patients below the 75th quartile with a median
survival of 3.5 years (95% CI 2.76-5.69) compared to 2.4 years (95%
CI 1.67-4.55) for patients above the 75th quartile (P=0.5).
Separating the survival of patients by the baseline
level of CEA showed no significant differences whether the UNL
(P=0.3) or 75th quartile (P=0.18) were applied. Patients who
achieved a best objective response of either a complete (CR) or
partial response (PR) had a baseline cfDNA concentration of 0.91
ng/µl (95% CI 0.76-0.98) compared to patients who only obtained
stable disease (SD) or progression (PD) with a level of 1.79 ng/µl
(95% CI 0.99-2.57) (P=0.02). The change in plasma cfDNA during HAI
treatment was not correlated to any clinical outcomes in term of
survival or response. Selectively looking at patients with the
longest survival (above the 75th quartile of survival=7.3 years)
(n=13) did not show any trends in cfDNA change.
The diagnostic accuracy of cfDNA is presented in
Table II by a contingency table
displaying the value of cfDNA above/below the baseline 75q for
patients with either response (CR/PR, n=56) versus patient with no
response (SD/PR, n=4) (P=0.03 Fisher exact test). The sensitivity,
specificity, positive and negative predictive values are 80, 75, 97
and 21%.
 | Table IIDiagnostic accuracy of baseline cfDNA
(above/below the 75th quartile) in cross tabulation with response
(CR/PR) versus not response (SD/PD) (P=0.03; Fisher exact
test). |
Table II
Diagnostic accuracy of baseline cfDNA
(above/below the 75th quartile) in cross tabulation with response
(CR/PR) versus not response (SD/PD) (P=0.03; Fisher exact
test).
| Response | >75q baseline
cfDNA | <75q baseline
cfDNA | Total |
|---|
| CR/PR (n=56) | 11 | 45 | 56 |
| SD/PD (n=4) | 3 | 1 | 4 |
| Total | 14 | 46 | |
Prognostic factors
In both uni- and multivariate model increasing
baseline level of cfDNA were associated with increased mortality
with HR of 2.39 (95% CI 1.51-3.76, P<0.001) and 1.90 (95% CI
1.07-3.38, P<0.03), respectively. The only other variable
associated with a sustained impact on survival in the multivariate
model was the mutational status of the KRAS oncogene where patients
with a KRAS mutation had a HR for mortality of 2.93 (95% CI
1.66-5.18, P<0.001) and 3.17 (95% CI 1.67-6.03, P<0.001) in
the univariate and multivariate analysis, respectively (Table III).
 | Table IIIUni and multivariate analysis
displaying the HR for mortality. |
Table III
Uni and multivariate analysis
displaying the HR for mortality.
| Variable | Univariate HR (95%
CI) | P-value | Multivariate HR
(95%CI) | P-value |
|---|
| Sex | | | | |
|
Male
(reference) | | | | |
|
Female | 0.69
(0.38-1.25) | 0.20 | 0.54
(0.28-1.02) | 0.06 |
| Age | | | | |
|
Continuous
variable | 0.99
(0.96-1.02) | 0.40 | 0.98
(0.94-1.01) | 0.20 |
| Site of
primary | | | | |
|
Colon
(reference) | | | | |
|
Rectum | 0.89
(0.48-1.68) | 0.70 | 0.90
(0.48-1.70) | 0.70 |
| Performance
status | | | | |
|
0
(reference) | | | | |
|
1 | 5.74
(2.24-14.74) | <0.01 | 2.19
(0.74-6.43) | 0.15 |
|
2 | 13.63
(2.84-65.52) | 0.01 | 3.84
(0.51-28.91) | 0.19 |
| cfDNA
(baseline) | | | | |
|
Continous
variable | 2.39
(1.51-3.76) | <0.01 | 1.90
(1.07-3.38) | 0.03 |
| CEA (baseline) | | | | |
|
Continous
variable | 1.49
(0.70-3.18) | 0.3 | 1.01
(0.45-2.23) | 0.90 |
| KRAS status | | | | |
|
Wild-type
(reference) | | | | |
|
Mutated | 2.93
(1.66-5.18) | <0.01 | 3.17
(1.67-6.03) | <0.01 |
Discussion
Due to the direct intrahepatic administration of
chemotherapy, HAI is an attractive treatment option for patients
with CRCLM. Over the past decades, the use of HAI has been
subjected to clinical trials both in first line treatment of
non-resectable metastases (17) and
as an adjuvant treatment subsequent to metastasectomy (18). Alongside chemo-embolization,
radiofrequency ablation (RFA), stereotactic body radiotherapy
(SBRT) and selective internal radiotherapy (SIRT), HAI constitutes
a loco-regional toolbox of treatment option for patients with
CRCLM. Seeking to increase the optimal use of these loco-regional
modalities, new prognostic and/or predictive circulating biomarkers
are needed (19).
Here, we have demonstrated that patients with CRCLM
and a high baseline level of cfDNA have an inferior outcome, both
in term of objective response and survival. Although the negative
prognostic impact of a high cfDNA level is well described in term
of survival (11), it is especially
interesting to report a possible predictive value of cfDNA, as
patients who subsequent developed a partial or complete response
had a lower baseline cfDNA compared to patients who only obtained
stable disease or progression. For patients with CRCLM not upfront
eligible for resection, a predictive marker can have a major
impact, as responding patients might be converted to resectability.
In contrast, patients not obtaining an objective response might
benefit mostly from purely systemic chemotherapy, avoiding the
invasive procedure and possible complications from the catheter
placement.
We have analyzed the total level of cfDNA using a
new fluorescent assay applied directly to the biological sample
(plasma). We have refined, optimized and validated this technique
since the first reported use in the literature in 2009(14), being an attractive option for cfDNA
analysis due to a high degree of laboratory feasibility, no DNA
preparation and low financial costs. Considering the total amount
of cfDNA, this allows for a detectable biomarker in practically all
patients, not restricting the analysis to patients with tumor
specific mutations. This is, in turn, limited by the potential
pitfalls of this assay being a potential contamination from
degenerated lymphocytes and falsely elevated levels due to various
medical disorders known to affect the total level of plasma cfDNA
(20).
We demonstrate that the total level of cfDNA has a
possible prognostic and predictive value, not considering any tumor
specific mutations in the blood. As reported from the clinical data
of this trial (13), patients with
KRAS wild-type status in archival tissue had a significant improved
survival, which is maintained in this translational supplement, as
increasing level cfDNA and the existence of KRAS mutation are the
only two independent variables associated with increased mortality
in the multivariate model (Table
III). As a limitation, with DFA analysis, we are unable to
quantify any RAS mutations in the blood stream, hence to analyze
any concordance between archival tissue and the blood stream.
Analysis of tumor specific cfDNA alterations could have further
applications in translational oncology, both in term of early
detection of recurrence or resistance to e.g. anti-EGFR therapy
(21).
CEA has been the hallmark of blood borne biomarkers
for patients with mCRC for decades, although the routinely use of
CEA plays a minor role in current guidelines (7,22,23).
For patients with CRCLM, CEA has been widely studied in patients
undergoing surgical resection of liver metastases and well
established as a prognostic marker for survival (4), yet a predictive value of CEA for any
loco-regional treatment has never been established.
In our analysis, cfDNA is a stronger prognostic
marker for mortality than CEA, as increasing CEA fails to show
independent association with mortality with a HR of 1.01 in
multivariate analysis. In contrast, increasing cfDNA levels show
sustained prognostic value in both uni- and multivariate model.
This is in concordance with a recent comparative study of CEA and
cfDNA for patients with mCRC, demonstrating a possible diagnostic
superiority of cfDNA compared to CEA (24). The majority of studies on cfDNA and
CRC has emphasis on either widespread metastatic disease, in a
palliative setting (11) or
patients with locally advanced rectal cancer scheduled for
chemo-radiation (25,26). Few studies have explored the utility
of cfDNA in the clinical setting of CRCLM, where some patients
might still be within the range of curability. We have previously,
also by DFA quantification, examined the cfDNA levels for 14
patients with CRCLM who received chemo-embolization, reporting a
numerical shorter survival for patients with a high baseline level
of cfDNA, but unable to display statistical significance with the
low sample size (27). The possible
efficacy of HAI treatment as a modality cannot be addressed in this
single armed study, and the relatively long survival can be a
consequence of selection bias.
In conclusion, we have demonstrated an inferior
outcome for patients with CRCLM with a high level of cfDNA who
undergo intrahepatic infusion with oxaliplatin and systemic
capecitabine. Cell free DNA in plasma could hold both prognostic
and predictive value for this group of patients emphasizing the
need for biobanking biological material for translational
analysis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Danish Cancer
Society, the Novo Nordisk Foundation and Aase og Ejnar Danielsens
Foundation.
Availability of data and materials
All raw data material from this study is available
for review upon contact to the corresponding author.
Authors' contributions
AKB, JVS, BVJ, DN, BSS, JSJ and KLGS designed the
study, AKB and BSS performed the laboratory analysis, AKB, BSS and
KLGS performed the data analysis, AKB drafted the manuscript, AKB,
JVS, BVJ, DN, BSS, JSJ and KLGS reviewed the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients consented verbal and written to
protocol inclusion. The protocol was approved by the regional
ethical committee and The Danish Data Protection Agency. The study
was conducted according to the principles of the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jamal A: Cancer
statistics 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kopetz S, Chang GJ, Overman MJ, Eng C,
Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM and
McWilliams RR: Improved survival in metastatic colorectal cancer is
associated with adoption of hepatic resection and improved
chemotherapy. J Clin Oncol. 27:3677–3683. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fong Y, Fortner J, Sun RL, Brennan MF and
Blumgart LH: Clinical score for predicting recurrence after hepatic
resection for metastatic colorectal cancer: Analysis of 1001
consecutive cases. Ann Surg. 230:309–321. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boysen AK, Spindler KL, Høyer M, Mortensen
FV, Christensen TD, Farkas DK and Ording AG: Metastasis directed
therapy for liver and lung metastases from colorectal cancer-a
population based study. Int J Cancer. 143:3218–3226.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ramming KP, Sparks FC, Eilber FR, Holmes
EC and Morton DL: Hepatic artery ligation and 5-fluorouracil
infusion for metastatic colon carcinoma and primary hepatoma. Am J
Surg. 132:236–242. 1976.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dzodic R, Gomez-Abuin G, Rougier P, Bonnay
M, Ardouin P, Gouyette A, Rixe O, Ducreux M and Munck JN:
Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin
administration: Comparative results with cisplatin using a rabbit
VX2 tumor model. Anticancer Drugs. 15:647–650. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6(224ra24)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leon SA, Shapiro B, Sklaroff DM and Yaros
MJ: Free DNA in the serum of patients and the effect of therapy.
Cancer Res. 37:646–650. 1977.PubMed/NCBI
|
|
11
|
Spindler KG, Boysen AK, Pallisgaard N,
Johansen JS, Tabernero J, Sørensen MM, Jensen BV, Hansen TF,
Sefrioui D, Andersen RF, et al: Cell free DNA in metastatic
colorectal cancer: A systematic review and meta analysis.
Oncologist. 22:1049–1055. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zarkavelis G, Boussios S, Papadaki A,
Katsanos KH, Christodoulou DK and Pentheroudakis G: Current and
future biomarkers in colorectal cancer. Ann Gastroenterol.
30:613–621. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vittrup BV, Nørgaard H, Bergenfeldt M,
Larsen FO, Høgdall E, Johansen JS, Sidenius Johansen JS, Hermann HK
and Nielsen DL: Hepatic arterial infusion (HAI) of oxaliplatin with
capecitabine in first line treatment of patients (pts) with liver
limited metastases from colorectal cancer (LLmCRC). Abstracts
Gastrointestinal Tumours, Colorectal. 29: Suppl 8(viii173)2018.
|
|
14
|
Boysen AK, Sørensen BS, Lefevre AC,
Abrantes R, Johansen JS, Jensen BV, Schou JV, Larsen FO, Nielsen D,
Taflin H, et al: Methodological development and biological
observations of cell free DNA with a simple direct fluorescent
assay in colorectal cancer. Clin Chim Acta. 487:107–111.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Goldshtein H, Hausmann MJ and Douvdevani
A: A rapid direct fluorescent assay for cell-free DNA
quantification in biological fluids. Ann ClinBiochem. 46:488–494.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Czeiger D, Shaked G, Sebbag G, Vakhrushev
A, Flomboym A, Lior Y, Belochitski O, Ariad S and Douvdevani A:
Elevated cell-free DNA measured by a simple assay is associated
with increased rate of colorectal cancer relapse. Am J Clin Pathol.
145:852–857. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mocellin S, Pilati P, Lise M and Nitti D:
Meta-analysis of hepatic arterial infusion for unresectable liver
metastases from colorectal cancer: The end of an era? J Clin Oncol.
25:5649–5654. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu J, Zhong Y, Weixin N, Xinyu Q, Yanhan
L, Li R, Jianhua W, Zhiping Y and Jiemin C: Preoperative hepatic
and regional arterial chemotherapy in the prevention of liver
metastasis after colorectal cancer surgery. Ann Surg. 245:583–590.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kelly CM and Kemeny NE: Liver-directed
therapy in metastatic colorectal cancer. Expert Rev Anticancer
Ther. 17:745–758. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Spindler KL, Appelt AL, Pallisgaard N,
Andersen RF, Brandslund I and Jakobsen A: Cell-free DNA in healthy
individuals, noncancerous disease and strong prognostic value in
colorectal cancer. Int J Cancer. 135:2984–2991. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Boussios S, Ozturk MA, Moschetta M,
Karathanasi A, Zakynthinakis-Kyriakou N, Katsanos KH, Christodoulou
DK and Pavlidis N: The developing story of predictive biomarkers in
colorectal cancer. J Pers Med. 9(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim IH, Lee JE, Yang JH, Jeong JW, Ro S,
Oh ST, Kim JG, Choi MH and Lee MA: Clinical significance of
discordance between carcinoembryonic antigen levels and RECIST in
metastatic colorectal cancer. Cancer Res Treat. 50:283–292.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hermunen K, Lantto E, Poussa T, Haglund C
and Österlund P: Can carcinoembryonic antigen replace computed
tomography in response evaluation of metastatic colorectal cancer?
Acta Oncol. 57:750–758. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Berger AW, Schwerdel D, Welz H, Marienfeld
R, Schmidt SA, Kleger A, Ettrich TJ and Seufferlein T: Treatment
monitoring in metastatic colorectal cancer patients by
quantification and KRAS genotyping of circulating cell-free DNA.
PLoS One. 12(e0174308)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Boysen AK, Wettergren Y, Sorensen BS,
Taflin H, Gustavson B and Spindler KG: Cell-free DNA levels and
correlation to stage and outcome following treatment of locally
advanced rectal cancer. Tumour Biol.
39(1010428317730976)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schou JV, Larsen FO, Sørensen BS, Abrantes
R, Boysen AK, Johansen JS, Jensen BV, Nielsen DL and Spindler KL:
Circulating cell-free DNA as predictor of treatment failure after
neoadjuvant chemo-radiotherapy before surgery in patients with
locally advanced rectal cancer. Ann Oncol. 29:610–615.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Boysen AK, Jensen M, Nielsen DT, Mortensen
FV, Sørensen BS, Jensen AR and Spindler KL: Cell-free DNA and
chemoembolization in patients with liver metastases from colorectal
cancer. Oncol Lett. 16:2654–2660. 2018.PubMed/NCBI View Article : Google Scholar
|