Introduction
Single-port surgery (SPS) is a recent advance in
minimally invasive techniques. The benefits of SPS included better
cosmetic outcomes, less postoperative pain, faster postoperative
recovery, and earlier discharge from the hospital, compared with
multi-port surgery (MPS) (1-3).
Recently, SPS can provide satisfactory oncological outcomes in
patients with colon cancer (4-6).
On the other hand, the usefulness of SPS for rectal
cancer is unknown. SPS for rectal cancer is somewhat technically
more challenging, and there is no clinical evidence to confirm the
safety and feasibility of SPS for rectal cancer. In addition, it is
unclear whether SPS is able to ensure the satisfactory oncological
clearance in patients with rectal cancer in comparison of MPS.
Therefore, in this systematic review, we aimed to compare the
feasibility and safety of SPS with those of MPS for rectal cancer
in terms of perioperative and short-term oncological outcomes.
Materials and methods
Literature research
We had systematically collected useful studies from
MEDLINE, PubMed and Cochrane Library from 2010 to 2018. The search
items were ‘Single incision’, ‘single port’, ‘single site’, ‘SILS’
and ‘rectal cancer’. Manual searches of references from relevant
articles were performed when necessary. MPS was defined as
laparoscopic surgery using three or more ports by a small incision.
SPS was defined as laparoscopic surgery using only one port by a
small incision. Articles were selected if the abstract contained
data on patients who underwent SPS for rectal cancer. Publications
were included if they were randomized controlled trials,
case-matched controlled trials, or comparative observational
studies, in which patients underwent SPS for rectal cancer. Studies
were excluded if they were non-comparative studies, or including
surgery involving colon cancer or rectosigmoid cancer. Review
articles, conference abstracts, case, letter and other unqualified
articles were excluded.
Outcome of interest
We used the following results to compare SPS and MPS
for rectal cancer: i) Patient profile including age, sex, body mass
index and previous abdominal surgery; ii) operative data including
operative time, blood loss, conversion rate and additional port;
iii) postoperative outcome including morbidity, mortality,
anastomotic leakage, postoperative hospital stay, reoperation and
readmission; iv) histopathological findings including tumor size,
number of harvested lymph node, length of resected specimen,
proximal margin, distal margin and positive resection margin.
Risk of bias evaluation
One randomized controlled trail quality was assessed
by the Cochrane Reviewers' Handbook with the Jaded score in three
metrics: Randomization, double blindness, and control. Four
non-randomized controlled trail qualities were assessed with the
Newcastle-Ottawa Scale from three aspects: Patient selection,
confirmation of exposure, and comparability of both groups.
Results
Study characteristics and quality
assessment
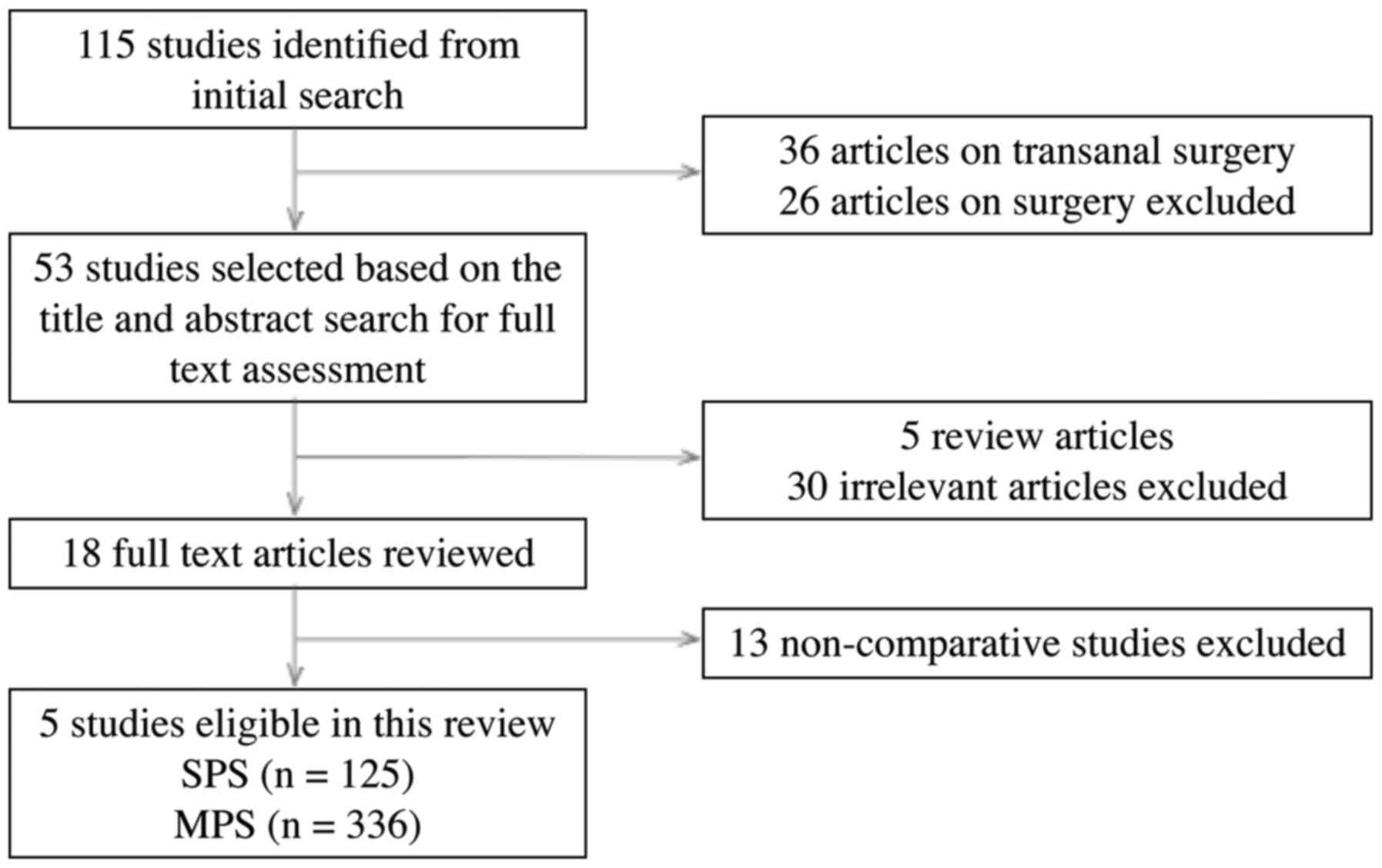
We found 115 potentially relevant publications by
using the above search strategy. Twenty-six publications on
robotic-assisted laparoscopic surgery and 36 publications on
transanal minimal invasive surgery were excluded. After reviewing
titles and abstracts of 53 articles, five review articles, 30
irrelevant articles, and 13 articles on surgical technique were
excluded. Finally, five studies were eligible in this review, which
included one randomized trial and four comparative studies. All
patients who underwent SPS or MPS were confirmed pathologically for
rectal cancer (7-11).
The flowchart of the selection process for studies included in this
review is presented in Fig. 1. We
considered that one randomized controlled trail had low quality,
and four non-randomized controlled trails had moderate or high
quality.
Patient profiles, operative details
and postoperative outcomes
Of the patients evaluated by these studies, 125
patients underwent SPS and 336 patients underwent MPS. Table I lists the profiles of the patients
from each study, including age, sex, body mass index and previous
abdominal surgery.
 | Table IPatient profiles. |
Table I
Patient profiles.
| | | Patients, n | Age, years | Sex, Female | Sex, Male | BMI,
kg/m2 | PAS, n (%) | |
|---|
| Year published | First author | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | Refs. |
|---|
| 2013 | Sourrouille et
al | 13 | 32 | 60 | 61 | 5 | 13 | 8 | 19 | 23.0 | 24.7 | NR | NR | (11) |
| 2015 | Bulut et
al | 20 | 20 | 69 | 73 | 8 | 8 | 12 | 12 | 24 | 24 | 3(15) | 7(35) | (8) |
| 2014 | Levic and Bulut | 36 | 194 | 69 | 68 | 19 | 61 | 17 | 133 | 23.8 | 25 | 13 (36.1) | 50 (25.8) | (9) |
| 2018 | Nerup et
al | 12 | 41 | 76 | 69 | 5 | 28 | 7 | 73 | 23.5 | 25 | 2 (16.7) | 14 (34.1) | (10) |
| 2018 | Tei et al | 44 | 49 | 66 | 63 | 15 | 20 | 29 | 29 | 23.6 | 22 | 13 (29.5) | 16 (32.7) | (7) |
The operative details show in the Table II. The operative time, blood loss
and the conversion rate to open surgery were described in five
studies. Levic and Bulu (9) and
Nerup et al (10) reported
that the operative time was significantly longer in the SPS group
than in the MPS group (295 min vs. 248 min, P=0.01 and 316 min vs.
269 min, P=0.004, respectively). Sourrouille et al (11) and Levic and Bulu (9) reported that blood loss was
significantly less in the SPS group than in the MPS group (100 ml
vs. 200 ml, P=0.01 and 35 ml vs. 100 ml, P=0.006, respectively).
The conversion rate to open surgery was lower in the SPS group than
in the MPS group (0.8 vs. 5.4%, respectively). 16.8% of patients in
the SPS group required an additional port to allow completion of
the operation.
 | Table IIOperative factors. |
Table II
Operative factors.
| | | Operative time, mean
(range), min | Blood loss, mean
(range), ml | Conversion to open
surgery, n (%) | Additional port, n
(%) | |
|---|
| Year published | First author | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | Refs. |
|---|
| 2013 | Sourrouille et
al | 290 (270-360) | 280 (240-353) | 100 (50-150) | 200 (100-300) | 1 (7.7) | 2 (6.3) | 1 (7.7) | NR | (11) |
| 2015 | Bulut et
al | 295 (108-465) | 264 (125-421) | 33 (0-300) | 100 (0-650) | 0 | 1(5) | 2(10) | NR | (8) |
| 2014 | Levic and
Bulut | 295 (108-465) | 248 (51-431) | 35 (0-400) | 100 (0-3142) | 0 | 13 (6.7) | 5 (13.9) | NR | (9) |
| 2018 | Nerup et
al | 316 (294-323) | 269 (236-309) | 50 (0-200) | 150 (62-250) | 0 | 2 (4.9) | NR | NR | (10) |
| 2018 | Tei et
al | 188 (116-343) | 203 (111-385) | 5 (5-650) | 5 (5-700) | 0 | 0 | 11(25) | 0 | (7) |
The postoperative outcomes show in Table III. The incidence of morbidity,
mortality, anastomotic leakage, postoperative hospital stay and
reoperation were reported in all five studies. Morbidity rate was
lower in the SPS group than in the MPS group (RR 0.69, 95% CI 0.43
to 1.11). Mortality rate was 2.4% in each group. The incidence of
anastomotic leakage was 9.7% in the SPS group and 10.2% in the MPS
group, respectively (RR 0.79, 95% CI 0.36 to 1.71). Postoperative
hospital stay was reported as 7 to 12 days in the SPS group and as
7 to 14 days in the MPS group, respectively. Reoperation rate was
8.8% in the SPS group and 13.4% in the MP group, respectively (RR
0.95, 95% CI 0.46 to 1.95). The incidence of readmission was
reported in four studies, 16.2% in the SPS group and 10.6% in the
MPS group, respectively (RR, 0.1.60; 95% CI, 0.69 to 3.69). The
postoperative pain was reported in two studies. Sourrouille et
al (11) reported that the
median visual analog scale score on postoperative days 2 was
significantly lower in the SPS group than in the MPS group (1.5 vs.
4, respectively, P=0.01) and the need for dose of morphine was
significantly lower in the SPS group than in the MPS group (2.5 vs.
4 mg, respectively, P=0.02). Bulut et al (8) reported that the NRS pain scores were
significantly reduced in the SPS group on postoperative days 2, 3
and 4 during both coughing and mobilization. In addition, the
patients in the SPS group suffered significantly less pain at rest
at 6 h after surgery and at postoperative days 1, 3 and 4. All five
studies had significant heterogeneity in random effect model by
I-square statistics.
 | Table IIIMorbidity and mortality |
Table III
Morbidity and mortality
| | Morbidity, n
(%) | Mortality, n
(%) | Anastomotic
leakage, n (%) | Postoperative
hospital stay, days | Reoperation, n
(%) | Readmission, n
(%) | |
|---|
| Year published | First author | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | Refs. |
|---|
| 2013 | Sourrouille et
al | 5 (38.5) | 9 (28.1) | 0 | 0 | 1 (7.7) | 3 (9.4) | 12 | 14 | 1 (7.7) | 2 (6.3) | NR | NR | (11) |
| 2015 | Bulut et
al | 7(35) | 8(40) | 2(10) | 0 | 4(25) | 4(25) | 7 | 8 | 2(10) | 2(10) | 4(25) | 1(5) | (8) |
| 2014 | Levic and
Bulut | 10 (27.8) | 80 (41.2) | 2 (5.6) | 6 (3.1) | 3 (8.3) | 22 (11.3) | 7 | 7 | 6 (16.7) | 37 (19.1) | 5 (13.9) | 22 (11.3) | (9) |
| 2018 | Nerup et
al | 4 (33.3) | 21 (51.2) | 2 (16.7) | 2 (4.9) | NR | NR | 7 | 8 | 0 | 2 (4.9) | 2 (18.2) | 5 (12.2) | (10) |
| 2018 | Tei et
al | 9 (20.5) | 13 (26.5) | 0 | 0 | 3 (6.8) | 4 (8.2) | 10 | 11 | 2 (4.6) | 2 (4.6) | NR | NR | (7) |
Pathological findings and oncological
outcomes
The pathological findings show in Table IV. Tumor size and the detail of
resected specimen were reported in four studies. The number of
harvested lymph node was reported in five studies. Levic and Bulu
(9) was reported that the number of
harvested lymph node was significantly lower in the SPS group than
in the MPS group (13 vs. 16, respectively, P=0.047). In the other
four studies, there was no significant difference in the number of
harvested lymph node. The length of resected specimen, proximal
resection margin, and distal margin were similar in both groups.
The rate of positive resection margin was 1% in the SPS group and
1.3% in the MPS group, respectively.
 | Table IVOncological outcomes. |
Table IV
Oncological outcomes.
| | | Tumor size, mm | Number of harvested
LN, mean (range) | Resected specimen,
mean (range), mm | Proximal margin,
mean (range), mm | Distal margin, mean
(range), mm | RM positive, n
(%) | |
|---|
| Year published | First author | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | SPS | MPS | Refs. |
|---|
| 2013 | Sourrouille et
al | NR | NR | 15 (11-18) | 14 (8-20) | NR | NR | NR | NR | NR | NR | 0 | 2 | (11) |
| 2015 | Bulut et
al | 25 | 40 | 14 (4-33) | 19 (7-33) | 210 (110-350) | 200 (140-250) | NR | NR | 32.5 (5-75) | 25 (5-65) | NR | NR | (8) |
| 2014 | Levic and
Bulut | NR | NR | 13 (3-33) | 16 (1-48) | 170 (100-350) | 190 (100-430) | NR | NR | 30 (5-75) | 25 (0-95) | 1 (2.8) | 1 (0.5) | (9) |
| 2018 | Nerup et
al | 20 | 25 | 12 (9-17) | 12 (7-15) | 260 (230-310) | 230 (200-260) | NR | NR | 40 (35-50) | 40 (20-50) | 0 | 0 | (10) |
| 2018 | Tei et
al | 35 | 45 | 23 (7-62) | 26 (6-68) | NR | NR | 120 (80-190) | 115 (75-210) | 30 (15-45) | 32 (12-42) | 0 | 1 (2.0) | (7) |
With regard to survival outcomes, only one study
reported 3-year relapse free survival and 5-year overall survival.
Tei et al (7) reported that
the 3-year relapse free survival and 5-year overall survival rates
were 94.7 and 97.4% in the SPS group, over the median follow-up
period of 40 months, respectively. In the MPS group, the 3-year
relapse free survival and 5-year overall survival rates were 78.6
and 86.1%, over the median follow-up period of 51 months,
respectively.
Discussion
Recently, surgery for colorectal cancer is shifting
to less invasive surgery because of better postoperative short-term
surgical results, which include: Lower postoperative morbidity,
reduced intraoperative blood loss, less pain, faster recovery and
better quality of life (12,13).
Moreover, laparoscopic surgery for colorectal cancer is considered
to be comparable to long-term results compared with open surgery
(12,13).
SPS for colorectal disease was first described in
2008 (14,15), and has been reported the better
short-term surgical outcomes in comparison to MPS since then.
However, most of the reports on SPS are concerned with colon cancer
(4-6),
and whether SPS is better than MPS for rectal cancer still remains
unclear.
This systematic review is to compare the clinical
outcomes of SPS vs. MPS in patients with rectal cancer. The major
findings of this analysis show that SPS for rectal cancer is a safe
and feasible approach (as deemed by 16.8% of patients requiring an
additional port and 0.8% requiring conversion to open surgery), and
yields adequate short-term surgical outcomes (e.g., morbidity of
28.0% and mortality of 2.4%) and satisfactory oncological outcomes
(e.g., R0 resection rate of 99.0%).
With regard to operative factors, two studies
reported that operative time was significantly longer in the SPS
group and two studies reported that the blood loss is significantly
less in the SPS group. Kim et al reported that the learning
curve of SPS for sigmoid colon cancer was 61-65 cases according to
multidimensional statistical analyses (16). Li et al reported that the
experienced MPS surgeons achieved the technical competence after
the 44th case of SPS plus one port in patients with sigmoid colon
cancer and upper rectal cancer (17). SPS for rectal cancer is technically
more challenging and require more cases for proficiency, and we
consider this result is likely little of statistical
significance.
The conversion rate to open surgery was
significantly lower in the SPS group than in the MPS group (0.8 vs.
5.4%, P=0.032). However, 16.8% in the SPS group had required an
additional port to complete the operation, due to fixation of
tumor, severe pelvic fibrosis or distal rectum division. In a
previous study, the conversion rate of SPS for rectal cancer was 8%
(18). Hirano et al reported
that SPS plus one port was safe and feasible for rectal cancer to
overcome the technical difficulties, including mobilization and
rectum division (19). We suggest
that it is most reasonable to insert an additional port at the time
when SPS is judged technically difficult.
With regard to postoperative outcomes, the morbidity
and mortality rate in the SPS group were 28.0 and 2.4%,
respectively, and comparable to the short-term outcomes of
conventional laparoscopic surgery for rectal cancer in a recent
randomized control trial (20).
Anastomotic leakage, reoperation, postoperative hospital stay and
readmission were similar in both groups, and also comparable to the
above trial.
Reduction of postoperative pain is very important
for early recovery, and it is well known that the transition from
open surgery to laparoscopic surgery is associated with reduction
of postoperative pain. Sourrouille et al (11) and Bulut et al (8) revealed reduction of postoperative pain
scores following SPS compared to MPS in their studies. Although it
is controversial whether SPS contributes to the reduction of
postoperative pain compared with MPS at present, this innovative
surgical technique may be involved in postoperative pain
reduction.
The maintenance of the surgical oncological outcome
is the most important factor in the treatment of rectal cancer. The
oncological outcomes, including number of harvested lymph nodes,
length of resected specimen, proximal dissection margin, distal
dissection margin, and residual tumor status did not differ between
groups. In particular, residual tumor status was negative in 99%
cases of the SPS group, and comparable to the recent randomized
controlled trials (20,21). With regard to long-term oncological
outcomes, Tei et al reported that the 3-year relapse-free
survival rate and 3-year overall survival rate in the SPS group
were 94.7 and 97.4%, over the median follow-up period of 40 months,
respectively (7). The other four
studies did not report for the long-term outcomes. The COLOR II
Study Group reported that the 3-year relapse-free survival rate and
3-year overall survival rate in the laparoscopic surgery group were
74.8 and 86.7%, respectively (22).
Jeong et al reported that the 3-year relapse-free survival
rate and 3-year overall survival rate in the laparoscopic surgery
group were 79.2 and 91.7%, respectively (23). The long-term outcomes of SPS for
rectal cancer should be needed for further research.
This systematic review being compared the clinical
outcomes between the SPS and MPS for rectal cancer has several
important limitations. First, five studies composed of one
randomized controlled study and four non-randomized studies with
small number of patients, which were not the highest quality of
evidence, were a limitation that might be affect the outcomes and
induce selection bias, although the majority of the assessed
outcomes across all papers had no dramatic conflicts in the
findings. Second, there is a difference in terms of preoperative
assessment, including tumor location (upper rectum, lower rectum,
or distance from anal verge), clinical TNM classification and
preoperative treatment. Third, there is a possibility that the
results may be influenced by various surgical techniques and
devices in the SPS group. Fourth, the long-term oncological outcome
is controversial because of insufficient follow-up period after
surgery.
This study confirmed the safety and feasibility of
SPS for rectal cancer, with slighter postoperative pain, lower
conversion rate to open surgery, lower postoperative complication
rate and satisfactory oncological clearance. In the future, more
randomized controlled trials with a large number of cases are
needed to demonstrate the clinical and prognostic impact of the SPS
for rectal cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All the datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
MTe designed and wrote the systematic review for the
topic highlight. TM, HF, CK, MW, HM, RK, and MTs collected the
literature. MTe and TS analyzed and interpreted the data and
provided the clinical suggestion. TS, TM, HF, CK, MW, HM, RK, and
MTs and JH critically revised the article. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that the have no competing
interests.
References
|
1
|
Papaconstantinou HT and Thomas JS:
Single-incision laparoscopic colectomy for cancer: Assessment of
oncologic resection and short-term outcomes in a case-matched
comparison with standard laparoscopy. Surgery. 150:820–827.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Champagne BJ, Papaconstantinou HT, Parmar
SS, Nagle DA, Young-Fadok TM, Lee EC and Delaney CP:
Single-incision versus standard multiport laparoscopic colectomy: A
multicenter, case-controlled comparison. Ann Surg. 255:66–69.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Poon JT, Cheung CW, Fan JK, Lo OS and Law
WL: Single-incision versus conventional laparoscopic colectomy for
colonic neoplasm: A randomized, controlled trial. Surg Endosc.
26:2729–2734. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takemasa I, Uemura M, Nishimura J,
Mizushima T, Yamamoto H, Ikeda M, Sekimoto M, Doki Y and Mori M:
Feasibility of single-site laparoscopic colectomy with complete
mesocolic excision for colon cancer: A prospective case-control
comparison. Surg Endosc. 28:1110–1118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Katsuno G, Fukunaga M, Nagakari K,
Yoshikawa S, Azuma D and Kohama S: Short-term and long-term
outcomes of single-incision versus multi-incision laparoscopic
resection for colorectal cancer: A propensity-score-matched
analysis of 214 cases. Surg Endosc. 30:1317–1325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tei M, Wakasugi M and Akamatsu H:
Comparison of perioperative and short-term oncological outcomes
after single- or multiport surgery for colorectal cancer.
Colorectal Dis. 17:O141–O147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tei M, Otsuka M, Suzuki Y, Kishi K,
Tanemura M and Akamatsu H: Safety and feasibility of single-port
laparoscopic low anterior resection for upper rectal cancer. Am J
Surg. 216:1101–1106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bulut O, Aslak KK, Levic K, Nielsen CB,
Rømer E, Sørensen S, Christensen IJ and Nielsen HJ: A randomized
pilot study on single-port versus conventional laparoscopic rectal
surgery: Effects on postoperative pain and the stress response to
surgery. Tech Coloproctol. 19:11–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Levic K and Bulut O: The short-term
outcomes of conventional and single-port laparoscopic surgery for
rectal cancer: A comparative non-randomized study. Minim Invasive
Ther Allied Technol. 23:214–222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nerup N, Rosenstock S and Bulut O:
Comparison of single-port and conventional laparoscopic
abdominoperineal resection. J Minim Access Surg. 14:27–32.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sourrouille I, Dumont F, Goéré D, Honoré C
and Elias D: Resection of rectal cancer via an abdominal
single-port access: Short-term results and comparison with standard
laparoscopy. Dis Colon Rectum. 56:1203–1210. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lacy AM, Garcia-Valdecasas JC, Delgado S,
Castells A, Taurá P, Piqué JM and Visa J: Laparoscopy-assisted
colectomy versus open colectomy for treatment of non-metastatic
colon cancer: A randomised trial. Lancet. 359:2224–2229.
2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY,
Ng SS, Lai PB and Lau WY: Laparoscopic resection of rectosigmoid
carcinoma: Prospective randomised trial. Lancet. 363:1187–1192.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Remzi FH, Kirat HT, Kaouk JH and Geisler
DP: Single-port laparoscopy in colorectal surgery. Colorectal Dis.
10:823–826. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bucher P, Pugin F and Morel P: Single port
access laparoscopic right hemicolectomy. Int J Colorectal Dis.
23:1013–1016. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim CW, Kim WR, Kim HY, Kang J, Hur H, Min
BS, Baik SH, Lee KY and Kim NK: Learning curve for single-incision
laparoscopic anterior resection for sigmoid colon cancer. J Am Coll
Surg. 221:397–403. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li J, Wang Y, Liu D, Zhou H, Mou T, Li G
and Deng H: Multidimensional analyses of the learning curve for
single-incision plus one port laparoscopic surgery for sigmoid
colon and upper rectal cancer. J Surg Oncol. 117:1386–1393.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gash K, Bicsak M and Dixon A:
Single-incision laparoscopic surgery for rectal cancer: Early
results and medium-term oncological outcome. Colorectal Dis.
17:1071–1078. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hirano Y, Hattori M, Douden K, Shimada M
and Hashizume Y: Short-term clinical and oncological outcomes after
single-incision plus one port laparoscopic anterior resection for
rectal cancer. Dig Surg. 35:111–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van der Pas MH, Haglind E, Cuesta MA,
Fürst A, Lacy AM, Hop WC and Bonjer HJ: COlorectal cancer
Laparoscopic or Open Resection II (COLOR II) Study Group.
Laparoscopic versus open surgery for rectal cancer (COLOR II):
Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol.
14:210–218. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kang SB, Park JW, Jeong SY, Nam BH, Choi
HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, et al: Open versus
laparoscopic surgery for mid or low rectal cancer after neoadjuvant
chemoradiotherapy (COREAN trial): Short-term outcomes of an
open-label randomised controlled trial. Lancet Oncol. 11:637–645.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA,
van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA,
Andersson J, Angenete E, et al: A randomized trial of laparoscopic
versus open surgery for rectal cancer. N Engl J Med. 372:1324–1332.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jeong SY, Park JW, Nam BH, Kim S, Kang SB,
Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, et al: Open versus
laparoscopic surgery for mid-rectal or low-rectal cancer after
neoadjuvant chemoradiotherapy (COREAN trial): Survival outcomes of
an open-label, non-inferiority, randomised controlled trial. Lancet
Oncol. 15:767–774. 2014.PubMed/NCBI View Article : Google Scholar
|