Introduction
The World Health Organization (WHO) has predicted
that by 2020 there will be ~10,000,000 cancer-related fatalities
(1). Prostate cancer (PCA) is the
most common neoplasm in men worldwide, with 1/7 men suffering from
the disease (2,3). In the USA in 2014, 2,819 deaths were
reported at a rate of 50.6 per 100,000 inhabitants (4). Cancer has been the second cause of
mortality in Cuba since 1958, with 56.9 deaths per 100,000
inhabitants recorded in 2018 (5,6).
According to data from the American Society of
Medical Oncology, the 5-year survival rate for PCA in the mid-1970s
was ~69%. However, in recent years, with the development of new
treatment options, an improved prognosis and quality of life has
been observed in these patients. The results vary according to
clinical stage, reaching 30% in the metastatic stage (7).
Treatment for PCA ranges from radical prostatectomy
and radiotherapy (RT) in the early stages, with a low recurrence
risk, to androgen deprivation combined with RT and/or chemotherapy
(CTX) in the advanced stages, with an intermediate to high
recurrence risk. Treatment is correlated with clinical stage,
including relapse risk, based on the new TNM scoring system of the
American Joint Committee on Cancer (AJCC) (8-10).
Tumour cells develop different mechanisms that allow
them to survive and replicate. The result is resistant PCA, which
may still respond to secondary hormonal manoeuvres.
Hormone-refractory PCA (HRPC) is a cancer in which hormonal therapy
is no longer effective in any form (11-14).
At the end of 2004, two studies demonstrated that
docetaxel-based CTX improved survival in HRPC patients (18.9 vs.
16.4 months for mitoxantrone and prednisone). Until then, the
various treatments used only resulted in symptom palliation
(15).
CTX is associated with partial multi-drug resistance
and a high percentages of toxicities such as neutropenia, anaemia
and thrombocytopenia. This causes a delay in treatment, and a
deterioration in the quality of life and nutritional status of the
patients. As a result, the number of hospitalisations increases, as
does the length of the hospital stay. Current supportive
treatments, such as colony-stimulating factor and human recombinant
erythropoietin, while improving patient outcome, are also linked to
adverse effects (16).
In vitro and in vivo studies have
suggested that oxidative stress and antioxidants play a key role in
the pathogenesis of chronic diseases, including PCA. Therefore they
are important in prevention therapy (17-20). Multiple
investigations carried out in specialised cancer treatment centres
have revealed a synergistic effect of antioxidants with standard
treatment. Antioxidants increase the concentration of various
anti-neoplastic drugs in tumour cells, but not in healthy ones. In
turn, antioxidants sensitise tumour cells to RT (21,22).
Oncoxin-Viusid (OV) is a nutritional supplement
formulated with more effective antioxidants (Table I), produced by Laboratorios
Catalysis. The antioxidants are treated by means of a molecular
activation process, increasing their biological activity.
Epigallocatechin gallate, a polyphenol present in green tea extract
with anticancer properties, is particularly noteworthy. Its effects
include the inhibition of the tissue necrosis factor and the
potentiating of nuclear factor κ-light-chain-enhancer of activated
B cells, which regulates anti-apoptotic genes and inhibits the
expression of cyclooxygenase 2. Epigallocatechin gallate blocks
growth factor signal transduction and inhibits the urokinase-type
plasminogen activator enzyme, which stimulates tumour
proliferation, decreases matrix metalloproteinase, and favours
tumour invasion, angiogenesis and metastasis. In addition, the OV
supplement restores cellular apoptosis by inducing P53, caspase-3
and B-cell lymphoma 2 (Bcl-2)-associated X protein expression and
inhibiting anti-apoptotic protein Bcl-2. It is also an
immunomodulator that stimulates the production of interferons and
interleukin 12, and increases the phagocyte action of macrophages
and T-helper cells. Multiple clinical and pre-clinical studies have
focused on OV, demonstrating its antitumour effect (23,24).
 | Table IChemical composition of
Oncoxin-Viusid. |
Table I
Chemical composition of
Oncoxin-Viusid.
| Chemical | Value,
mga |
|---|
| Glycine | 2,000 |
| Glucosamine | 2,000 |
| Arginine | 640 |
| Cystine | 204 |
| Malic acid | 1,200 |
| Monoammonium
glycyrrhizinate | 200 |
| Ascorbic acid | 120 |
| Sodium
methylparaben | 100 |
| Zinc sulfate | 80 |
| Grean tea
extract | 25 |
| Calcium
penthotenate | 12 |
| Pyridoxine | 4 |
| Manganese
sulphate | 4 |
| Cinnamon
extract | 3 |
| Folic acid | 400 |
| Cyanocobalamin | 2 |
In Argentina, a clinical trial on OV in
hormone-responsive PCA was carried out. The results when
hormone-therapy is associated with OV showed a greater tolerance
and a good response to treatment. However, the effects and safety
of OV in patients with HRPC are unknown. This proof-of-concept,
phase II, prospective, non-randomised and open-label clinical trial
was aimed to identify the effect of the OV nutritional supplement
on quality of life, onco-specific treatment tolerance and
progression-free survival (PFS), as well as annual overall survival
(OS) in patients with advanced clinical stage PCA (25).
Materials and methods
Study design
A proof-of-concept, phase II, descriptive,
prospective, non-randomised and open-label clinical trial was
conducted on 25 male patients with a histological diagnosis of HRPC
at the General Calixto Garcia University Hospital in Havana, Cuba,
between June 2017 and March 2018.
Inclusion and exclusion criteria of
patients
The trial was comprised by 25 men over 18 years old
with compensated intercurrent diseases, Karnofsky index >70 and
parameter laboratory according to undergoing CTX. All patients
authorized the inclusion via informed consent. The Patients
couldn't with another oncospecific product in investigation or have
hipersensibility of Taxol. They neither have brain metastasis or
carrier of human immunodeficiency virus (HIV).
HRPC
Patients with testosterone production suppression by
orchiectomy or hormonal suppression, whose testosterone values were
<0.3 ng/ml and were still undergoing disease progression, were
considered hormone-resistant, according to the 2008 progression
criteria published by The Prostate Cancer Clinical Trials Working
Group (1). The present study
included hormone-resistant patients; hormone resistance was defined
as an absence of therapeutic response after 6 months of hormone
therapy.
The initial study was planned to be conducted in a
different center and for technical reasons was transferred to
General Calixto Garcia University Hospital, for this reason was not
possible to include the placebo, in order to prevent nutritional
product lapse. Then it was considered a preliminary study to
another more stretching study.
All 25 participating patients were treated with a 75
mg/m2 IV dose of docetaxel in 500 ml 0.9% NaCl every 3
weeks, and 5 mg prednisone twice daily (orally). Patients received
a minimum of 6 cycles of CTX; if there was a good response, it was
continued for 8 cycles. RT was administered to the abdominal lymph
nodes in 2 patients (total dose of 74 g cobalt 60), following 6
cycles of CTX.
Supportive treatment with OV
The 25 participants underwent treatment with a 75
mg/m2 IV dose of docetaxel in 500 ml NaCl 0.9% every 3
weeks, and prednisone 5 mg twice daily (orally). They received a
minimum of 6 cycles of CTX and, if there was a good response,
continued for 8 cycles. A daily 50-75 ml (25 ml in 2 or 3 doses) OV
oral solution was administered as supportive treatment following
meals. It was prescribed on an ongoing basis during the
onco-specific treatment, including CTX suspension periods, and was
administered ≥1 month after the end of the treatment.
Objectives of clinical trial
The objectives of the clinical trial were as
follows: i) To identify the quality of life of the enrolled
patients; ii) to analyse clinical, humoral and imaging variables;
iii) to determine CTX interruptions, as well as the number and
severity of adverse reactions; iv) to estimate the annual OS rate
in patients with advanced stage clinical PCA.
Data collection and processing
Data from the case report file were entered into the
database created for this purpose and processed using SPSS software
version 21.0 IBM.
Statistical analysis
A descriptive analysis of the data was carried out.
In the case of continuous quantitative variables, such as body mass
index (BMI), laboratory tests and QLQ-30 and -PR25 assessment
scales, the mean, standard deviation and standard error were
calculated, and the t-test was used for related samples, to compare
the variables' mean value at different points in the treatment. In
addition, Cohen's d was estimated to measure the clinical effects
magnitude. For discrete quantitative variables, such as the pain
scale or International Prostate Symptom Score (IPSS), the median,
mode, minimum and maximum were estimated, and the absolute change
and Wilcoxon signed-rank test were used to determine the variation
of the median at different time intervals during treatment.
P<0.05 was considered to indicate a statistically significant
difference. Absolute and relative frequencies were calculated for
the qualitative variables.
The initial variables included age and concurrent
diseases. The general condition of patients was assessed based on
the Karnofsky Scale, including patients with a score of ≥70.
Clinical stage was evaluated based on the AJCC's TNM scale, updated
in January 2018, which includes the risk of relapse.
Response variables included quality of life,
nutritional status, pain, prostate symptoms, prostate specific
antigen (PSA) and Response Evaluation Criteria in Solid Tumors
(RECIST).
The European Organisation for Research and Treatment
of Cancer Quality of Life Questionnaires (EORTC QLQ-C30 and -PR25)
were used to assess the quality of life of patients. Efficacy was
considered when ≥50% of patients on the OV treatment showed no
signs of deterioration in their quality of life.
Nutritional status was evaluated by BMI (BMI =
weight/height2), pain, according to an analogical
numerical scale, and prostate symptoms, according to the IPSS
questionnaire. A 50% decrease in the PSA baseline was considered an
acceptable humoral response. RECIST criteria were used to assess
measurable or quantifiable lesions by radiological means, all of
which were used to assess disease progression.
Toxicity was assessed according to the WHO criteria.
CTX interruptions were measured in terms of cycles, number,
frequency and causes. Relative variation was defined as the change
expressed in the percentage of one variable between its initial and
final value.
The PFS and OS rate per year were evaluated using
the Kaplan-Meier method. Mean and median time was estimated for
these curves.
Results
Patient characteristics
The study included 25 male patients with HRPC. They
were between the ages of 52 and 88 years, with an average age of 73
years. Concurrent diseases affected 23 patients, 92% of the
analysed sample, of which 52% had >1 pathology. Cardiovascular
diseases predominated, particularly high blood pressure (44%). Out
of a total of 25 cases classified as high risk of relapse at stage
IV, 96% presented a T category of 2b or higher with bone
metastases, with only one patient presenting with a T2a extension
of the primary tumour. Nevertheless, visceral and non-regional
lymph node metastases appeared in few patients (n=6). There were
more patients with a high histological grade. The inclusion took
into account that patients had a Karnofsky index (KPS) of 70-90 to
ensure treatment compliance. The PSA sample mean was 62 ng/ml
(Table II).
 | Table IIPatient characteristics (n=25). |
Table II
Patient characteristics (n=25).
| Variables | Value |
|---|
| Age, years | |
|
Mean ±
SD | 72.7±8.3 |
|
Range | 52-88 |
| Concurrent
pathologies by system, n (%) | |
|
HBP | 11(44) |
|
Bronchial
asthma | 2(8) |
|
Gastritis | 7(28) |
|
Ulcer | 9(36) |
|
Prostatic
hyperplasia | 7(28) |
|
Renal
insufficiency | 1(16) |
|
Atrophic
pyelonephritis of left kidney | 1(16) |
|
Osteoarthritis | 4(16) |
|
Herniated
disc | 2(8) |
|
Pathological
fracture | 1(16) |
|
Sickle cell
anaemia | 1(4) |
|
Diabetes
mellitus | 4(16) |
|
Neuropathy | 3(12) |
|
Hemiplegia | 1(16) |
|
Hemiparesis | 1(16) |
| TNM, n (%) | |
|
T2a | 1(4) |
|
T2b | 20(80) |
|
T2c | 3(12) |
|
T4 | 1(4) |
|
M1 | 3(12) |
|
M2 | 24(96) |
|
M3 | 3(12) |
| Mean PSA ± SD,
ng/l | 61.9±22.5 |
| Karnofsky
performance status scale, n (%) | |
|
70 (unable
to carry on normal activity or | 13(52) |
|
to do active
work) |
|
80 (normal
activity with effort) | 9(36) |
|
90 normal
activity | 3(12) |
Treatments administered,
interruptions, adverse reactions to CTX and treatment response
Treatment administered
The 25 participants underwent treatment with
docetaxel. Of them, 23 received 6-8 treatment cycles, according to
response and toxicity (OV group). All of them had been pre-treated
with hormone therapy and RT, like a high risk of relapse. Radiation
was administered at the end of CTX in only 2 patients (Table III).
 | Table IIITreatments administered,
interruptions, response and status at 1 year (n=25). |
Table III
Treatments administered,
interruptions, response and status at 1 year (n=25).
| Treatment | Value |
|---|
| Docetaxel + OV,
n | 23 |
| Docetaxel + RT +
OV, n | 2 |
| Interruptions | |
|
Transient
interruptions of CTX + OV, n (%) | 4(16) |
|
Mean
duration of interruptions, days | 13 |
|
Transient
interruptions of OV, n (%) | 3(12) |
|
Mean
duration of interruptions, days | 7 |
|
Permanent
interruptions of CTX + OV, n (%) | 9(36) |
| Response to
treatment, n (%) | |
|
Disease
progression | 11(44) |
|
Partial
response | 8(32) |
|
Stable
disease | 2(8) |
|
Not
evaluable | 4(16) |
| Patient status at 1
year, n (%) | |
|
Alive | 16(64) |
|
Deceased | 4(16) |
|
Lost to
follow-up | 5(20) |
Interruptions
A minimum of 6 treatment cycles were received by 20
patients (80% of participants). Four transient interruptions of
onco-specific + OV treatment lasting ~13 days on average were
caused by urinary tract infection in 2 patients, which were
resolved with antibiotic treatment, and 2 patients who travelled
outside the province. There were 3 transient interruptions of OV
treatment, due to viral diarrhoeal infection, acute cholecystitis
and transitory anorexia, which lasted 7 days on average. A total of
9 permanent interruptions occurred, 5 due to loss of patients to
follow-up and 4 due to patient mortality due to disease progression
(Table II).
Adverse effects
The most frequent adverse effects associated with
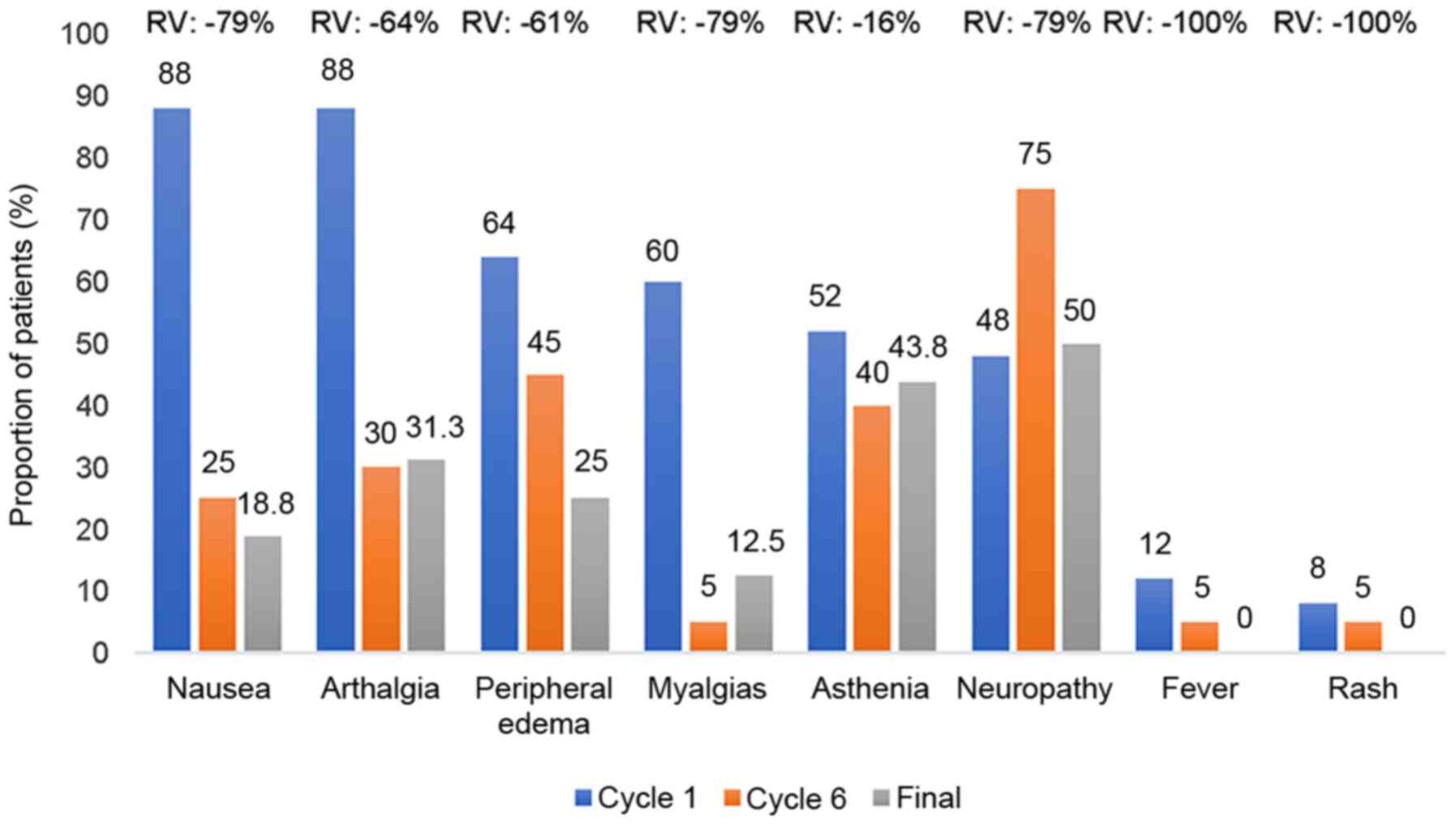
CTX were nausea and arthralgias, which afflicted 22 patients (88%)
at the beginning of the treatment, followed by peripheral oedema
present in 16, myalgia in 15 and asthenia in 13 patients. In
Fig. 1, the adverse reactions were
significantly decreased, when comparing the last treatment cycle
with the first. The only exception was neuropathy, which remained
virtually the same over time. On average, 79.1% were mild
reactions, 20.7% were moderate and only 0.2% were severe. In terms
of causes, 4.7% of events were not treatment-related, in 25.2% of
cases it was improbable that they were, in 28.7% it was possible
and in 46.1% probable. Of these patients, 77.4% recovered without
squeal.
Response to treatment
Of the 25 patients studied, 44% showed signs of
progression, 4 of which died from the disease. In the remaining
worsening cases, the symptoms and number of bone lesions increased,
and so did the PSA. Ten patients showed some response to treatment,
8 of which had a partial response, despite experiencing a decrease
in IPSS, an increase in BMI and a new bone lesion. However, the PSA
decreased without reaching normal values. The disease remained
stable in 2 patients, while 4 were not assessed due to loss to
follow-up (Table II).
Nutritional status
Body mass index was the indicator used for patient
follow-up. No significant body weight loss occurred during
treatment. The t-test for correlated samples was used to contrast
the presence of mean differences between the initial BMI and the
third, sixth and final cycle. The P-values obtained were 0.34, 0.53
and 0.32, respectively, all >0.05. This supports the argument
that the differences observed between the BMI mean throughout the
study were due to chance and not to significant nutritional changes
in the patients during treatment (data non shown).
Pain assessment at the different
cycles (Table IV)
A visual numerical pain scale was used, which at the
start of treatment had a median of 7, positioning the patients'
pain level at severe. At this point, 20 patients (80%) reported
having pain of >6, the most frequent score being recorded in
46.2% of participants. A marked and sustained decrease in pain was
observed as treatment cycles progressed, with the pain between the
first and second cycles being moderate, and that in the third cycle
being mild. The pain continued to decrease until the eighth cycle,
where no patient scored >4 in their assessment, meaning that
100% of patients experienced mild pain at the end of the treatment.
The Wilcoxon signed-rank test results showed statistically
significant differences between the mean pain scores between the
treatment onset, and the third, sixth and eighth cycles. For each
of these three comparisons, the result was P<0.001 (see Table II).
Prostate symptoms
At the beginning of treatment, the IPSS score that
assesses prostate symptom severity was a median of 25, ranking
patients at the severe symptom level. This indicator began to
decrease after the first cycle, with the symptom level being
moderate in the second cycle and mild in the seventh cycle.
Wilcoxon signed-rank test results showed that the differences in
the mean ISSP scores between the start of the treatment, and the
third, sixth and eighth cycles were statistically significant
(P<0.001; Table III).
Other clinical symptoms
Other dominant symptoms included oedema and
functional impairment of the lower limbs, affecting 72 and 52% of
patients, respectively. Symptomatic remission was observed in 78%
of patients suffering from oedema. The 13 patients who exhibited
functional impairment at the beginning of the study recovered their
functional capacity. It was related with the KPS, as shown in
Table I, however it was preferred
to be included in the QLQs to evaluate all those symptoms in
context.
Evolution of PSA levels
PSA levels only normalised in the 2 patients who
received a combined CTX and RT treatment. However, prostate antigen
levels were reduced by analysing their numbers between the first,
and the third, sixth and final treatment cycles. The t-test results
for mean differences of related samples were significant for all 3
cases (P<0.05), and the mean PSA of the treatment group at the
end of the study had dropped by 50% from the baseline (Table V).
 | Table VComparison of PSA between treatment
initiation and cycles 3, 6 and final cycle. |
Table V
Comparison of PSA between treatment
initiation and cycles 3, 6 and final cycle.
| | Mean |
|---|
| PSA at the
beginning and end of treatment | Estimate, ng/l | n | Standard
deviation | Standard error | Mean
difference | P-value (mean
difference) |
|---|
| Initial PSA | 61.9 | 25 | 22.5 | 4.5 | 20.42 |
<0.001a |
| PSA in cycle 3 | 41.5 | 25 | 27.7 | 5.5 | | |
| Initial PSA | 59.7 | 20 | 24.3 | 5.4 | 31.18 | 0.017a |
| PSA in cycle 6 | 41.4 | 20 | 29.9 | 6.7 | | |
| Initial PSA | 61.4 | 16 | 23.1 | 5.8 | 31.31 | 0.001a |
| PSA final
cycle | 30.5 | 16 | 24.0 | 6.0 | | |
Evolution of other laboratory
parameters
A significant decrease in the mean enzyme lactate
dehydrogenise (LDH) value of patients between the beginning and end
of treatment (P=0.028), which is indicative of the decrease in
chronic inflammation and oxidative stress. The heamogram values, as
well as leukocyte and platelet counts, remained stable. In
addition, no evidence of CTX-related haematological toxicity was
identified. In terms of liver function tests, alanine
aminotransferase and aspartate aminotransferase mean levels were
slightly higher than baseline values, but without statistical
significance (P=0.498 and P=0.059). Creatinine was slightly
increased, although not significantly different from the baseline
levels. No significant changes were observed in total protein,
albumin, calcium and glycaemia, which remained within the normal
ranges, confirming the absence of a nutritional or metabolic impact
on patients.
Quality of life
The EORTC QLQ-C 30 (version 3) and QLQ-PR 25
questionnaires were used for patients at the beginning of, and 1
year after, their inclusion in the study, to measure their quality
of life. Of the 16 patients who reached 1 year of treatment, 15
cases (representing 60% of the initial sample) showed an improved
quality of life, and 1 suffered no deterioration of this indicator
from the beginning of the study. As shown in Table V, the mean total quality of life
rose by 83.5% at the end of treatment, as compared with its initial
values. This increase was not only statistically significant
(P<0.001), but also showed an effect size of 1.9 (>0.8),
which on the Cohen scale represents a large-scale, clinically
perceptible effect. Similar behaviour, although more modest, was
observed in the scales corresponding to physical, cognitive, social
and emotional functions, where a significant increase was observed
in the mean value of the dimensions from a statistical point of
view (P<0.05), with a Cohen d index of 0.5-0.9. In other words,
a medium-to-high and clinically perceptible effect size was
observed in all cases. Similarly, there was a significant reduction
in parameters associated with symptoms such as fatigue, pain,
insomnia, anorexia and constipation (P<0.05).
Results of the supplementary QLQ-25 questionnaire
for patients with PCA showed a statistically significant decrease
in mean values achieved between the start of and 1-year of
treatment for urinary symptoms, incontinence, intestinal symptoms
and hormone treatment-related symptomatology; the effect size was
large from a clinical point of view. Dimensions associated with
sexual activity and function did not change significantly
(P>0.05; Table VI).
 | Table VIComparison of the mean values on the
quality life questionnaires scales (QLQ-C30 and PR25). |
Table VI
Comparison of the mean values on the
quality life questionnaires scales (QLQ-C30 and PR25).
| QLQ C30 | Initial evaluation
at start | Evaluation after
one year | Mean relative
variation, % | P-value (mean
difference) | Effect size
(Cohen's d) |
|---|
| Overall | 40.6 | 74.5 | 83.5 | <0.001 | 1.9 |
| Physical
function | 77.5 | 86.7 | 11.9 | 0.018 | 0.7 |
| Emotional
wellbeing | 77.6 | 87.0 | 12.1 | 0.04 | 0.5 |
| Cognitive
function | 87.5 | 99.0 | 13.1 | 0.022 | 0.9 |
| Social
function | 82.3 | 92.7 | 12.6 | 0.036 | 0.7 |
| Fatigue | 36.1 | 14.6 | -59.6 | 0.001 | -1.3 |
| Pain | 45.8 | 21.9 | -52.2 | <0.001 | -1.5 |
| Insomnia | 47.9 | 14.6 | -69.5 | <0.001 | -1.4 |
| Anorexia | 37.5 | 8.3 | -77.9 | <0.001 | -1.6 |
| Constipation
PR25 | 19.7 | 4.2 | -78.7 | 0.029 | -0.9 |
| Urinary
symptoms | 40.1 | 9.6 | -76.0 | <0.001 | -2.6 |
| Incontinence | 14.6 | 0.0 | -100.0 | 0.030 | -0.9 |
| Intestinal
symptoms | 5.2 | 1.0 | -80.1 | 0.015 | -0.8 |
| Hormonal
treatment-symptoms | 18.8 | 9.7 | -48.1 | 0.001 | -1.4 |
| Sexual
activity | 64.6 | 53.1 | -17.8 | 0.060 | -0.7 |
OS and PFS
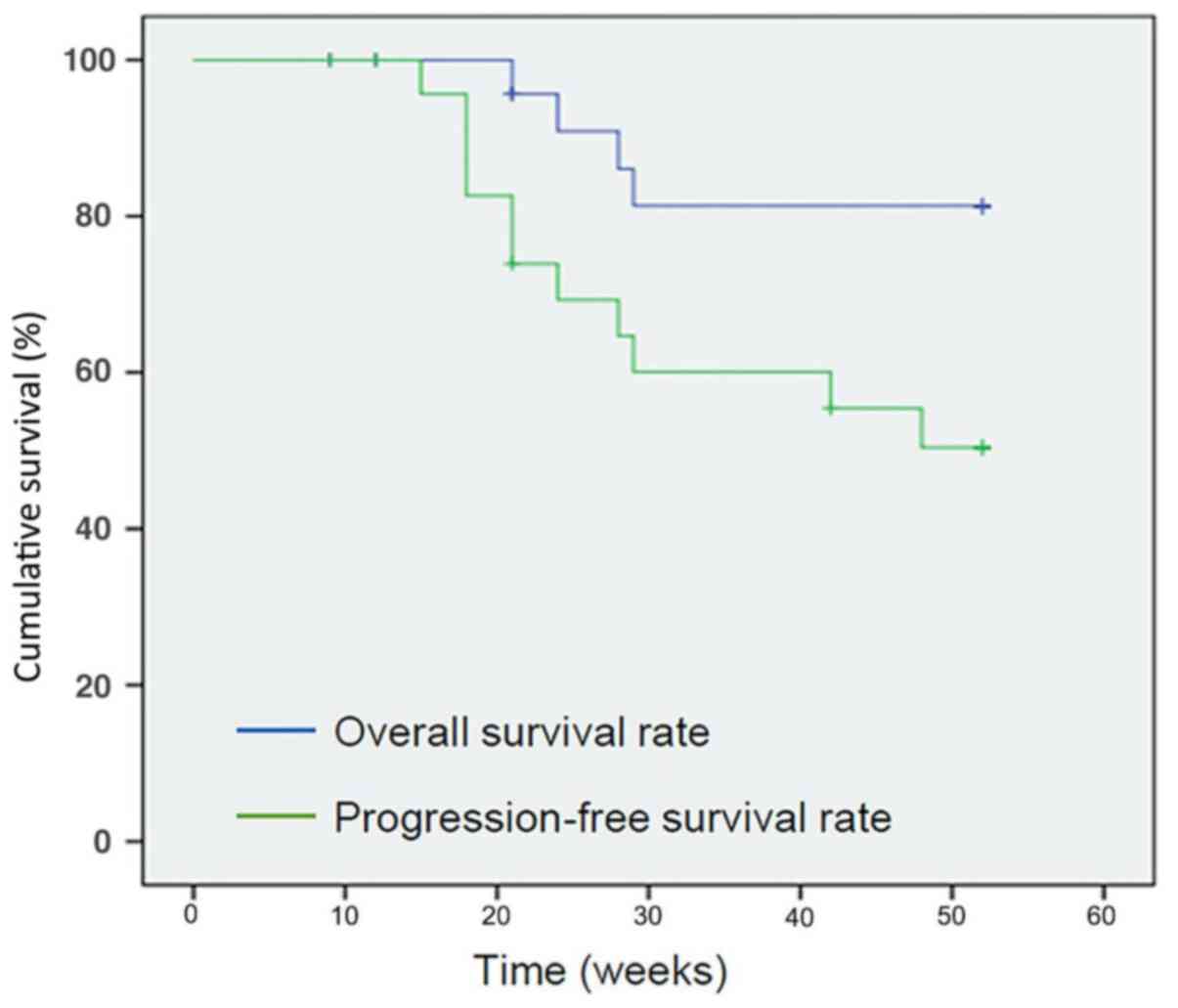
PFS
The estimated probability that an individual would
remain disease- or relapse-free from the date of entry into the
study to t1=26 weeks (6 months) and t2=52 weeks (1 year) was 0.69
and 0.50, respectively. At the end of the study 14 patients had not
yet developed signs of progression, exhibiting a PFS rate of 59%.
The mean PFS time was 39 weeks [95% confidence interval (CI), 33-45
weeks] with a mean standard error of 3 weeks (Fig. 2).
OS
The estimated probability that an individual would
survive from the date of entry into the study to t1=26 weeks (6
months) and t2=52 weeks (1 year) was 0.90 and 0.81, respectively.
At the end of the study, 16 patients were still alive, exhibiting
an OS rate of 64%. The mean survival time was 47 weeks (95% CI,
43-51 weeks), with a mean standard error of 2 weeks (Fig. 2).
Discussion
In this proof-of-concept, phase II, prospective,
non-randomised and open-label clinical trial, we explored the
efficacy and safety of OV in 25 male patients with a histological
diagnosis of HRPC.
PCA patients are generally diagnosed in locally
advanced stages (III-IV) at the age of >50 years old and with
associated chronic co-morbidities. HRPC exhibits significant
heterogeneity. Cases only showing an elevated PSA are markedly
different from cases with metastatic disease. The expression of the
PSA in these cases is lower than that of hormone-sensitive tumours
and may not correlate with cell proliferation. When hormone therapy
has failed, the course of the disease is aggressive, with a
variable progression rate of 18-24 months and survival rate of
24-36 months. The prognostic change according molecular subtypes
through a genetic variability: 17 Genes assay to predict PCA
aggressiveness in the context of Gleeson grade heterogeneity,
multifocality and biopsy under sample (26-29).
It is important to emphasise that the patients in
the present study displayed unfavourable prognostic factors:
Advanced age, high PSA and Gleason score, metastatic disease stage
and affected performance status. (Table II). Patient ages was 52-88 years
old, which approaches the range recorded in national statistics (60
and 80 years old). In Cuba, cancer continues to be the second
leading cause of mortality, exceeded only by cardiovascular
diseases, which explains the prevalence of cardiovascular
pathologies (56%) in the sample. The ages and clinical stages found
in the present study coincide with those found in studies focusing
on the primary and secondary medical assistance of Cuba (30-32). The clinical
symptoms exhibited by patients and the mean PSA value of 65.9 ng/ml
were due to advanced disease. Studies have shown that patients with
a PSA of a >49 are 6 times more likely to have a positive scan
(33,34).
The KPS scale was first used in 1949 on patients
receiving CTX (35). Since then it
has been used to predict cancer patient evolution. Performance
status was affected in 52% of patients, who were unable to carry
out their daily tasks or work (KPS of 70). A significantly worse
prognosis has been reported in HRPC patients with a <80%
Karnofsky score. In a study of gastric tumours, a statistical
correlation was observed between KPS and patient survival (36,37).
In the 90s, several studies reported an
amplification of the Bcl-2 gene in HRPC. Docetaxel is a
second-generation taxane that acts at the micro-tubule level and
promotes apoptosis through the induction of Bcl phosphorylation.
Two phase III clinical trials, one American (Southwest Oncology
Group) and a second Canadian and European (TAX 327), demonstrated
the superiority of docetaxel over mitoxantrone. The significant
improvement in pain control and quality of life was relevant (22%).
The PSA response rate was 45-48% (P<0.001). The mean survival of
patients treated with docetaxel every 3 weeks was 18.9 months. The
most common non-haematological adverse effects included alopecia
(50-65%), fatigue (49-53%), nausea (36-41%), diarrhoea (32-34%) and
neuropathy (24-30%). Subsequently, treatment was considered to
achieve symptom control, improved survival and low toxicity
(38-41).
The patients of the study were at an intermediate
and high risk, and they were treated following the protocol. Only 2
patients received radiation at the end of treatment. In a
retrospective study, 1,024 patients with intermediate-risk PCA were
treated with radiation with or without neoadjuvant and concurrent
Androgen-Deprivation therapy (ADT). Multivariate analysis revealed
that a primary Gleason pattern 4, percentage of positive biopsy
scores of ≥50, and presence of >1 intermediate-risk factor (Tic,
T2b-c, PSA 10-20 ng/ml, Gleason score 7) were significant
predictors of increased incidence of distant metastasis. The
authors then used these factors to separate the patients into
unfavourable and favourable intermediate-risk groups, and
determined that the unfavourable intermediate-risk group had a
worse PSA recurrence-free survival, distant metastasis, and
PCA-specific mortality than the favourable intermediate-risk group.
Thus, the study concluded that external beam radiation therapy +
ADT + docetaxel was a reasonable treatment option in appropriate
men with high- and very-high-risk disease (42).
The 2015 version of the guidelines added systemic
therapeutic options for men with progressive castration-naïve PCA.
Docetaxel combined with ADT was an option for men with high-volume
metastatic disease, based on results from the phase III ECOG 3805
trial, also known as the Chemohormonal Therapy Versus Androgen
Ablation Randomized Trial for Extensive Disease in Prostate Cancer
(CHAARTED), where a total of 790 randomly allocated men with
metastatic, androgen-stimulated PCA were treated with docetaxel
plus ADT or ADT alone. The patients in the combination arm
experienced a longer OS than those in the ADT arm [57.6 vs. 44.0
months; hazard ratio (HR), 0.61; 95% CI, 0.47-0.80; P<0.001].
Subgroup analysis showed that the survival benefit was more
pronounced in the 65% of participants with high-volume disease (HR,
0.60; 95% CI, 0.45-0.81; P<0.001). Men with low-volume disease
in CHAARTED may have benefited from the inclusion of docetaxel in
terms of survival (HR, 0.60; 95% CI, 0.32-1.13; P=0.11), although
the median OS was not reached in either arm of the study, and the
number of patients was low. The Systemic Therapy in Advancing or
Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE)
trial, a multi-arm, multistage phase III trial, included patients
with both M0 and M1 castration-naïve PCA starting ADT. The extent
of metastatic disease was not evaluated in the 1,087 men with M1
disease, but the median OS for all patients with M1 disease was 5.4
years in the ADT/docetaxel arm vs. 3.6 years in the ADT arm (a
difference of 1.8 years between groups, as compared with a 1.1-year
difference in CHAARTED). The strong statistical power of STAMPEDE
(n=2,962) had a clear survival advantage to the upfront CTX
approach (43).
The European GETUG-AFU 15 trial compared ADT and ADT
+ docetaxel treatment, but no survival benefit was identified
(median OS, 58.9 vs. 54.2 months; HR, 1.01; 95% CI, 0.75 1.36)
(41). Retrospective subset
analysis from this trial showed that participants with a
high-volume metastatic disease showed a non-significant 20%
reduction in the risk of mortality, with no reduction observed in
the low-volume subgroup (44).
Different authors have attempted to establish a
model to determine prognosis of tumours. Nguyen et al
(45) suggested that the number of
unfavourable risk factors is significantly associated with
PCA-specific mortality. PCA is the leading cause of mortality in
men with a minimum of three risk factors. Therefore, novel agents
should be considered for clinical trials designed to assess whether
they can prolong survival. A previous study showed that survival
remains disappointingly low in men presenting with M1 disease who
receive long-term ADT alone, despite active treatment with
supplement Oncoxin, even the majority of patients was M1 disease
being available at first failure of ADT (45,46).
Antioxidants had been used for the prevention and
treatment of cancer (47-50). They work by
restoring the natural antioxidants in the body, which are often
depleted following the completion of CTX, resulting in decreased
side effects and increased survival time for patients undergoing
CTX. The nutritional supplement OV is a composite formulation that
contains antioxidants. The extract of green tea, present in the OV
supplement, is an antioxidant studied for the prevention of cancer.
Its antitumour effect is mainly due to catechins, and particularly
the epigallocatechin-3-gallate, which is found in a high
concentration in green tea. Green tea extract is the most studied
and most active in the inhibition of oncogenesis and reduction of
oxidative stress. OV also contains vitamin C. It is an essential
nutrient acting as an antioxidant and a co-factor for various
enzymes. Heaney et al (51)
concluded that vitamin C supplementation may exert adverse effects
during cancer treatment. The redox active from of vitamin C has a
therapeutic effect on tumour cells and synergistic effects with
CTX. This antitumour effect is based on the induction of apoptosis
and cell cycle arrest (52-55).
The quality of life of patients has become a
consideration in oncology, as a consequence of the development of
highly aggressive treatments. It now is thought that the effect of
the therapeutic strategy on the patient's quality of life should be
an endpoint of clinical trials. Quality of life is important in the
research of advanced PCA (56).
According to QLQ-30, the overall quality of life of the patients
improved significantly (P<0.001) with clear clinical evidence
(Cohen's d of 1.9), which affected all aspects of the individual:
Physical, emotional, cognitive and social. Anorexia, asthenia and
weight loss are common in advanced stages of the disease. In the
present study, fatigue, oedema and bone pain led to functional
impairment, with difficulty walking observed in the studied
patients. However, a significant decrease in fatigue, pain and
anorexia, as well as a lack of weight loss (BMI), occurred when the
two treatments were combined. The literature supports that
nutritional care should be integrated into oncology, due to its
significant contribution to quality of life. Nutritional
intervention increases the tolerance and response to cancer
treatment (57,58).
A previous study with 640 patients provided
evaluable information on protocol-defined progression that led to
further treatment. An evaluation of men in a post-docetaxel setting
should consider the type of progression, duration of treatment, and
known pre-treatment prognostic factors. The study also provided
evidence of benefits resulting from the continuation of CTX beyond
progression, but only for men who exhibited isolated worsening of
pain. A nomogram was constructed and internally validated with a
concordance index of 0.70(59).
However, when OV was used alongside CTX, even the patients in the
present study that had exhibited moderate and severe pain
pre-treatment, saw benefits in their quality of life post-OV
treatment. OV is a vitamin-rich nutritional supplement with
carbohydrate-protein nutritional requirements, which improves
asthenia, and therefore quality of life. A preclinical study on OV
in Her2-positive breast cancer led to weight gain and improvement
of quality of life in participants (60).
The IPSS is used to evaluate changes in the severity
of symptoms and efficacy of treatment. A significant improvement in
urinary symptoms was shown by QLQ 25. The same was suggested by the
ISSP in lower urinary tract diseases, where a significant
improvement was observed in the third and second treatment cycles.
In 2006, a randomized, double blinded, placebo-controlled study was
performed as a 1-year proof-of-principal trial to assess the safety
and efficacy of catechins for chemoprevention in PCA. That was the
first study showing that catechins have a potent in vivo
chemopreventive effect in human PCA. A secondary observation was
the significant improvement of lower urinary tract symptoms, as
determined by the IPSS and Quality of Life Scale (61,62).
OV contains green tea catechins, which explain the similar results
obtained in both studies. Urinary obstruction is associated with
the T2b tumour size of the prostate gland. The improvement in
prostate symptoms suggested tumour reduction following combination
treatment. In a preclinical study of colorectal cancer metastasis
in the liver, it was demonstrated that Ocoxin oral solution slows
down tumour growth (63). It may be
used in combination with a standard therapy to potentiate
antiproliferative action in acute myeloid leukaemia and lung cancer
(64,65).
Although a high PSA was observed in the majority of
cases, it decreased in 80% of them, with a decrease observed in 50%
of the baseline. Improved survival was observed in patients with
HRPC and a >50% drop in PSA levels. This correlated with a 68%
response in the measurable disease (66-68). In the trial,
9,346 (INT-0162) the value following androgen deprivation was a
strong independent predictor of survival in new metastatic PCA
(69).
LDH is released into the blood from different
tissues, mainly the liver. It is considered a prognostic and
predictive biomarker for visceral metastatic disease. High values
suggest a poor prognosis. Visceral metastasis only occurred in 2
patients, both of whom succumbed to the disease. In general, cancer
produces chronic inflammation with high LDH levels. Treatment with
CTX and RT releases free radicals and produces oxidation. However,
this enzyme decreased significantly in the present study, pointing
to a potential compensatory effect induced by OV in this context
(70), and suggesting a better
response to combination therapy. The study on metastatic renal cell
carcinoma demonstrated LDH as a biomarker for survival (71).
The response to treatment with symptomatic and
PSA-lowering outcomes was significant. However, according to the
RECIST criteria, a partial response to treatment predominated (8
patients). This criterion assumed more value in the disease
progression analysis. The typical full response, partial response,
stable disease and progressive disease criteria are not very useful
in HRPC. This is due to the fact that 80-90% of patients do not
have bi-dimensional measurable disease, based on imaging tests.
Bone metastases are difficult to quantify. The mixed responses, in
which the regression of certain osseous metastases is followed by
the progression of others, and interindividual variability in the
interpretation of the explorations, mean that they are not
systematically used to evaluate treatment response. Angulo et
al (72) in his report on
survivors of castration-resistant PCA reached the conclusion that
the treatment extends survival expectations in a clinical practice
setting, and that prognostic predictors can be identified in these
patients. The study found that younger patients without metastasis
at diagnosis had a better prognosis. Patients with higher PSA
levels (>45 ng/ml; P=0.09) and a Gleason pattern 5 in the biopsy
had a less favourable outcome (69). Schröder reported similar findings
(73).
de Bono et al (74) found differences in metastatic
castration-resistant PCA between favourable and unfavourable
prognostic factors. Patients with unfavourable pre-treatment
circulating tumour cells (CTCs; 57%) had a shorter OS (median OS,
11.5 vs. 21.7 months). The unfavourable post-treatment CTC count
also predicted a shorter OS (median OS, 6.7-9.5 vs. 19.6-20.7
months). According to de Bono et al (74) results which were based on patients
with unfavourable prognostic factors, the mean estimate of the
post-treatment survival time was 47 weeks (74). Different authors have attempted to
establish a model to determine the prognosis of tumours (75,76).
Overall, treatment adherence was positive: 80% of
the cases completed the 6 CTX cycles with a decrease in toxicity
frequency and intensity observed throughout the treatment. The most
frequent toxicities were arthralgia, myalgia, asthenia, anorexia
and neuropathy. The neuropathy seemed to increase during treatment;
it was an effect of neuropathy caused by pathologies present in the
patients, including diabetes and nervous system disease. It was
described as the accumulative effect over 400 mg/m2,
which explain the increment by cycles. Although, determining the
causes of the slow performance status and pain through the physical
exam was challenging, and there was no physiopathology test to
confirm, it was up to the patients to describe their symptoms. The
neuropathy was an imprecise sign, even though it was compensated at
the end of the study. Rashes disappeared during treatment. No
patients exhibited neutropenia, which is the most frequent
docetaxel-related haematological toxicity (77,78).
The findings in the PCA patients could be an due to
the supportive treatment with OV, given its anti-inflammatory and
immunomodulator properties. Several studies have shown a decrease
in the CTX and RT toxicities, with an increase in the OS rate, as a
result of the synergism of the treatments. In a Japanese clinical
study carried out in terminal stage patients of hepatocellular
carcinoma, it was observed that 21% of patients were alive at the
end of the treatment in the treatment group, whereas in the control
group they were dead (79). Another
clinical study in Bangladesh demonstrated more tolerance to cancer
treatment, which was found to improve patient survival and quality
of life (80). A clinical study of
pancreatic cancer proved that OV reduced the stromal-mediated
chemoresistance (81). More
recently, clinical studies of head and neck and cervico-uterine
cancer reported a decrease in toxicity and improvement in the
quality of life of patients following supportive OV treatment
(82-84).
A study by Tan (84)
reviewed recent abstracts and literature through Medline/Pub Med,
using the following key words: Androgen-independent/HRPC, novel
treatment options, Phase II, III trials, and meeting
abstracts/presentations. Tan concluded that there is a need to
improve on this survival benefit, since, with the standard
treatment, the relapse-free survival among responders is often
short (6 months) and patients often exhibit cancer progression,
which leads to mortality. There is a need to develop less toxic
drugs that would significantly improve survival (85,86).
OV, when used as supportive therapy, decreased the toxicity of
docetaxel and improved patient survival.
In conclusion, the OV nutritional supplement, when
used in combination with onco-specific treatment in patients with
PCA, was found to be highly efficient, as it significantly improved
the overall quality of life of patients, promoted greater tolerance
to CTX, and reduced the occurrence of related adverse events.
Therefore, it contributed to a greater number of treatment cycles
being completed per patient. The above results, combined with the
fact that the prostate antigen decreased, nutritional status was
preserved, and haematological and hepatic complications were
avoided, resulted in high survival rates and a delayed onset of
signs of progression.
Acknowledgements
The authors would like to thank Mr. David Márquez
Soriano (Molecular Biologist, Catalysis S.L., C/Macarena 14, 28016
Madrid, Spain) for his general support and help for facilitating
the communication between Spain and Cuba.
Funding
The present study was supported in part by a grant
from Laboratorios Catalysis (Madrid, Spain), who provided the
Oncoxin-Viusid for the study protocol, but played no role in the
study design, collection and interpretation of data, writing of the
manuscript or submission for publication.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MIFR contributed in conducting the clinical trial,
is the corresponding author and contributed to writing the paper.
The author has been involved in contributions to conception and
design, acquisition of data, analysis and interpretation of data,
and drafting the manuscript or revising it critically for important
intellectual content. LBM, FOC, FHC, CSR and AHG contributed as
research participants. They were involved in acquisition of data,
and discussion and interpretation of the results. CSR and IBL
assisted with imaging realization and interpretation. EVG and IBL
helped with lab tests and imaging realization and interpretation.
KPM contributed to the pharmacy labours, and provided important
support in the treatment, control of the trail and revising the
manuscript. JJL contributed to conception, design, biostatistical
analysis and data management. ES was involved in the study concept
and design; acquisition of data; analysis and interpretation of
data; drafting of the manuscript; critical revision of the
manuscript for important intellectual content and statistical
analysis, and corresponding author. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics and
Clinical Research Committee of General Calixto Garcia University
Hospital (approval no. 000181/12). At recruitment, all patients
signed the informed consent form which explained the objective and
design of the study and provided information regarding the product
of research. It also explained that the patient could abandon the
trial without any damage or deny the medical assistance. The
consent referred to the confidentiality of the patient with a code
number.
Patient consent for publication
All patients that participated in the present study
provided written informed consent for the publication of any
associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Center MM, Jemal A, Lortet-Tieulent J,
Ward E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bell KJ, Del Mar C, Wright G, Dickinson J
and Glasziou P: Prevalence of incidental prostate cancer: A
systematic review of autopsy studies. Int J Cancer. 137:1749–1757.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brawley OW: Prostate cancer epidemiology
in the United States. World J Urol. 30:195–200. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sanso JF, Soberate S, Alonso GP and Torres
VRM: Mortality from cancer in Cuba. Revista Cubana de Salud
publica. 36:78–94. 2010.
|
|
6
|
MINSAP: Dirección de registros médicos y
estadísticas de salud. Anuario estadístico de salud. La Habana:
52-68, 2018 (In Spanish). Access date March 16, 2020.
|
|
7
|
SEER database of epidemiological
surveillance in the EEUU, 2008-2014. urihttps://seer.cancer.gov/archive/csr/1975_2014/results_merged/sect_23_prostate.pdfsimplehttps://seer.cancer.gov/archive/csr/1975_2014/results_merged/sect_23_prostate.pdf.
Access date March 16, 2020.
|
|
8
|
Mohler JL, Armstrong AJ, Bahnson RR,
D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano
CS, Horwitz EM, et al: Prostate cancer, version 1.2016. J Natl
Compr Canc Netw. 14:19–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
European Association of Urology. Mottet N,
Bellmunt J, Briers E, Bolla M, Bourke L, Cornford P, et al.
Guidelines on Prostate Cancer, 2017. EAU Guidelines. ISBN
978-90-79754-91-5.urihttps://uroweb.org/wp-content/uploads/09-Prostate-Cancer_2017_web.pdfsimplehttps://uroweb.org/wp-content/uploads/09-Prostate-Cancer_2017_web.pdf.
Access date March 16, 2020.
|
|
10
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA: Grading Committee: The 2014
International Society of Urological Pathology (ISUP): Consensus
conference on Gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Amin MB, Edge S, Greene F, Byrd DR,
Bokland RK, Washington RK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC et al, (eds). AJCC Cancer Staging Manual, 8th
ed. Springer International Publishing, Berlin, 2017.
|
|
12
|
Devlin HL and Mudryj M: Progression of
prostate cancer: Multiple pathways to androgen independence. Cancer
Lett. 274:177–186. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Leibowitz-Amit R and Joshua AM: Targeting
the androgen receptor in the management of castration-resistant
prostate cancer: Rationale progress and future directions. Cur
Oncol. 19 (Suppl 3):S22–S31. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Montgomery RB, Mostaghel EA, Vessella R,
Hess DL, Kalhorn TF, Higano CS, True LD and Nelson PS: Maintenance
of intratumoral androgens in metastatic prostate cancer: A
mechanism for castration-resistant tumor growth. Cancer Res.
68:4447–4454. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Michaud JE, Billups KL and Partin AW:
Testosterone and prostate cancer: An evidence-based review of
pathogenesis and oncologic risk. Ther Adv Urol. 7:378–387.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512.
2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moro Soria A, Laborí Cardá C, López AB and
Hernández JG: El cáncer de próstata resistente a castración.
Mecanismos de progresión y nuevos tratamientos. Rev Cub Urol.
1:106–122. 2012.(In Spanish).
|
|
18
|
Oh B, Figtree G, Costa D, Eade T, Hruby G,
Lim S, Elfiky A, Martine N, Rosenthal D, Clarke S and Back M:
Oxidative stress in prostate cancer patients: A systematic review
of case control studies. Prostate Int. 4:71–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khandrika L, Kumar B, Koul S, Maroni P and
Koul HK: Oxidative stress in prostate cancer. Cancer Lett.
282:125–136. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
García Triana BE, Saldaña Bernabeu A and
Saldaña García L: El estrés oxidativo y los antioxidantes en la
prevención del cáncer. Revista Habanera de Ciencias Médicas.
12:187–196. 2012.(In Spanish).
|
|
21
|
Vallejo-Zamudio E, Rojas-Velásquez A and
Torres-Bulgarin O: Una poderosa herramienta en la medicina
preventiva del cáncer: Los antioxidantes. El Residente. 12:104–111.
2017.(In Spanish).
|
|
22
|
Singh K, Bhori M, Arfat Y, Bhat G and
Marar T: Antioxidants as precision weapons in war against cancer
chemotherapy induced toxicity. Exploring the armory of obscurity.
Saudi Pharm J. 26:177–190. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Simone CB II, Simone NL, Simone V and
Simone CB: Antioxidants and other nutrients do not interfere with
chemotherapy or radiation therapy and can increase kill and
increase survival, part 2. Altern Ther Health Med. 13:40–47.
2007.PubMed/NCBI
|
|
24
|
González A, et al: Prevention and
antitumor treatment. In: Antiviral and Others Degenerative
Diseases. Pharmaceutical Laboratory Catalysis, S.L.(C/ Macarena 14,
Madrid, CP 28016). January 2001. Scientific Department. Unpublished
book. Available upon request to the laboratory.
|
|
25
|
Eficacia en cáncer del Oncoxin+Viusid.
Revista mundial de salud y medicina 176, 2014 (In Spanish). Access
date March 16, 2020.
|
|
26
|
Corte AM, Rodriguez E and Kobeleienky M:
Prospective open study on safety and effectiveness of supplement on
tumor L markers and quality of life in patients of cancer of the
prostate. Rev Solidaridad. Argentina, 2013.
|
|
27
|
Azzouni F and Mohler J: Biology of
castration-recurrent prostate cancer. Urol Clin North Am.
39:435–520. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Helfand BT and Catalon WJ: The
epidemiology and clinical implications of genetic variation in
prostate cancer. Urol Clin North Am. 41:277–297. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tomlins SA, Alshalalfa M, Davicioni E,
Erho N, Yousefi K, Zhao S, Haddad Z, Den RB, Dicker AP, Trock BJ,
et al: Characterization of 1577 primary prostate cancers reveals
novel biological and clinic pathologic insights into molecular
subtypes. Eur Urol. 68:555–567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Klein EA, Cooperberg MR, Magi-Galluzzi C,
Simko JP, Falzarano SM, Maddala T, Chan JM, Li J, Cowan JE, Tsiatis
AC, et al: A 17-gene assay to predict prostate cancer
aggressiveness in the context of Gleason grade heterogeneity, tumor
multifocality, and biopsy undersampling. Eur Urol. 66:550–560.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Correa Zaporta D. Characterization of
patients with prostate cancer. Two consultories. Reyna Polyclinic.
Thesis General Integral Medicine Specialty 2016-2017 (not
publishable). General Calixto García University. Avenida
Universidad y J Plaza, Ciudad de la Havana, CP10400, Cuba (In
Spanish).
|
|
32
|
Rodríguez Borrego LD: Detección del cáncer
de próstata en la comunidad Policlínico Docente Wilfredo Santana.
Tesis Especialidad de Urología, La Habana. Rev Cub Urol.
7(e47)2018.
|
|
33
|
Borrego Díaz L, González Sapsin K, Borrego
Pino L, Dovale Borjas B and González Sapsin K: Diagnóstico tardío
del cáncer en adultos mayores en el hospital V. I. Lenin. Correo
Científico Médico de Holguín 12, 2018.
|
|
34
|
García PHA and Varela R: Validez
diagnóstica del antígeno prostático específico para la presencia de
metástasis en pacientes con cáncer de próstata. Urol Colomb.
19:13–18. 2010.
|
|
35
|
Salman JW, Schoots IG, Carlsson SV,
Jenster G and Roobol MJ: Prostate specific antigen as a tumor
marker in prostate cancer: Biochemical and clinical aspects. Adv
Exp Med Biol. 867:93–114. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Karnofsky DA, Abelarman WH, Graver LF and
Burchenal JH: The use of the nitrogen mustards in the palliative
treatment of carcinoma. With particular reference to bronchogenic
carcinoma. Cancer. 1:634–656. 1948.
|
|
37
|
Pérez-Cruz PE and Francisco Acevedo C:
Escalas de estado en cáncer. Gastroenterol Latinoam. 25:219–226.
2014.
|
|
38
|
Herrera-Guerrero MI, Torres Gómez A and
Allende Pereza S: Correlación del estado funcional de Karnofsky con
la supervivencia de pacientes con tumores de origen
gastrointestinal en un servicio de cuidados paliativos. Cir Gen.
36:134–137. 2014.
|
|
39
|
Petrylak DP: The current role of
chemotherapy in metastatic hormone-refractory prostate cancer.
Urology. 65 (Suppl 5):S3–S7, Discussion 7-8. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: Updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zumsteg ZS, Spratt DE, Pei I, Zhang Z,
Yamada Y, Kollmeier M and Zelefsky MJ: A new risk classification
system for therapeutic decision making with intermediate-risk
prostate cancer patients undergoing dose-escalated external beam
radiation therapy. Eur Urol. 64:895–902. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
van Soest RJ and de Wit R: Irrefutable
evidence for use of docetaxel in newly diagnosed metastatic
prostate cancer: Result from de STAMPEDE and CHAARTER trials. BMC
Med. 13(304)2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gravis G, Boher JM, Joly F, Soulié M,
Albiges L, Priou F, Latorzeff I, Delva R, Krakowski I, Laguerre B,
et al: Androgen deprivation therapy (ADT) plus docetaxel versus ADT
alone in metastatic non castrate prostate cancer: Impact of
metastatic burden and long-term survival analysis of the randomized
phase 3 GETUG-AFU15 trial. Eur Urol. 70:256–262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nguyen PL, Chen MH, Catalona WJ, Moul JW,
Sun L and D'Amico AV: Predicting prostate cancer mortality among
men with intermediate to high-risk disease and multiple
unfavourable risk factors. Int J Radiat Oncol Biol Phys.
73:659–664. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
James ND, Sydes MR, Mason MD, Clarke NW,
Dearnaley DP, Millman MRS, Parker C, Ritchie AW, Russell JM,
Staffurth J, et al: Docetaxel and/or zoledronic acid for
hormone-naive prostate cancer: First overall survival results from
STAMPEDE (NCT00268476). J Clin Oncol. 33 (Suppl)(5001)2015.
|
|
47
|
Van Poppel H and Tombal B: Chemoprevention
of prostate cancer with nutrient and supplement. Cancer Manag Res.
3:91–100. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sarkar, et al: Perspective for cancer
prevention with natural component. Endocrin Relative Cancer.
17:R195–R212. 2010.
|
|
49
|
Amin AR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Coronado HM, Vega y León S, Gutiérrez TR,
Vázquez FM and Radilla VC: Antioxidantes: Perspectiva actual para
la salud humana. Rev Chil Nutr. 42:206–212. 2015.(In Spanish).
|
|
51
|
Heaney ML, Gardner JR, Karasavvas N, Golde
DW, Scheimberg DA, Smith EA and O'Connor OA: Vitamin C antagonizes
the cytotoxic effects of antineoplastic drug. Cancer Res.
68:8031–8038. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Roomi WM, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: In vivo antitumor effect of
ascorbic acid, lysine, proline and green tea extract on human colon
cancer cell HCT 116 xenograіs in nude mice: Evaluation of tumor
growth and immunohistochemistry. Oncol Rep. 13:421–425.29.
2005.PubMed/NCBI
|
|
53
|
Tang Y, Zhao DY, Elliot S, Zhao W, Curiel
TJ, Beckman BS and Burrow ME: Epigallocatechin-3 gallate induces
growth inhibition and apoptosis in human breast cancer cells
through surviving suppression. Int J Oncol. 31:705–711.
2007.PubMed/NCBI
|
|
54
|
Peng G, Dixon DA, Muga SJ, Smith TJ and
Wargovich MJ: Green tea polyphenol (-)-epigallocatechin-3-gallate
inhibits cyclooxygenasa-2 expression in colon carcinogenesis. Mol
Carcinog. 45:309–319. 2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Frömberg A, Gutsch D, Schulze D,
Vollbracht C, Weiss G, Czubayko F and Aigner A: Ascorbate exerts
anti-proliferative effects through cell cycle inhibition and
sensitizes tumor cells towards cystostatic drugs. Cancer Chemoter
Pharmacol. 67:1157–1166. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ferriols Lisart R, Ferriols Lisart F, Alós
Almiñana M and Magraner Gil J: Calidad de vida en oncología
clínica. Farm Hosp. 19:315–322. 1995.
|
|
57
|
Marín Caro MM, Laviano A and Pichard C:
Nutritional intervention and quality of life in adult oncology
patients. Clin Nutr. 26:289–301. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Faria A, Coriat J, Rueda M, Cardona C and
Rosselli D: Nutritional supplements as modifiers of morbidity and
mortality in patients with cancer. Latin American Nutrition Files.
67:169–177. 2017.
|
|
59
|
Armstrong JA, Garrett-Mayer E, De Wit R,
Tannock I and Mario Eisenberger M: Prediction of survival following
first-line chemotherapy in men with castration-resistant metastatic
prostate cancer. Clin Cancer Res. 16:203–211. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hernández-García S, González V, Sanz E and
Pandiella A: Effect of ocoxin oral solution in HER2-overexpressing
breast cancer. Nutr Cancer. 67:1159–1169. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Preciado-Estrella DA, Kaplan SA, Iturriaga
Goyón E, Ramón-Trejo E and Mayorga Gómez E: Auza Benavides A y
colaboradores: Comparación del Índice Internacional de Síntomas
Prostáticos versus Escala Visual Análoga Gea® para la
evaluación de los síntomas de la vía urinaria inferior. Rev Mex
Urol. 77:372–382. 2017.(In Spanish).
|
|
62
|
Bettuzzy S, Brauzi M, Rizzy F, Castagnetti
G, Peracchia G and Corti A: Chemoprevention of human prostate
cancer by oral administration of green catechins in volunteers with
high grade prostate intraepithelial neoplasm: A preliminary report
from a one year proof of principle study. Cancer Res. 66:1234–1240.
2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Márquez J, Mena J, Hernández-Unzueta I,
Benedicto A, Sanz E, Arteta B and Olaso E: Ocoxin® oral
solution slows down tumor growth in an experimental model of
colorectal cancer metastasis to the liver in balb/c mice. Oncol
Rep. 35:1265–1272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Diaz-Rodriguez E, Hernández-Garcia S, Sanz
E and Pandiella A: Antitumor effect of ocoxin on acute myeloid
leukemia. Oncotarget. 7:6231–6242. 2016.
|
|
65
|
Diaz-Rodríguez E and Sanz E: Pandiella: A
Efecto antitumoral de Oncoxina un suplemento nutricional que
contiene compuestos naturales, en el cáncer de pulmón de células
pequeñas. Revista Internacional de Oncología. 5:113–123. 2018.
|
|
66
|
Parker C, Gillessen S, Heidenreich A and
Horwich A: ESMO Guidelines Committee: Cancer of the prostate: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 26 (Supl 5):v69–v77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wolf AM, Wender RC, Etzioni RB, Thompson
IM, D'Amico AV, Volk RJ, Brooks DD, Dash C, Guessous I, Andrews K,
et al: American cancer society guideline for the early detection of
prostate cancer: Update 2010. CA Cancer J Clin. 60:70–98.
2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kontos CK, Adamopoulos PG and Scorilas A:
Prognostic and predictive biomarkers in prostate cancer. Expert Rev
Mol Diagn. 15:1567–1576. 2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Hussain M, Tangen CM, Higano C,
Schelhammer PF, Faulkner J, Crawford ED, Wilding G, Akdas A, Small
EJ, Donnelly B, et al: Absolute prostate-specific antigen value
after androgen deprivation is a strong independent predictor of
survival in new metastatic prostate cancer: Data from Southwest
Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 24:3984–3990.
2006.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Pérez-Peña J, Diaz-Rodríguez E, Sanz E and
Pandiella A: Papel Central de la regulación del ciclo celular en la
acción antitumoral de la oncoxina. Nutrients 11, 2019.
|
|
71
|
Amstrong AJ, Goerge DJ and Halbi S: Serum
lactate dehydrogenase (LDH) as a biomarker for survival with mTOR
inhibition in patients with metastatic renal cells carcinoma (RCC).
J Clin Oncol. 28 (Suppl 15)(S4631)2010.
|
|
72
|
Angulo J, Romero I, Díaz-Puente MT, Enrech
S, Díez R and Molina T: Supervivencia del cáncer de próstata
resistente a la castración en la práctica clínica y el papel del
tratamiento. Rev Colomb Cancerol. 21:95–103. 2017.
|
|
73
|
Schröder FH, Hugosson J, Roobol MJ,
Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H,
Zappa M, et al: Prostate-cancer mortality at 11 years of follow-up.
N Engl J Med. 366:981–990. 2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Steyerberg EW, Moons KG, Vander Windt DA,
Hayden JA, Perel P, Schroter S, Riley RD, Hemingway H and Altman
DG: PROGRESS Group: Prognosis Research Strategy (PROGRESS) 3:
Prognostic model research. PLoS Med. 10(e1001381)2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Weiner AB, Matulewicz RS, Eggener SE and
Schaeffer EM: Increasing incidence of metastatic prostate cancer in
the United States (2004-2013). Prostate Cancer Prostatic Dis.
19:395–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Pérez BP, Corral JJ and Fernández de
Tejerina CMA: Neurotoxicidad por quimioterapia. Capitulo 6:
112-116.
|
|
78
|
Al Martin, UPA Rodríguez, LPA Rodríguez:
Manejo de la toxicidad neurológica Manual de la SEOM de cuidados
continuos, 2nd edition, pp 156. urihttp://www.seom.org/seomcms/images/stories/recursos/MANUAL_SEOM_CUIDADOS_CONTINUOS_Segunda_edicion.pdfsimplehttp://www.seom.org/seomcms/images/stories/recursos/MANUAL_SEOM_CUIDADOS_CONTINUOS_Segunda_edicion.pdf.
Access date March 16, 2020.
|
|
79
|
Hernández-Unzueta I, Benedicto A, Olaso E,
Sanz E, Viera C, Arteta B and Márquez J: Ocoxin oral
solution® as a complement to irinotecan chemotherapy in
the metastatic progression of colorectal cancer to the liver. Oncol
Lett. 13:4002–4012. 2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Uddin DM, Islam M, Mahmood I, Gosha AK,
Khatun RA and Kundu S: Findings of the 3-month supportive treatment
with Oncoxin solution beside the standard modalities of patients
with different neoplastic diseases. TAJ. 22:172–175. 2009.
|
|
81
|
Hernández-Unzueta I, Benedicto A, Romayor
I, Herrero A, Sanz E, Arteta B, Olaso E and Márquez J: Ocoxin oral
solutions exerts and antitumor effect in pancreatic cancer and
reduce the stromal mediated chemotherapy. Pancreas. 48:555–567.
2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Rivas CI, Alert SJ, Alfonso G, Candanedo
H, Cuervo Y, Mestre B, Cabello JR, Lence J, Lugoyo M and Sanz E:
Oncoxin-Viusid with radiotherapy and chemotherapy in patients with
head and neck cancer: Results from a phase II, randomised, double
blind study. J Cancer Sci Ther. 10(10)2018.
|
|
83
|
Lorente R, Duran D, Viamonte J, Lence AJ,
Reyes R and Sans E: Efficacy of Oncoxin-Viusid on the reduction of
adverse reactions to chemotherapy and radiotherapy in patients
diagnosed with cervical cancer and endometrial adenocarcinoma. J
Cancer Ther. 11:276–295. 2020.
|
|
84
|
Tan W: Promising new treatment option for
metastatic androgen independent prostate cancer. Actas Urol Esp.
31:680–685. 2007.(In Spanish). PubMed/NCBI View Article : Google Scholar
|
|
85
|
Huguet Pérez J, Maroto Rey P, Palou
Redorta J and Villavicencio Mavrich H: Hormone-refractory prostate
cancer. Modifications of the therapeutic strategies since
chemotherapy proved its usefulness. Actas Urol Esp. 30:123–133.
2006.(In Spanish). PubMed/NCBI View Article : Google Scholar
|
|
86
|
Berlin A and Fernández MI: Advances in the
treatment of castration-resistant prostate cancer: Emphasis in new
hormonal therapies. Rev Med Chile. 143:223–236. 2015.(In Spanish).
PubMed/NCBI View Article : Google Scholar
|