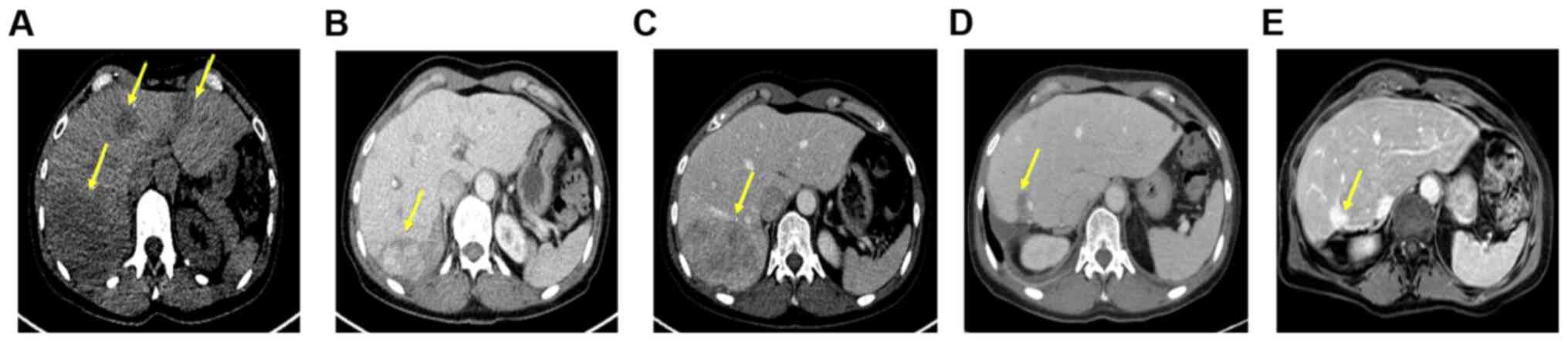
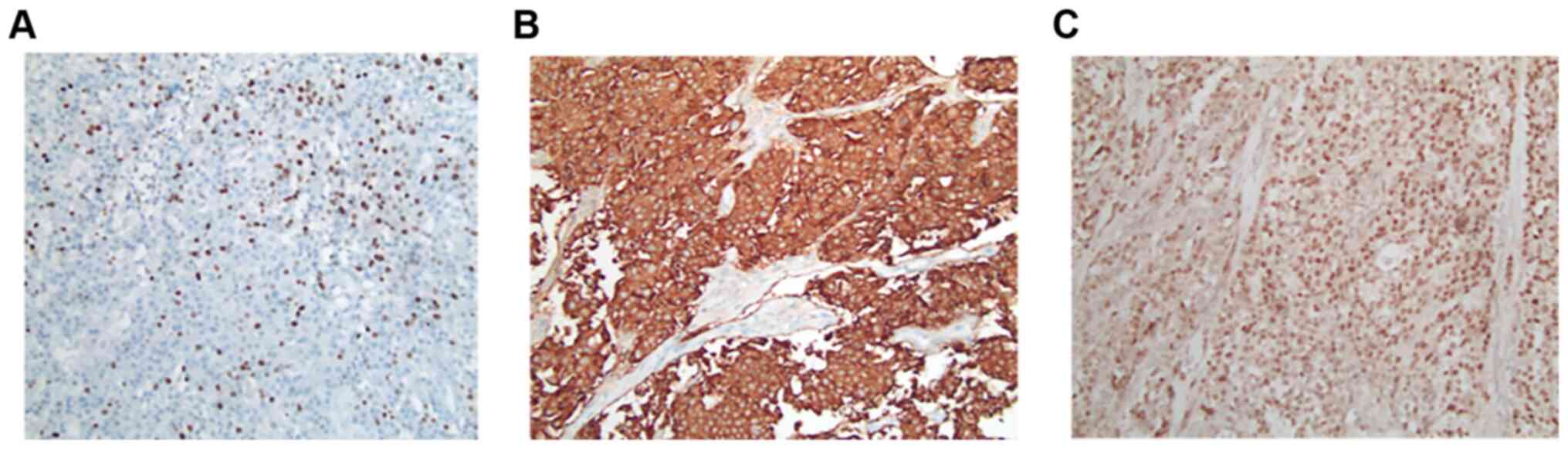
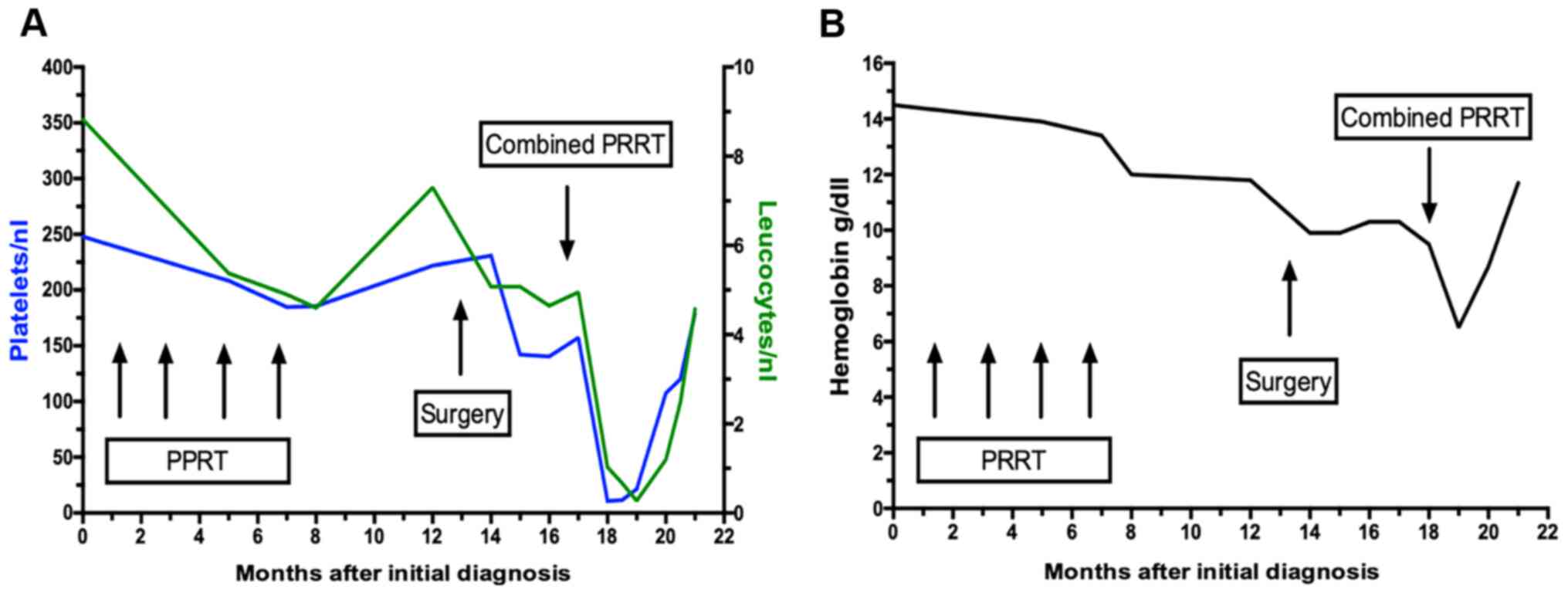
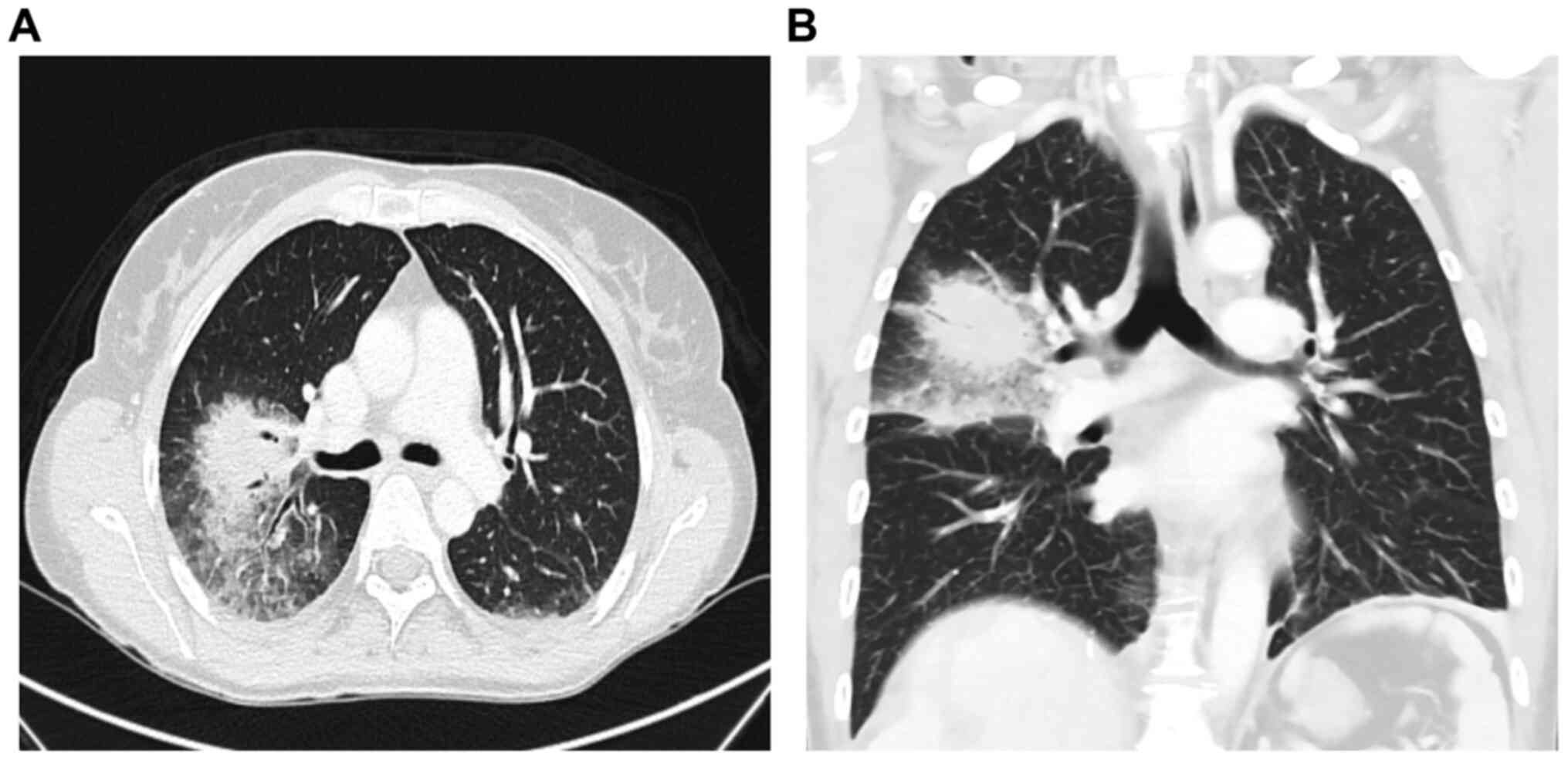
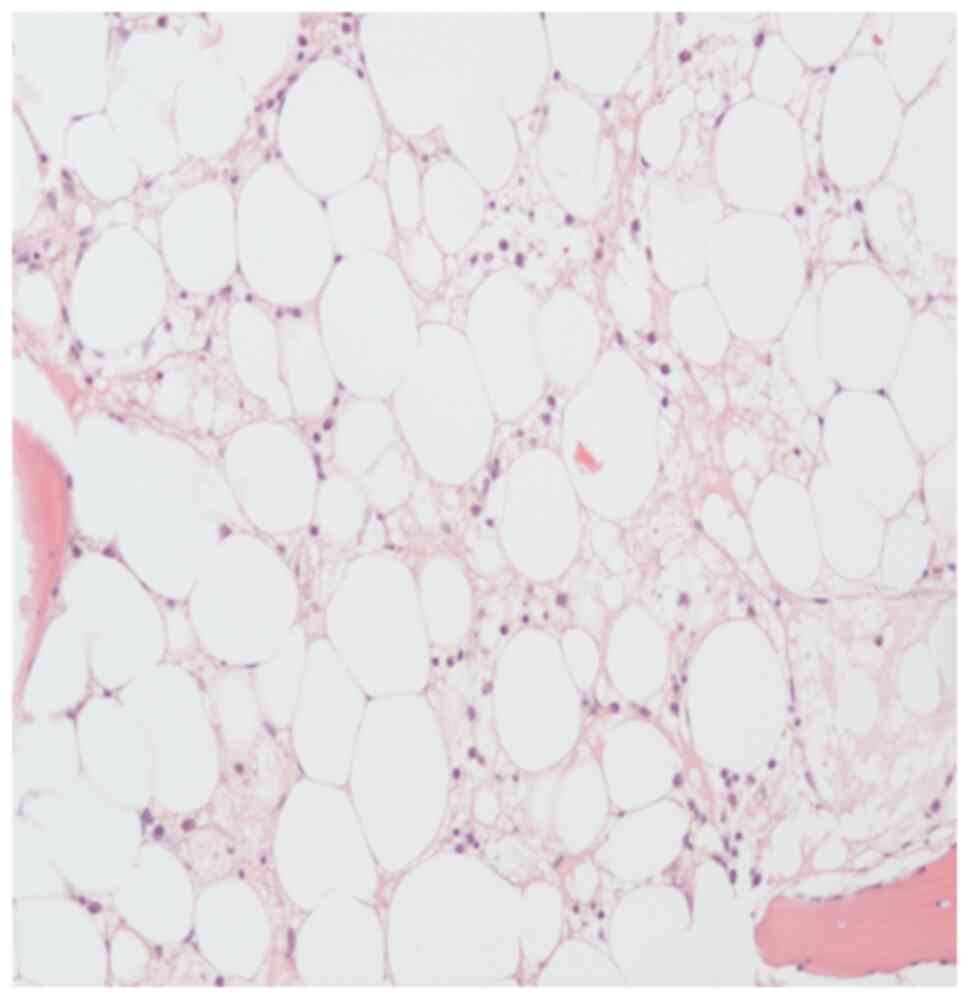
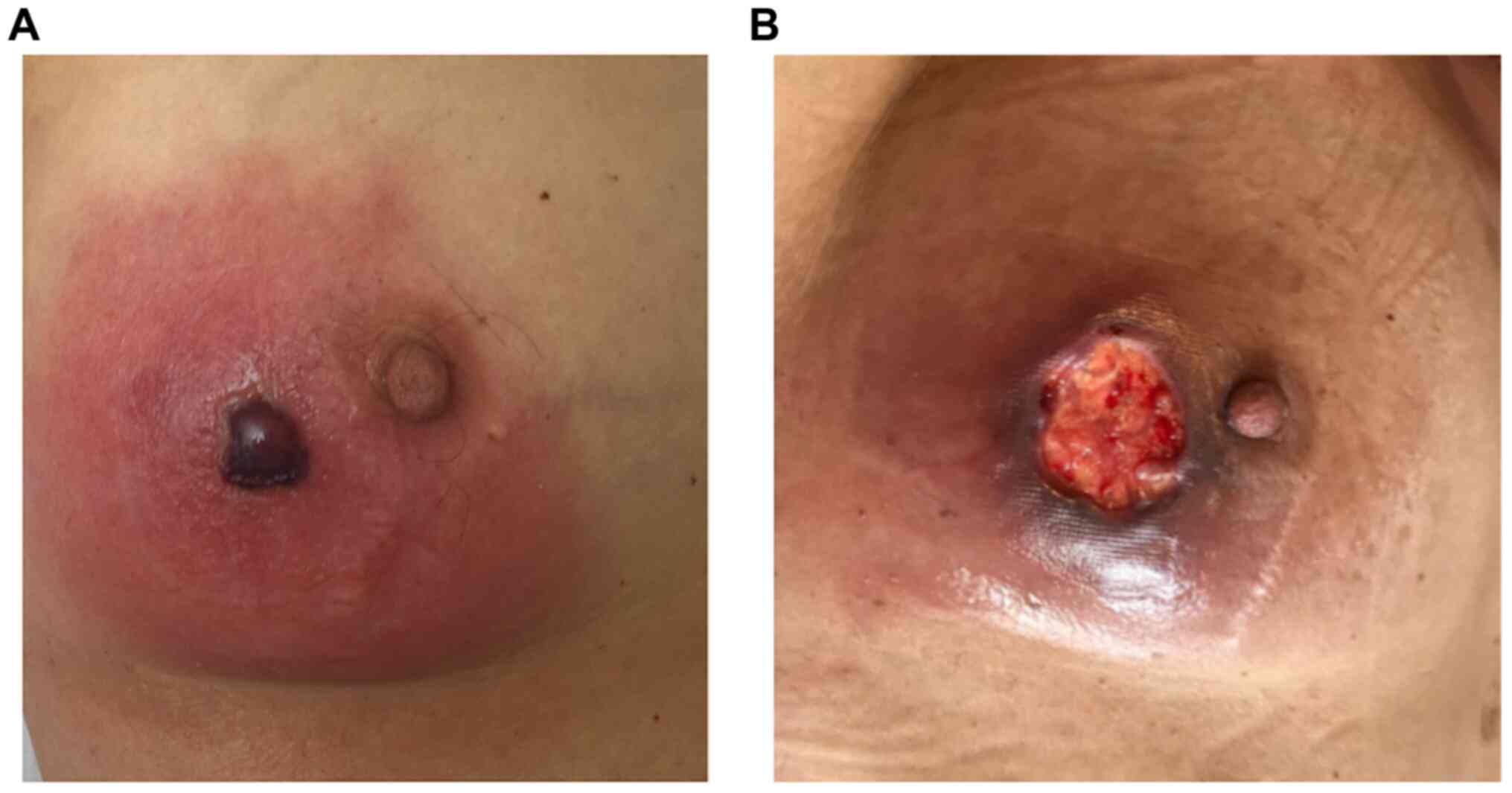
|
1
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: Classification of tumours of the digestive system. 4th
edition ed. Geneva, Switzerland: WHO Press; 2010.
|
|
2
|
Vélayoudom-Céphise FL, Duvillard P, Foucan
L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D,
Debaere T, et al: Are G3 ENETS neuroendocrine neoplasms
heterogeneous? Endocr Relat Cancer. 20:649–657. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heetfeld M, Chougnet CN, Olsen IH, Rinke
A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D and Walter
T: Other Knowledge Network members. Characteristics and treatment
of patients with G3 gastroenteropancreatic neuroendocrine
neoplasms. Endocr Relat Cancer. 22:657–664. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fani M, Maecke HR and Okarvi SM:
Radiolabeled peptides: Valuable tools for the detection and
treatment of cancer. Theranostics. 2:481–501. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kwekkeboom DJ, de Herder WW, Kam BL, van
Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO and
Krenning EP: Treatment with the radiolabeled somatostatin analog
[177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J
Clin Oncol. 26:2124–2130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Strosberg J, El-Haddad G, Wolin E,
Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H,
et al: Phase 3 trial of 177 Lu-dotatate for midgut
neuroendocrine tumors. N Engl J Med. 376:125–135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van der Zwan WA, Bodei L, Mueller-Brand J,
de Herder WW, Kvols LK and Kwekkeboom DJ: GEPNETs update:
Radionuclide therapy in neuroendocrine tumors. Eur J Endocrinol.
172:R1–R8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang J, Kulkarni HR, Singh A, Niepsch K,
Muller D and Baum RP: Peptide receptor radionuclide therapy in
grade 3 neuroendocrine neoplasms: Safety and survival analysis in
69 patients. J Nucl Med. 60:377–385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mahjoub AR and O'Reilly EM: Emerging
therapies for pancreas neuroendocrine cancers. Chin Clin Oncol.
2:2304–3865. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Strosberg JR, Fine RL, Choi J, Nasir A,
Coppola D, Chen DT, Helm J and Kvols L: First-line chemotherapy
with capecitabine and temozolomide in patients with metastatic
pancreatic endocrine carcinomas. Cancer. 117:268–275.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fine RL, Gulati AP, Krantz BA, Moss RA,
Schreibman S, Tsushima DA, Mowatt KB, Dinnen RD, Mao Y, Stevens PD,
et al: Capecitabine and temozolomide (CAPTEM) for metastatic,
well-differentiated neuroendocrine cancers: The pancreas center at
Columbia university experience. Cancer Chemother Pharmacol.
71:663–670. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Claringbold PG, Brayshaw PA, Price RA and
Turner JH: Phase II study of radiopeptide 177Lu-octreotate and
capecitabine therapy of progressive disseminated neuroendocrine
tumours. Eur J Nucl Med Mol Imaging. 38:302–311. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Claringbold PG, Price RA and Turner JH:
Phase I-II study of radiopeptide 177Lu-octreotate in combination
with capecitabine and temozolomide in advanced low-grade
neuroendocrine tumors. Cancer Biother Radiopharm. 27:561–569.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Claringbold PG and Turner JH: Pancreatic
neuroendocrine tumor control: Durable objective response to
combination 177Lu-octreotate-capecitabine-temozolomide radiopeptide
chemotherapy. Neuroendocrinology. 103:432–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bison SM, Haeck JC, Bol K, Koelewijn SJ,
Groen HC, Melis M, Veenland JF, Bernsen MR and de Jong M:
Optimization of combined temozolomide and peptide receptor
radionuclide therapy (PRRT) in mice after multimodality molecular
imaging studies. EJNMMI Res. 5(62)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kesavan M, Claringbold PG and Turner JH:
Hematological toxicity of combined 177Lu-octreotate radiopeptide
chemotherapy of gastroenteropancreatic neuroendocrine tumors in
long-term follow-up. Neuroendocrinology. 99:108–117.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Thakral P, Sen I, Pant V, Gupta SK, Dureja
S, Kumari J, Kumar S, Un P and Malasani V: Dosimetric analysis of
patients with gastro entero pancreatic neuroendocrine tumors (NETs)
treated with PRCRT (peptide receptor chemo radionuclide therapy)
using Lu-177 DOTATATE and capecitabine/temozolomide (CAP/TEM). Br J
Radiol. 91(20170172)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stueven AK, Kayser A, Wetz C, Amthauer H,
Wree A, Tacke F, Wiedenmann B, Roderburg C and Jann H: Somatostatin
analogues in the treatment of neuroendocrine tumors: Past, present
and future. Int J Mol Sci. 20(3049)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Claringbold PG and Turner JH:
NeuroEndocrine tumor therapy with lutetium-177-octreotate and
everolimus (NETTLE): A phase I study. Cancer Biother Radiopharm.
30:261–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pfeifer AK, Gregersen T, Grønbæk H, Hansen
CP, Müller-Brand J, Herskind Bruun K, Krogh K, Kjaer A and Knigge
U: Peptide receptor radionuclide therapy with Y-DOTATOC and
(177)Lu-DOTATOC in advanced neuroendocrine tumors: Results from a
Danish cohort treated in Switzerland. Neuroendocrinology.
93:189–196. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Imhof A, Brunner P, Marincek N, Briel M,
Schindler C, Rasch H, Mäcke HR, Rochlitz C, Müller-Brand J and
Walter MA: Response, survival, and long-term toxicity after therapy
with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in
metastasized neuroendocrine cancers. J Clin Oncol. 29:2416–2423.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bodei L, Cremonesi M, Grana CM, Fazio N,
Iodice S, Baio SM, Bartolomei M, Lombardo D, Ferrari ME, Sansovini
M, et al: Peptide receptor radionuclide therapy with
(1)(7)(7)Lu-DOTATATE: The IEO phase I-II study. Eur J Nucl Med Mol
Imaging. 38:2125–2135. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bodei L, Cremonesi M, Zoboli S, Grana C,
Bartolomei M, Rocca P, Caracciolo M, Mäcke HR, Chinol M and
Paganelli G: Receptor-mediated radionuclide therapy with
90Y-DOTATOC in association with amino acid infusion: A phase I
study. Eur J Nucl Med Mol Imaging. 30:207–216. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shapiro M, Wald U, Simchen E, Pomeranz S,
Zagzag D, Michowiz SD, Samuel-Cahn E, Wax Y, Shuval R, Kahane Y, et
al: Randomized clinical trial of intra-operative antimicrobial
prophylaxis of infection after neurosurgical procedures. J Hosp
Infect. 8:283–295. 1986.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Waldherr C, Pless M, Maecke HR, Haldemann
A and Mueller-Brand J: . The clinical value of
[90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of
neuroendocrine tumours: A clinical phase II study. Ann Oncol.
12:941–945. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Waldherr C, Pless M, Maecke HR, Schumacher
T, Crazzolara A, Nitzsche EU, Haldemann A and Mueller-Brand J:
Tumor response and clinical benefit in neuroendocrine tumors after
7.4 GBq (90)Y-DOTATOC. J Nucl Med. 43:610–616. 2002.PubMed/NCBI
|
|
27
|
van Essen M, Krenning EP, Kam BL, de
Herder WW, van Aken MO and Kwekkeboom DJ: Report on short-term side
effects of treatments with 177Lu-octreotate in combination with
capecitabine in seven patients with gastroenteropancreatic
neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 35:743–748.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kong G, Thompson M, Collins M, Herschtal
A, Hofman MS, Johnston V, Eu P, Michael M and Hicks RJ: Assessment
of predictors of response and long-term survival of patients with
neuroendocrine tumour treated with peptide receptor
chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging.
41:1831–1844. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
O'Toole D, Kianmanesh R and Caplin M:
ENETS 2016 consensus guidelines for the management of patients with
digestive neuroendocrine tumors: An update. Neuroendocrinology.
103:117–118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Newman CB, Melmed S, Snyder PJ, Young WF,
Boyajy LD, Levy R, Stewart WN, Klibanski A, Molitch ME and Gagel
RF: Safety and efficacy of long-term octreotide therapy of
acromegaly: Results of a multicenter trial in 103 patients-a
clinical research center study. J Clin Endocrinol Metab.
80:2768–2775. 1995.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lamberts SW, van der Lely AJ, de Herder WW
and Hofland LJ: Octreotide. N Engl J Med. 334:246–254.
1996.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bodei L, Cremonesi M, Grana C, Rocca P,
Bartolomei M, Chinol M and Paganelli G: Receptor radionuclide
therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in
neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 31:1038–1046.
2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bodei L, Mueller-Brand J, Baum RP, Pavel
ME, Hörsch D, O'Dorisio MS, O'Dorisio TM, Howe JR, Cremonesi M,
Kwekkeboom DJ and Zaknun JJ: The joint IAEA, EANM, and SNMMI
practical guidance on peptide receptor radionuclide therapy (PRRNT)
in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 40:800–816.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bergsma H, Konijnenberg MW, van der Zwan
WA, Kam BL, Teunissen JJ, Kooij PP, Mauff KAL, Krenning EP and
Kwekkeboom DJ: Nephrotoxicity after PRRT with
(177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 43:1802–1811.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kashyap R, Jackson P, Hofman MS, Eu P,
Beauregard JM, Zannino D and Hicks RJ: Rapid blood clearance and
lack of long-term renal toxicity of 177Lu-DOTATATE enables
shortening of renoprotective amino acid infusion. Eur J Nucl Med
Mol Imaging. 40:1853–1860. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kesavan M and Turner JH: Myelotoxicity of
peptide receptor radionuclide therapy of neuroendocrine tumors: A
decade of experience. Cancer Biother Radiopharm. 31:189–198.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ploeckinger U, Kloeppel G, Wiedenmann B
and Lohmann R: Representatives of 21 German NET Centers. The German
NET-registry: An audit on the diagnosis and therapy of
neuroendocrine tumors. Neuroendocrinology. 90:349–363.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Garcia-Carbonero R, Capdevila J,
Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso
Orduña V, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez
A, Llanos-Muñoz M, et al: Incidence, patterns of care and
prognostic factors for outcome of gastroenteropancreatic
neuroendocrine tumors (GEP-NETs): Results from the national cancer
registry of Spain (RGETNE). Ann Oncol. 21:1794–1803.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lombard-Bohas C, Mitry E, O'Toole D,
Louvet C, Pillon D, Cadiot G, Borson-Chazot F, Aparicio T, Ducreux
M, Lecomte T, et al: Thirteen-month registration of patients with
gastroenteropancreatic endocrine tumours in France.
Neuroendocrinology. 89:217–222. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008.PubMed/NCBI View Article : Google Scholar
|