Introduction
Neuroendocrine neoplasia (NEN) are a rare and
heterogeneous group of tumors. According to the World Health
Organization (WHO) classification, NEN are stratified into
low/moderate-[grade (G) 1/2] or high-grade (G3) neuroendocrine
tumors (NET) and neuroendocrine carcinoma (NEC) (1). Well-differentiated NET (G1 and G2) are
characterized by a low proliferative index, retain the expression
of somatostatin receptors (SSTR) and are associated with a good
prognosis compared with that in other malignancies. By contrast, G3
NET feature a high Ki-67 proliferation index of >20% and are
associated with a poor prognosis.
The systemic treatment of patients with G3 NET has
been under investigated. To date, no data from prospective clinical
trials are available, and current recommendations for the treatment
of G3 NET primarily relies on retrospective analyses and case
series. Overall, G3 NET show low objective response rates to
platinum-based therapies, when compared with that in NECs (2). Therefore, alternative, less toxic
chemotherapy regimens, such as capecitabine/temozolomide are
recommended (3). Data on second- or
third-line therapy in the treatment of G3 NET are even rarer, and
current recommendations are primarily based on expert opinions
rather than on systematic clinical studies. In the case of
SSR-positive tumors, peptide receptor radionuclide therapy (PRRT)
has been recommended by several expert research groups. PRRT is a
tumor-targeted systemic radiotherapy that enables the specific
delivery of radionuclides directly into tumor cells inducing tumor
cell death. The high-level expression of SSR on the tumor cell
surface in NEN provides the rational for a therapy with
radioisotope-labeled somatostatin analogs (4). While PRRT has emerged as a highly
effective and well-tolerated treatment in SSR-positive,
well-differentiated NET (5-7),
few data exist on patients with high-grade NET. Zhang et al
(8) reported a median
progression-free survival (PFS) time of 9.6 months and a median
overall survival (OS) time of 19.9 months in 69 patients with G3
NET treated with PRRT. Notably, in these patients PRRT was
well-tolerated without any decline in renal function,
hepatotoxicity or grade 3/4 hematotoxicity. Combinations of PRRT
with systemic chemotherapy (e.g. capecetabine with and without
temzolomide) might be associated with both additive and synergistic
effects, since chemotherapeutic agents might serve as a
radiosensitizer, as well as targeting cells non-responsive to PRRT
(9). However, at present, there are
only a few case reports and small number of case series, which have
reported the outcome of patients treated with a combination of PRRT
and chemotherapy. In the present case study, a patient with disease
progression following 4 cycles of PRRT, who was subsequently
treated with a combination of PRRT and capecitabine/temozolomide at
our institution has been described.
Case report
The case of a 58-year-old female patient who was
diagnosed with a G3 NET of unknown primary location and synchronous
liver metastases in October 2017 (Table
I) has been described. The proliferation according to Ki-67 was
high (20%). Multi-slice computed tomography (CT) and
DOTATOC-positron emission tomography (PET)/CT revealed multiple
SSR-positive liver metastases; however, it did not provide any
evidence of a primary tumor. Immunohistochemical analysis of a
biopsy obtained from a liver metastasis showed strong expression of
synaptophysin and a slightly weaker expression of chromogranin.
Staining for serotonin, CDX2 and TTF1 were negative and membranous
PD-L1 expression was found in <1% of tumor cells.
 | Table ICourse of disease. |
Table I
Course of disease.
| Year | Month | Therapy | Staging |
|---|
| 2017 | October | - | G3 NET CUP with
synchronous hepatic metastases: First biopsy of a hepatic
metastasis, Ki 67 20%, Synaptophysin+++, CGA++, SSTR-2A+++ |
| | November | SSA therapy
(Somatuline 120 mg) every 28 days | Staging CT and
DOTATOC-PET: SSR-positive multiple hepatic metastases primarily in
the right liver lobe. No evidence of primary tumor |
| | | - | Second biopsy of a
hepatic metastasis: Ki67 35-40%, Synaptophysin+++, CGA++,
SSTR-2A+++, ISLET1-positive, TTF1- and CDX2-negative |
| | December | First cycle PRRT
7,4 GBq 177LU-DOTATOC | - |
| 2018 | February | Second cycle PRRT
7,4 GBq 177LU-DOTATOC | - |
| | April | Third cycle PRRT
7,6 GBq 177LU-DOTATOC | Staging CT: Hepatic
progressive disease |
| | | - | Staging CT and
DOTATOC-PET: Hepatic progressive disease |
| | June | Fourth cycle PRRT
7,7 GBq 177LU-DOTATOC | - |
| | July | Continuation of SSA
therapy | Staging CT: Partial
remission with hepatic tumor size reduction |
| | September | - | Staging CT and
DOTATOC-PET: Partial further hepatic tumor size reduction |
| | December | - | Staging CT and
DOTATOC-PET: Hepatic progressive disease (progress of right lobe
liver metastasis). No pathological lymph node enlargement |
| 2019 | January | Right
hemihepatectomy | Histopathology of
liver specimen: Ki 67 >20%, synaptophysin +++, CGA++, MLH1+,
MSH2+, MSH6+, PMS2+serotonin, CDDX2 and TTF1 negative. PD-L1+ |
| | May | - | Staging CT and
DOTATOC-PET: Hepatic and lymphatic progressive disease (>20%
according to RECIST) |
| | June | Fifth PRRT 6,941
GBq 177Lu-DOTATOC in combination with capecitabine (540
mg/m2) and temzolomide (150 mg/m2) | - |
| | July | Hospitalization due
to clinical complications (neutropenic fever, transfusion
obligatory pancytopenia, right sided necrotizing mastitis, fungal
pneumonia) after PRRT in combination with
capecitabine/temzolomide | Staging CT: Partial
remission (55% according to RECIST). No pathological lymph node
enlargement. No primary tumor detectable |
| | August | Continuation of SSA
therapy | - |
| | October | - | MR: Partial
remission (68% according to RECIST) |
Treatment with lanreotide Autogel (120 mg) was
administered every 28 days. In addition, the patient underwent 4
cycles of PRRT with 7.4 gigabecquerel (GBq) 177LU
LU-DOTATOC over a period of 6 months (last dose June 2018).
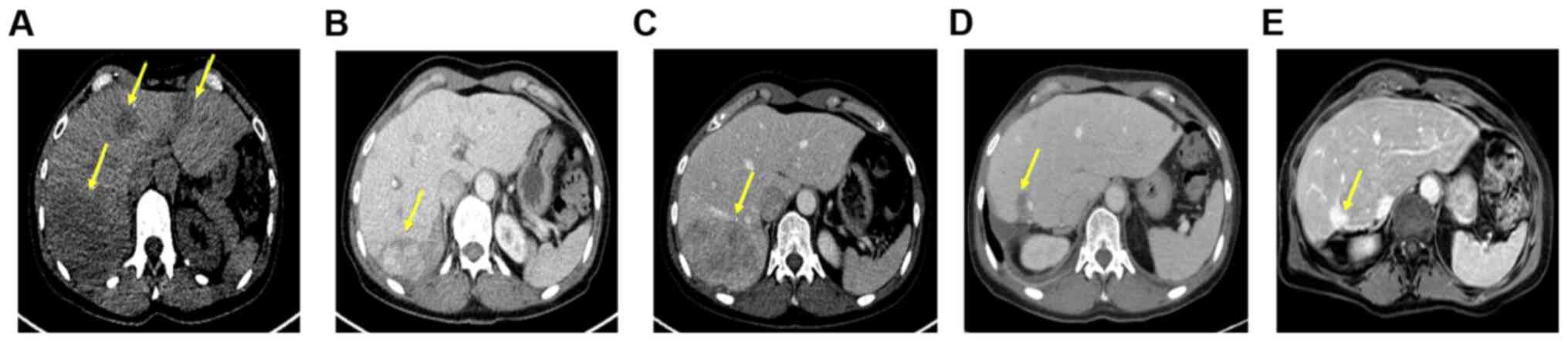
Notably, this treatment resulted in a partial remission lasting
until December 2018 (Fig. 1A-C). At
this time point a follow-up DOTATOC-PET/CT scan revealed disease
progression in the liver (only in the right lobe, with stable
disease on the left-hand side). No other distant metastases was
evident. Based on the short duration of tumor control, another
systemic treatment was not administered; however, the patient was
admitted to undergo hemihepatectomy to resect the progressive
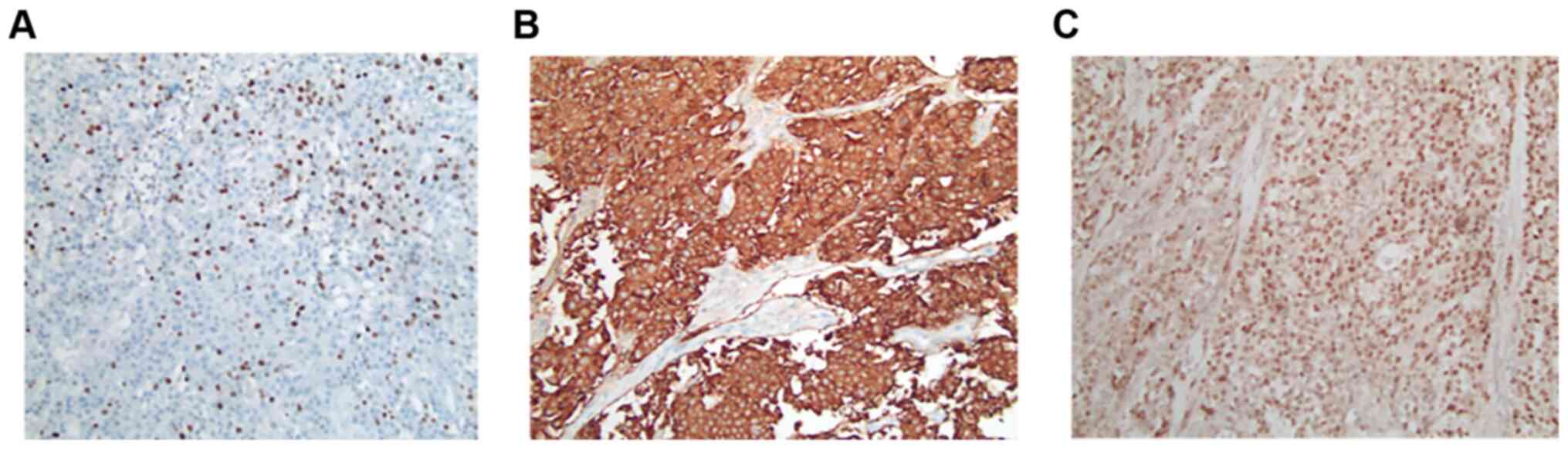
lesions. Histopathological analysis of the resected tumor confirmed
the diagnosis of NET with Ki-67 >20%, leading to the diagnosis
of G3 NET (Fig. 2A-C). However, a
DOTATOC-PET/CT scan performed four months following surgery showed
further hepatic and lymphatic progression with an increase in tumor
size of >20% according to the Response Evaluation Criteria in
Solid Tumors (RECIST). Considering the initial partial response to
PRRT and the systemic progression at that time point, systemic
therapy was not administered but simultaneously continuation of
PRRT sessions. Capecitabine/temzolomide was chosen as the
chemotherapeutic agent due to the high response rates observed in
patients with NET (10,11) and since it represents the most
common therapy regime used in studies investigating PRRT in
combination with chemotherapy (12-14).
At the time point of treatment initiation, the patient was in good
general condition [Eastern Cooperative Oncology Group (ECOG) 0] and
had recovered from the side effects of previous therapies.
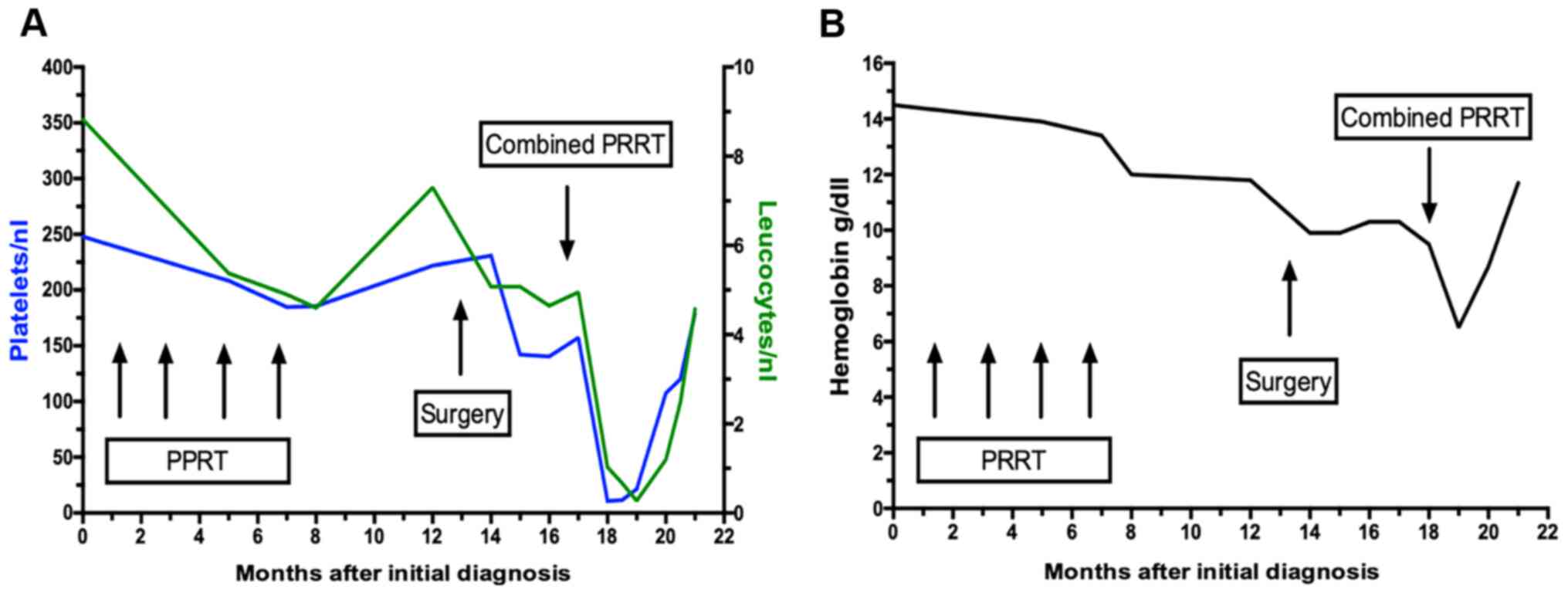
Nevertheless, the therapy was administered at a reduced dose, since
(reversible) anemia and a lower platelet and leucocyte count had
occurred, as some of the side effects from the initial 4 cycles of
PRRT (Fig. 3A and B).
As timing between chemotherapy and PRRT has been
found to have an impact on outcome parameters in animal studies,
wean already established protocol was used (15,16).
Of note, this particular protocol was selected, as it has been
reported that therapy was only accompanied by modest reversible
myelosuppression, which was not greater than that in conventional
PRRT therapies. Therefore, the combination of PRRT plus
capecitabine/temozolomide was administered according to the
protocol recently published by Strosberg et al (10) using 750 mg/m2
capecitabine (which was reduced to 538 mg/m2) and
temzolomide 200 mg/m2 (which was reduced to 150
mg/m2). Chemotherapy with oral capecitabine started five
days prior to PRRT. In particular, 7.0 GBq
177-LU-DOTATOC was administered intravenously, followed
by oral temozolamide in the last five days of the 14-day period of
the capecitabine cycle. Dosimetric calculations revealed that the
radiation absorbed doses were 1.09 milligrays (mGy)/megaBq (MBq)
for the kidneys, 0.288 mGy/MBq for the liver, 0.41 mGy/MBq for the
spleen and 0.03 mGy/MBq for bone marrow, while hepatic metastases
demonstrated a higher uptake of 4.56 mGy/MBq, which was in line
with previously published results from patients receiving
combinations of chemotherapy and PRRT (17).
The treatment was initially well-tolerated without
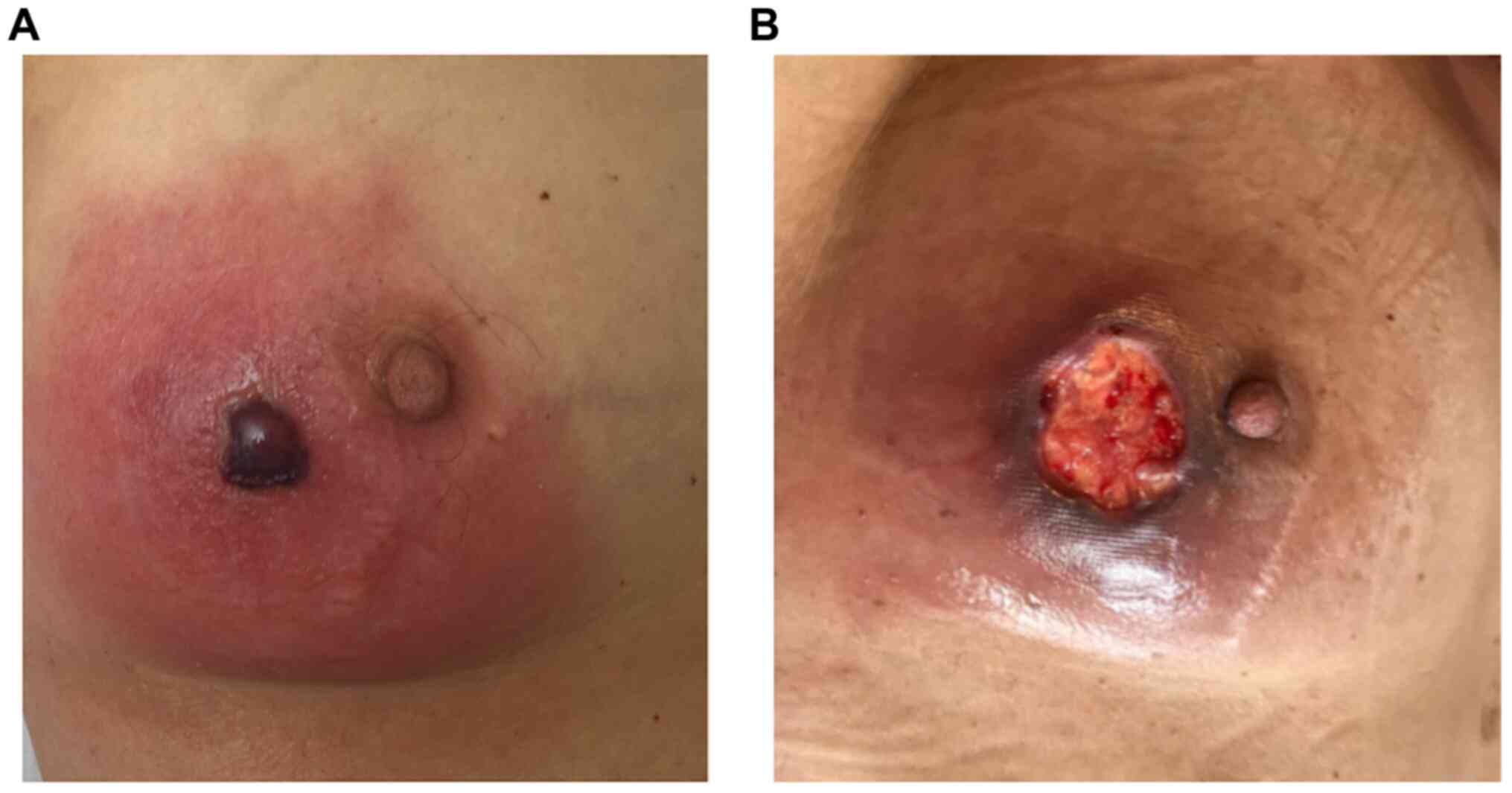
any side effects. However, 23 days after PRRT, the patient was
hospitalized due to recurrent episodes of fever, dyspnea, as well
as pain, redness and swelling in the right mamma. Laboratory
testing revealed pancytopenia and slightly elevated inflammatory
markers, while an ultrasound of the mamma showed distinct edema
with induration of the tissue without evidence of an abscess
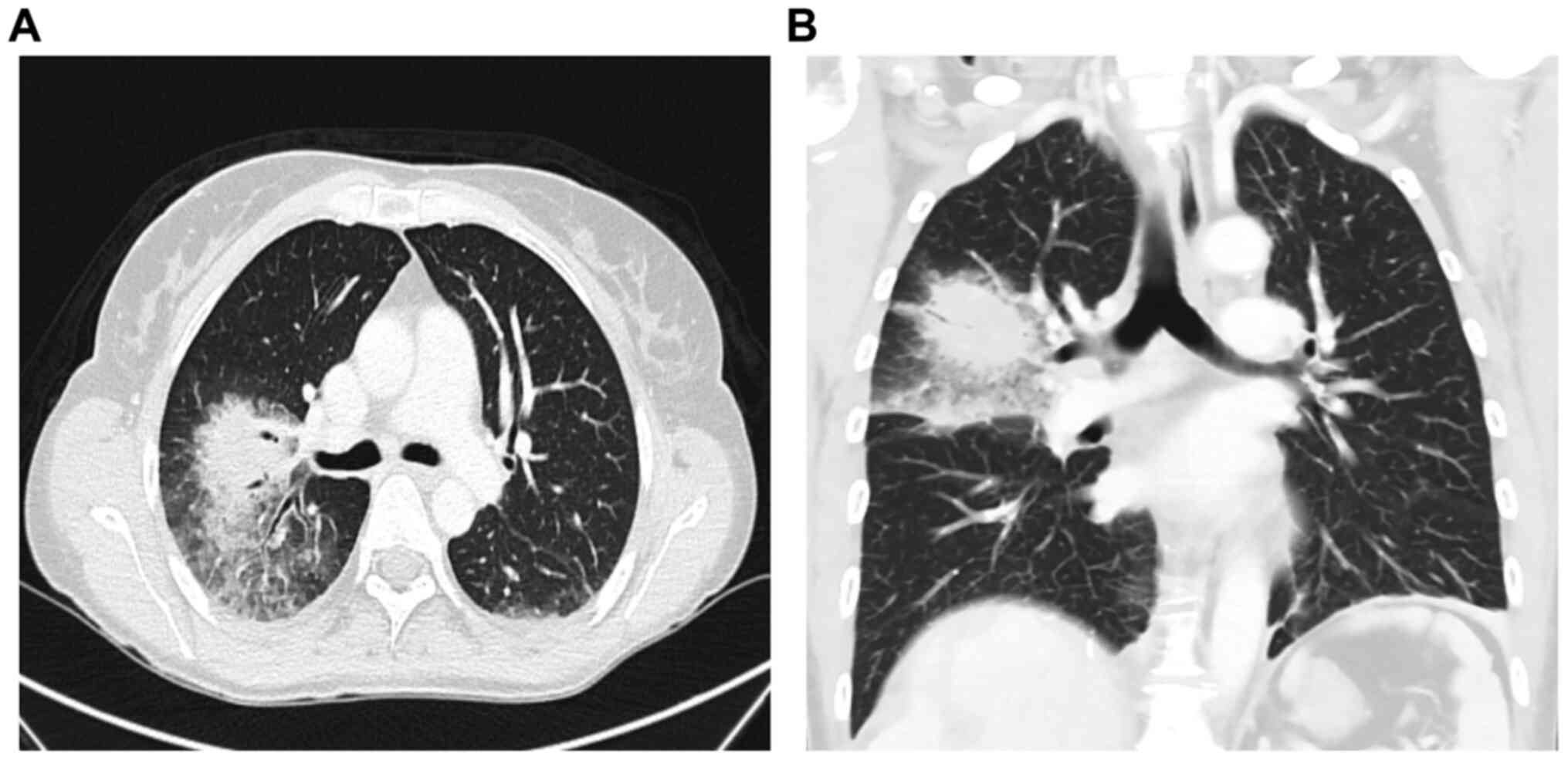
formation. Chest CT revealed a mass-like pulmonary infiltrate in
the right upper lobe with surrounding ground glass opacity,
suggesting fungal pneumonia (Fig.
4A and B).
Blood, sputum and swab cultures did not identify any
pathogens. For further evaluation of the pancytopenia, a bone
marrow puncture was performed, which revealed toxic bone marrow
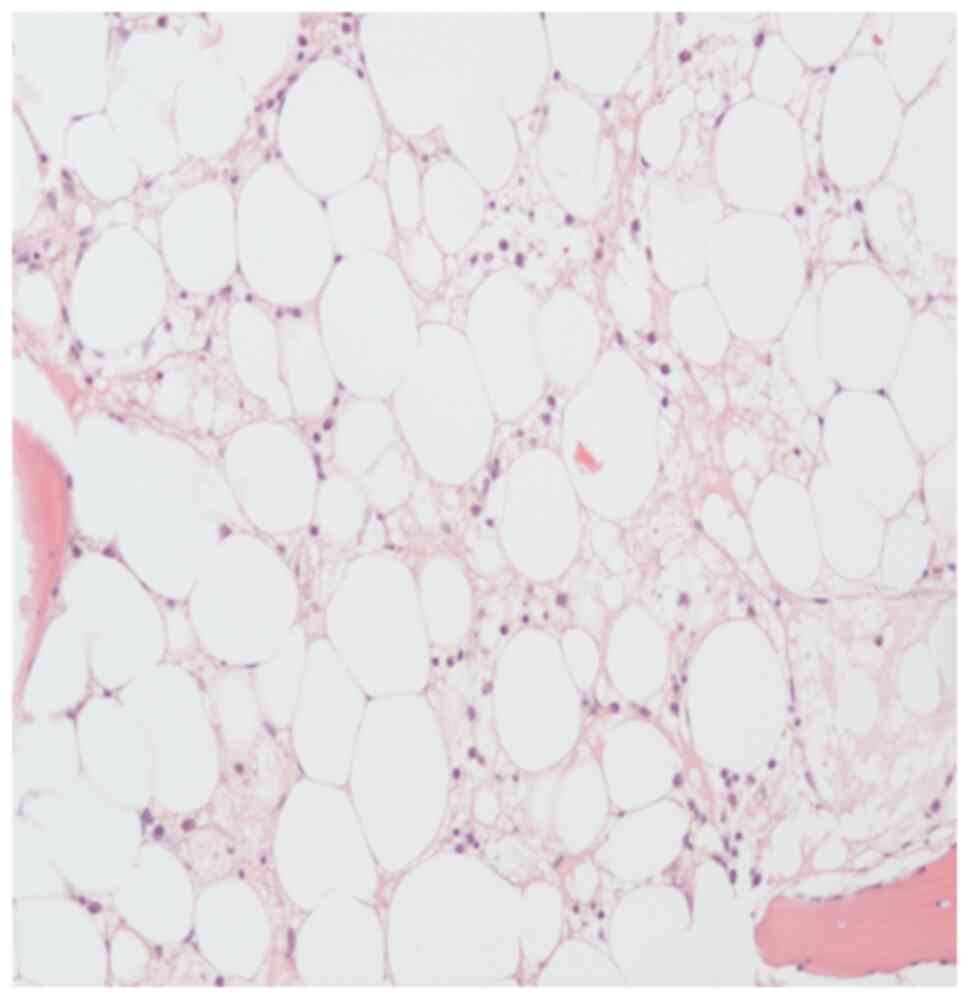
damage (Fig. 5). As a result of the
clinical investigations, neutropenic fever, a right-sided
necrotizing mastitis and fungal pneumonia, as clinical
complications of toxic bone marrow aplasia (most likely due to
hematoxicity of PRRT and chemotherapy) was diagnosed. The patient,
treated in an external rural hospital at that time, was then sent
to an Oncology unit in a tertiary University hospital. An empirical
combination therapy with piperacillin/tazobactam, vancomycin,
aciclovir and Caspofungin was initiated and was later changed to a
combination of vancomycin, ciprofloxacin and voriconazole.
Furthermore, stimulation therapy with granulocyte-colony
stimulating factor was performed, along with the administration of
several red blood cell and platelet transfusions. After 11 days,
the blood cells started to regenerate (Fig. 3A and 3B). Follow-up imaging two weeks later
revealed a clear regression of the infiltrations in the right upper
lobe. The inflammatory markers decreased, along with an improvement
in the healing process of the wound tissue of the mamma (Fig. 6).
Despite the critical clinical condition caused by
combination PRRT, CT staging conducted one month following PPRT
plus capecitabine/temozolomide showed a liver tumor mass reduction
of at least 55% according to RECIST, without any signs of
pathological lymph node enlargement. Subsequent magnetic resonance
imaging four months later revealed a further tumor reduction of at
least 68% according to RECIST (Fig.
1D and E).
In consideration of the severe bone marrow damage
and the critical condition of the patient, the combination of PRRT
plus capecitabine/temozolomide was discontinued and somatostatin
analogue (SSA)-therapy was restarted again. The blood count
stabilized and remained normal. The following staging
investigations in October 2019 revealed further sustained tumor
response. To date, the patient is alive and fully recovered from
the therapy-related side effects.
Discussion
The present case report described the case of a
patient diagnosed with a G3 NET of unknown primary origin
accompanied by synchronous liver metastases. The patient received a
combination of PRRT and capecitabine/temozolomide chemotherapy, as
part of a multi-modal treatment concept at our institution. After
receiving only one cycle of therapy, the patient exhibited severe
bone marrow toxicity, as well as neutropenic fever and critical
infectious complications (necrotizing mastitis and fungal
pneumonia); however, demonstrated an effective tumor response. The
patient in the present case report provides several notable
aspects: First, the combination of chemotherapy and PRRT was
associated with an effective tumor response, leading to a sustained
tumor control >5 months after only one cycle; second, this
response was achieved in a patient with high-grade NET,
representing a cohort of patients with limited treatment options;
third, the toxicity of the treatment exceeded the toxicity reported
in the current literature by far, highlighting the requirement for
careful patient selection and close monitoring of patients
receiving PRRT in combination with chemotherapy.
Until now, different experimental approaches and
strategies have been investigated to optimize the effectiveness of
PRRT and to minimize potential side effects (18). Research groups, such as Claringbold
et al (12-14)
have tried to combine PRRT with chemotherapy (capecitabine with and
without temzolomidect) in cases of patients with advanced low-grade
GNETs, in which either of the two treatment options alone failed
(12-14,16).
With the intention to use chemotherapy, as a radiosensitizing agent
to enhance the efficacy of PRRT, effective tumor control rates were
achieved, with disease control in up to 55% of the patients
(13,19). A study, investigating pNET in
particular, revealed an overall response rate of 80%, including
complete remission in 13% and partial response in 70% of the cases
(14). Accordingly, the effective
tumor response of at least 68% tumor reduction was in line with
previous studies.
Both combined PRRT and PRRT alone have been
presented as procedures leading to an increase in long-term
survival with a low complication rate (20-28).
The patient in the present case study received the combination of
177Lu-octreotate and capecitabinec and temozolomide,
which was considered feasible and safe, regarding the acute and
subacute side effects (12-14).
According to previous studies, acute side effects are typically
mild and self-limiting (most commonly nausea), whereas long-term
side effects include loss of renal function, myelodysplastic
syndrome and acute leukemia. However, hematological toxicity was
the most significant potential adverse event following PRRT, caused
by irradiation of the bone marrow and primarily presenting as
reversible, limited grade cytopenia. Current research studies
suggest that WHO grade 3 or 4 toxicity could only occur in up to
15% of patients. According to Kesavan et al (16) this number was not significantly
increased in patients receiving PRRT in combination with
radiosensitizing chemotherapy, which has the potential to enhance
the efficiency of the therapy. Research by Kesavan et al
(16) retrospectively analyzed
long-term outcomes of the two cohorts from their
177Lu-octreotate and chemotherapy study (37 patients
treated with capecitabine/temzolomide and 28 patients treated with
177Lu-octreotate and capecitabine). In both cohorts,
only modest reversible myelosuppression was observed. In patients
treated with capecitabine/temzolomide, long-term follow-up revealed
significant thrombocytopenia in 2.7% (n=1), neutropenia in 2.7%
(n=1) and anemia in 10.8% (n=4), while no short-term hematological
toxicity grade 3/4 (n=0) was reported. In patients receiving
177Lu-octreotate and capecitabine, long-term
hematotoxicity, such as anemia and thrombytopenia was only detected
in 3.5% of the cases (n=1). However, an exact measure of the
adverse events due to PRRT plus chemotherapy can be challenging,
which is why the procedure is still considered investigational
(29).
The patient in the present case report developed
severe bone marrow toxicity, along with critical infectious
complications (necrotizing mastitis and fungal pneumonia) after
only one session of PRRT in combination with
capecitabine/temzolomidect at a reduced dose. Despite the fact that
only one cycle of combined PRRT, at a reduced dose was
administered, severe bone marrow damage was observed, leading to
myelotoxic cytopenia most likely caused by prior therapy with PRRT,
which was not seen in association with previous SSA therapy
(30,31). Fig.
3A and B revealed the
myelotoxic damage after two PRRT sessions causing a lower platelet
and leucocyte count,counts as well as persistent anemia after
several months. However, an increased radiation uptake can be
excluded, as dosimetric calculations revealed the radiation
absorption doses, which were in line with previously published
results from patients receiving combinations of chemotherapy and
PRRT (17). Therefore, it was
concluded Therefore, we can conclude that the patient in the
current study was already predisposed to develop pancytopenia
during PRRT in combination with capecitabine/temzolomide.
Pretreatment with radiation-based therapy or alkylating agents has
also been considered a significant factor to predict myelotoxicity,
as research by Kesavan et al (16) showed a significant
differencesignificance between increased risk of short- and
long-term toxicity and the presence and number of previous
treatments. Thus, a reduced dose of capecitabine/temzolomide was
administered to the patient in the present case report.
As aforementioned, there are several approaches to
prevent adverse effects of PRRT, such as using amino acid infusion
or gelofusine and optimization of antiemetic regimens (32-35).
Furthermore, it has been suggested that early therapy with
PRRT-containing regimens could not only improve the outcome, but
also reduce myelotoxicity (36).
However, early treatment with PRRT was not successful in preventing
severe bone marrow damage in the patient in the present case
report, suggesting the requirement for additional approaches to
prevent myelotoxicity. In this regard, establishment of specific
algorithms incorporating predictors for myelotoxicity are highly
desirable to select optimal treatment strategies, with respect to
dosage and the number of cycles for each individual patient.
Another primary finding of the present case report
was that the tumor reduced in size by at least 55% after only one
month, followed by a further reduction of up to 68% (Fig. 1D). This supports several previous
studies, which consider PRRT in combination with radiosensitizing
chemotherapy an effective therapeutic option in this challenging
disease (12-14).
The rapid response seen in the patient in the current case report
indicates the requirement for close clinical and radiological
monitoring in patients treated with such regimens, to adjust the
therapeutic strategy according to its efficacy and toxicity.
Long-term follow-up would be a requirement to investigate
sustainability of the tumor response after one cycle, as well as
the occurrence of long-term adverse effects.
However, the present case report has some
limitations, as only one patient with radiosensitizing chemotherapy
in combination with PRRT was treated at our institute, which makes
further conclusions difficult. Furthermore, the primary tumor in
the patient is still unknown. However, there is a high incidence
of, CUP (10-15%) in patients with NET (37-40)
and no correlation between an improveda therapy response and/or
higher toxicity with respect to tumor origin after PRRT
combinationcombined with chemotherapy has been analyzed or reported
yet (12-14,19).
Despite the high tumor reduction rate and several
successful approaches to reduce the side effect profile in the
field of radio sensitizing chemotherapy in combination with PRRT,
the serious problem of myelotoxicity could not be addressed.
Clinical trials on this type of therapy are rare, but are urgently
required to further investigate the toxicity, as well as to develop
preventive measures and predictors of response and long-term
survival in patients receiving a combination of PRRT and systemic
chemotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BÖ, HA, IS, PEG, MTM, UF, FT, HJ and CR were
involved treated the patient. BÖ, HJ and CR wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of patient data and images according to the
Declaration of Helsinki.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: Classification of tumours of the digestive system. 4th
edition ed. Geneva, Switzerland: WHO Press; 2010.
|
|
2
|
Vélayoudom-Céphise FL, Duvillard P, Foucan
L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D,
Debaere T, et al: Are G3 ENETS neuroendocrine neoplasms
heterogeneous? Endocr Relat Cancer. 20:649–657. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heetfeld M, Chougnet CN, Olsen IH, Rinke
A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D and Walter
T: Other Knowledge Network members. Characteristics and treatment
of patients with G3 gastroenteropancreatic neuroendocrine
neoplasms. Endocr Relat Cancer. 22:657–664. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fani M, Maecke HR and Okarvi SM:
Radiolabeled peptides: Valuable tools for the detection and
treatment of cancer. Theranostics. 2:481–501. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kwekkeboom DJ, de Herder WW, Kam BL, van
Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO and
Krenning EP: Treatment with the radiolabeled somatostatin analog
[177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J
Clin Oncol. 26:2124–2130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Strosberg J, El-Haddad G, Wolin E,
Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H,
et al: Phase 3 trial of 177 Lu-dotatate for midgut
neuroendocrine tumors. N Engl J Med. 376:125–135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van der Zwan WA, Bodei L, Mueller-Brand J,
de Herder WW, Kvols LK and Kwekkeboom DJ: GEPNETs update:
Radionuclide therapy in neuroendocrine tumors. Eur J Endocrinol.
172:R1–R8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang J, Kulkarni HR, Singh A, Niepsch K,
Muller D and Baum RP: Peptide receptor radionuclide therapy in
grade 3 neuroendocrine neoplasms: Safety and survival analysis in
69 patients. J Nucl Med. 60:377–385. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mahjoub AR and O'Reilly EM: Emerging
therapies for pancreas neuroendocrine cancers. Chin Clin Oncol.
2:2304–3865. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Strosberg JR, Fine RL, Choi J, Nasir A,
Coppola D, Chen DT, Helm J and Kvols L: First-line chemotherapy
with capecitabine and temozolomide in patients with metastatic
pancreatic endocrine carcinomas. Cancer. 117:268–275.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fine RL, Gulati AP, Krantz BA, Moss RA,
Schreibman S, Tsushima DA, Mowatt KB, Dinnen RD, Mao Y, Stevens PD,
et al: Capecitabine and temozolomide (CAPTEM) for metastatic,
well-differentiated neuroendocrine cancers: The pancreas center at
Columbia university experience. Cancer Chemother Pharmacol.
71:663–670. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Claringbold PG, Brayshaw PA, Price RA and
Turner JH: Phase II study of radiopeptide 177Lu-octreotate and
capecitabine therapy of progressive disseminated neuroendocrine
tumours. Eur J Nucl Med Mol Imaging. 38:302–311. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Claringbold PG, Price RA and Turner JH:
Phase I-II study of radiopeptide 177Lu-octreotate in combination
with capecitabine and temozolomide in advanced low-grade
neuroendocrine tumors. Cancer Biother Radiopharm. 27:561–569.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Claringbold PG and Turner JH: Pancreatic
neuroendocrine tumor control: Durable objective response to
combination 177Lu-octreotate-capecitabine-temozolomide radiopeptide
chemotherapy. Neuroendocrinology. 103:432–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bison SM, Haeck JC, Bol K, Koelewijn SJ,
Groen HC, Melis M, Veenland JF, Bernsen MR and de Jong M:
Optimization of combined temozolomide and peptide receptor
radionuclide therapy (PRRT) in mice after multimodality molecular
imaging studies. EJNMMI Res. 5(62)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kesavan M, Claringbold PG and Turner JH:
Hematological toxicity of combined 177Lu-octreotate radiopeptide
chemotherapy of gastroenteropancreatic neuroendocrine tumors in
long-term follow-up. Neuroendocrinology. 99:108–117.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Thakral P, Sen I, Pant V, Gupta SK, Dureja
S, Kumari J, Kumar S, Un P and Malasani V: Dosimetric analysis of
patients with gastro entero pancreatic neuroendocrine tumors (NETs)
treated with PRCRT (peptide receptor chemo radionuclide therapy)
using Lu-177 DOTATATE and capecitabine/temozolomide (CAP/TEM). Br J
Radiol. 91(20170172)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stueven AK, Kayser A, Wetz C, Amthauer H,
Wree A, Tacke F, Wiedenmann B, Roderburg C and Jann H: Somatostatin
analogues in the treatment of neuroendocrine tumors: Past, present
and future. Int J Mol Sci. 20(3049)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Claringbold PG and Turner JH:
NeuroEndocrine tumor therapy with lutetium-177-octreotate and
everolimus (NETTLE): A phase I study. Cancer Biother Radiopharm.
30:261–269. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pfeifer AK, Gregersen T, Grønbæk H, Hansen
CP, Müller-Brand J, Herskind Bruun K, Krogh K, Kjaer A and Knigge
U: Peptide receptor radionuclide therapy with Y-DOTATOC and
(177)Lu-DOTATOC in advanced neuroendocrine tumors: Results from a
Danish cohort treated in Switzerland. Neuroendocrinology.
93:189–196. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Imhof A, Brunner P, Marincek N, Briel M,
Schindler C, Rasch H, Mäcke HR, Rochlitz C, Müller-Brand J and
Walter MA: Response, survival, and long-term toxicity after therapy
with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in
metastasized neuroendocrine cancers. J Clin Oncol. 29:2416–2423.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bodei L, Cremonesi M, Grana CM, Fazio N,
Iodice S, Baio SM, Bartolomei M, Lombardo D, Ferrari ME, Sansovini
M, et al: Peptide receptor radionuclide therapy with
(1)(7)(7)Lu-DOTATATE: The IEO phase I-II study. Eur J Nucl Med Mol
Imaging. 38:2125–2135. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bodei L, Cremonesi M, Zoboli S, Grana C,
Bartolomei M, Rocca P, Caracciolo M, Mäcke HR, Chinol M and
Paganelli G: Receptor-mediated radionuclide therapy with
90Y-DOTATOC in association with amino acid infusion: A phase I
study. Eur J Nucl Med Mol Imaging. 30:207–216. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shapiro M, Wald U, Simchen E, Pomeranz S,
Zagzag D, Michowiz SD, Samuel-Cahn E, Wax Y, Shuval R, Kahane Y, et
al: Randomized clinical trial of intra-operative antimicrobial
prophylaxis of infection after neurosurgical procedures. J Hosp
Infect. 8:283–295. 1986.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Waldherr C, Pless M, Maecke HR, Haldemann
A and Mueller-Brand J: . The clinical value of
[90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of
neuroendocrine tumours: A clinical phase II study. Ann Oncol.
12:941–945. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Waldherr C, Pless M, Maecke HR, Schumacher
T, Crazzolara A, Nitzsche EU, Haldemann A and Mueller-Brand J:
Tumor response and clinical benefit in neuroendocrine tumors after
7.4 GBq (90)Y-DOTATOC. J Nucl Med. 43:610–616. 2002.PubMed/NCBI
|
|
27
|
van Essen M, Krenning EP, Kam BL, de
Herder WW, van Aken MO and Kwekkeboom DJ: Report on short-term side
effects of treatments with 177Lu-octreotate in combination with
capecitabine in seven patients with gastroenteropancreatic
neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 35:743–748.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kong G, Thompson M, Collins M, Herschtal
A, Hofman MS, Johnston V, Eu P, Michael M and Hicks RJ: Assessment
of predictors of response and long-term survival of patients with
neuroendocrine tumour treated with peptide receptor
chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging.
41:1831–1844. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
O'Toole D, Kianmanesh R and Caplin M:
ENETS 2016 consensus guidelines for the management of patients with
digestive neuroendocrine tumors: An update. Neuroendocrinology.
103:117–118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Newman CB, Melmed S, Snyder PJ, Young WF,
Boyajy LD, Levy R, Stewart WN, Klibanski A, Molitch ME and Gagel
RF: Safety and efficacy of long-term octreotide therapy of
acromegaly: Results of a multicenter trial in 103 patients-a
clinical research center study. J Clin Endocrinol Metab.
80:2768–2775. 1995.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lamberts SW, van der Lely AJ, de Herder WW
and Hofland LJ: Octreotide. N Engl J Med. 334:246–254.
1996.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bodei L, Cremonesi M, Grana C, Rocca P,
Bartolomei M, Chinol M and Paganelli G: Receptor radionuclide
therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in
neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 31:1038–1046.
2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bodei L, Mueller-Brand J, Baum RP, Pavel
ME, Hörsch D, O'Dorisio MS, O'Dorisio TM, Howe JR, Cremonesi M,
Kwekkeboom DJ and Zaknun JJ: The joint IAEA, EANM, and SNMMI
practical guidance on peptide receptor radionuclide therapy (PRRNT)
in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 40:800–816.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bergsma H, Konijnenberg MW, van der Zwan
WA, Kam BL, Teunissen JJ, Kooij PP, Mauff KAL, Krenning EP and
Kwekkeboom DJ: Nephrotoxicity after PRRT with
(177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 43:1802–1811.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kashyap R, Jackson P, Hofman MS, Eu P,
Beauregard JM, Zannino D and Hicks RJ: Rapid blood clearance and
lack of long-term renal toxicity of 177Lu-DOTATATE enables
shortening of renoprotective amino acid infusion. Eur J Nucl Med
Mol Imaging. 40:1853–1860. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kesavan M and Turner JH: Myelotoxicity of
peptide receptor radionuclide therapy of neuroendocrine tumors: A
decade of experience. Cancer Biother Radiopharm. 31:189–198.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ploeckinger U, Kloeppel G, Wiedenmann B
and Lohmann R: Representatives of 21 German NET Centers. The German
NET-registry: An audit on the diagnosis and therapy of
neuroendocrine tumors. Neuroendocrinology. 90:349–363.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Garcia-Carbonero R, Capdevila J,
Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso
Orduña V, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez
A, Llanos-Muñoz M, et al: Incidence, patterns of care and
prognostic factors for outcome of gastroenteropancreatic
neuroendocrine tumors (GEP-NETs): Results from the national cancer
registry of Spain (RGETNE). Ann Oncol. 21:1794–1803.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lombard-Bohas C, Mitry E, O'Toole D,
Louvet C, Pillon D, Cadiot G, Borson-Chazot F, Aparicio T, Ducreux
M, Lecomte T, et al: Thirteen-month registration of patients with
gastroenteropancreatic endocrine tumours in France.
Neuroendocrinology. 89:217–222. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008.PubMed/NCBI View Article : Google Scholar
|