Introduction
Endometrial cancer is a malignancy of the corpus
uteri and its morbidity is increasing in Japan (1). Currently it is the most prevalent
gynecological cancer in Japan as well as in other developed
countries. Globally, nearly 382,000 individuals are newly diagnosed
with endometrial cancer each year, and ~90,000 die from it
(2). Most patients with endometrial
cancer are cured by surgery alone or with adjuvant chemotherapy,
and thus the prognosis of endometrial cancer is better than the
prognosis of other gynecological cancers (3). However, because the prognosis of
advanced endometrial cancer is still poor (3), new therapeutic modalities are urgently
needed.
We have developed a peptide vaccine that is
personalized according to each patient's HLA-A locus type and
pre-vaccination immunity to a vaccine peptide panel (4-6).
The peptide panel consists of 31 different cytotoxic T-lymphocyte
(CTL)-epitope peptides, and a maximum of 4 peptides are selected
and used as vaccines with Montanide ISA51VG adjuvant. Clinical
trials of the vaccines, named the personalized peptide vaccines,
were conducted in patients with various cancers, including
gynecological cancers, and the results showed that the vaccines
were both feasible and safe (4-8).
For further development of the personalized peptide vaccines, the
identification of new biomarkers will be important. A recent trend
in the identification of new biomarkers is ‘liquid biopsy’ using
cell-free plasma/serum specimens and circulating tumor cells.
Therefore, we focused on circulating cell-free DNA (cfDNA) in the
plasma and investigated the cfDNA integrity of patients with
advanced endometrial cancer during treatment with the personalized
peptide vaccination.
Patients and methods
Patients and plasma samples
Frozen plasma samples from 32 patients with advanced
endometrial cancer who were enrolled in clinical trials of the
personalized peptide vaccination during the period from December
2008 to March 2018 were used in this study. Clinical stages of the
patients were as follows: Stage III (n=2), stage IV (n=3) and
recurrent (n=27). Histology of the patients was as follows: 19
endometrioid carcinoma (7 G1, 7 G2, 5 G3), 8 serous carcinoma, 1
clear cell carcinoma, 1 adenosquamous cell carcinoma, and 1
neuroendocrine carcinoma. Histology of the remaining two patients
was unknown. The clinical protocols of the personalized peptide
vaccination have been reported previously (7,8). The
clinical study was approved by the Kurume University Ethics
Committee and registered with the UMIN Clinical Trial Registry
under trial numbers UMIN1482, 10068, 11230, and 14855. Written
informed consent was obtained from all participants included in the
study. The plasma samples used were obtained before and after the
first vaccination cycle, which consisted of weekly injection for 6
or 8 weeks.
cfDNA integrity
The analytical method of the cfDNA integrity was
described elsewhere (9,10). After thawing the plasma samples,
insoluble materials were removed by centrifugation at 16,000 x g
for 5 min at 4˚C. The supernatants were diluted 1:40 with distilled
water, and 1.5 µl of each sample was subjected to subsequent
polymerase chain reaction (PCR) in a total volume of 15 µl. Short
(115-bp) and long (247-bp) Alu fragments were amplified and
quantitatively analyzed using real-time PCR (StepOne plus; Thermo
Fisher Scientific, Inc.) with THUNDERBIRD SYBR qPCR mix (Toyobo).
The PCR primer pairs were as follows: 5'-CCTGAGGTCAGGAGTTCGAG-3'
(forward) and 5'-CCTGAGGTCAGGAGTTCGAG-3' (reverse) for Alu-115;
5'-GTGGCTCACGCCTGTAATC-3' (forward) and 5'-CAGGCTGGAGTGCAGTGG-3'
(reverse) for Alu-247. The PCR protocol consisted of an initial
denaturation at 95˚C for 10 min, followed by 40 cycles of
amplification at 95˚C for 30 sec, 64˚C for 30 sec, and 72˚C for 30
sec. An arbitrary cutoff value of delta Rn=0.65 was used to obtain
cycle threshold (Ct) values (11).
Short 115-bp and long 247-bp PCR fragments of Alu reflect total
cfDNA and necrotic cell (mainly tumor cell)-derived cfDNA,
respectively. The cfDNA integrity was calculated according to the
formula: cfDNA integrity = 2(Ct value of Alu-115-Ct value of
Alu-247).
Measurement of peptide-reactive IgG
and CTLs
Quantitation of vaccinated peptide-reactive IgG in
the plasma and CTLs was described previously (7). The IgG responses were measured by the
LUMINEX beads method and the CTL responses were measured by an
ELISPOT assay of interferon-gamma-secreting cells. If the IgG
levels or spot number were increased to ≥2-fold the pre-vaccination
level, the response was considered augmented.
Statistical analysis
The cfDNA and cfDNA-integrity levels of the pre- and
post-vaccination were compared by Wilcoxon's signed rank test. The
relationship between the cfDNA integrity and pathogenetic types
were analyzed by Wilcoxon's rank sum test. The survival curves were
plotted by the Kaplan-Meier method. We compared the high and low
cfDNA-integrity groups at before and after the first vaccination
cycle by using a Cox's proportional hazard model. The contribution
of other factors, including cfDNA integrity as a continuous
variable, to the overall survival (OS) was also analyzed by using a
Cox's proportional hazard model. The statistical analyses were
performed using JMP Pro version 14 software (SAS, Inc.).
Results
Alteration of the circulating cfDNA
integrity during the peptide vaccination
To analyze the cfDNA integrity, genomic DNA
fragments of the Alu element, which is the most abundant repetitive
element in the human genome, in the plasma were quantified by
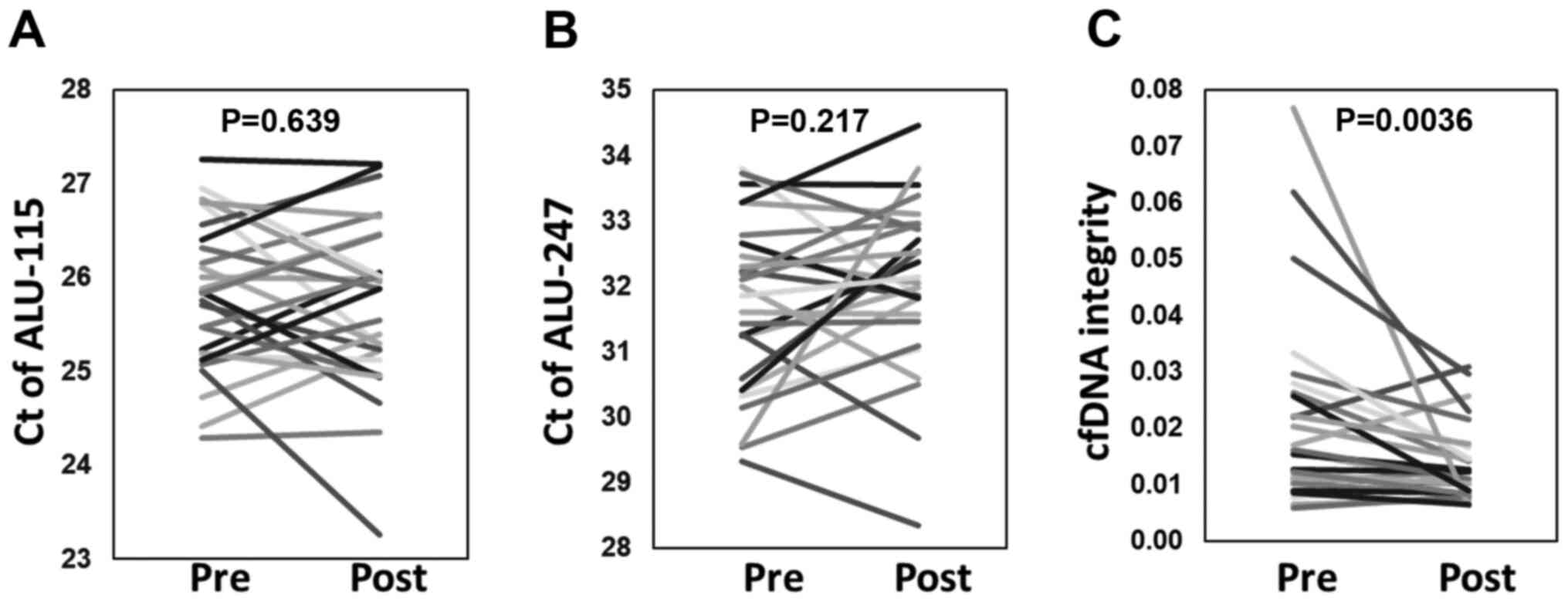
real-time PCR. Fig. 1 shows the Ct
values of short 115-bp (Alu-115) and long 247-bp (Alu-247) PCR
fragments of the Alu element and cfDNA integrity of plasma samples
obtained at before and after the first vaccination cycle. The
cfDNA-integrity values after one cycle of vaccination were
significantly decreased (P=0.0036). In contrast, such alteration
was not observed in the Ct values of Alu-115 and 247. We therefore
analyzed the relative contributions of Alu-115 and 247 to the
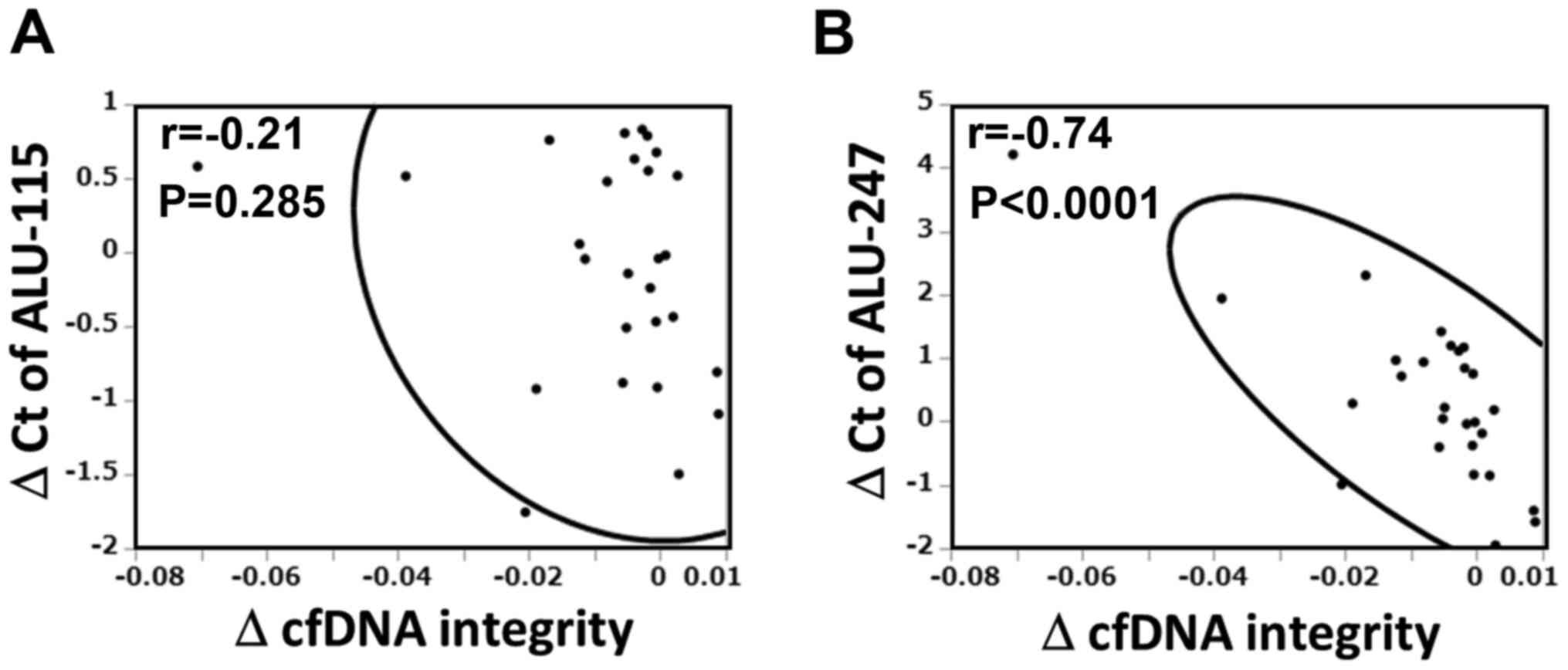
alteration of cfDNA integrity. As shown in Fig. 2, the change in Alu-247, but not
Alu-115, was significantly corelated with the change in cfDNA
integrity (r=-0.741, P<0.0001).
Relationship between the circulating
cfDNA integrity and pathogenetic types
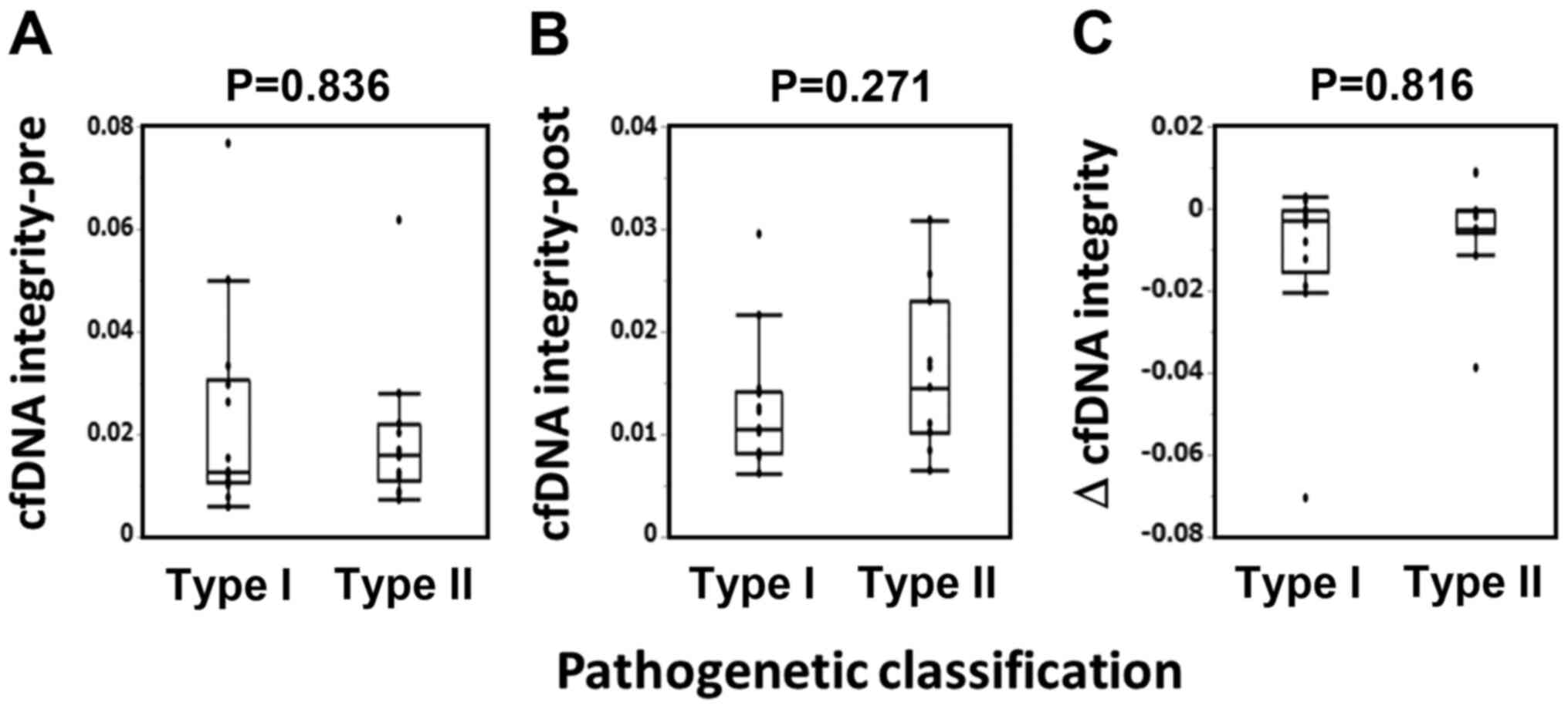
Next, we analyzed the relationship between the cfDNA
integrity and pathogenetic types. Pathogenetic classification of
the patients were as follows: Type I (n=14) consisted of 14
endometrioid carcinoma (7 grade 1 and 7 grade 2); type II (n=14)
consisted of 5 endometrioid carcinoma grade 3, 8 serous carcinoma,
and 1 clear cell carcinoma. As shown in Fig. 3, there were no significant
correlation between the pathogenetic types and either the
pre-vaccination values, post-one cycle vaccination values, or
changes in cfDNA integrity.
Relationship between the circulating
cfDNA integrity and prognosis
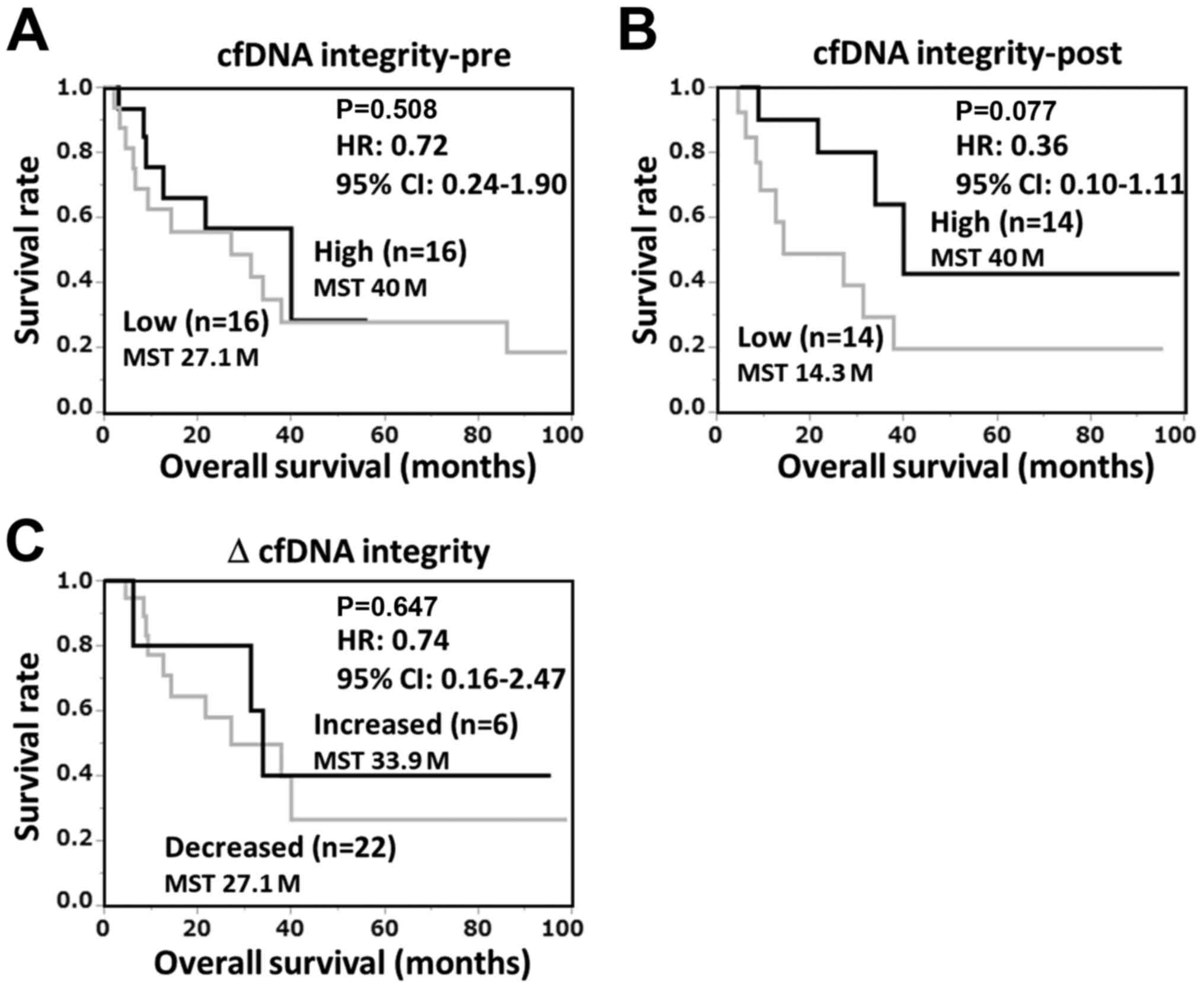
To examine the relationship between the circulating
cfDNA integrity and prognosis, the patients were divided into high
and low cfDNA-integrity groups and their OS was analyzed by
Kaplan-Meier plot. ‘High’ and ‘low’ were respectively defined as
the upper and lower median values of cfDNA. As shown in Fig. 4, there were no significant
correlations between OS and either the pre-vaccination values,
post-one cycle vaccination values, or changes in cfDNA integrity.
The median survival time (MST) of the post-vaccination high
cfDNA-integrity group was longer than that of the low
cfDNA-integrity group (40 and 14.3 months, respectively), although
the difference was not statistically significant. Thus, we further
analyzed the contribution of various factors, including cfDNA
integrity as continuous variables, to the OS by Cox's proportional
hazard analysis (Table I). Among
the various factors, only post-vaccination cfDNA integrity was
significantly correlated with OS (P=0.038). Vaccine-induced IgG or
CTL responses were not significantly correlated with OS.
 | Table ICox's proportional hazard analysis of
various factors with OS. |
Table I
Cox's proportional hazard analysis of
various factors with OS.
| Factors | Hazard ratio (95%
CI) | P-value |
|---|
| Age (1-year
increase) | 0.98 (0.93-1.05) | 0.661 |
| Lymphocyte (>1,200
or less) | 0.68 (0.26-1.74) | 0.418 |
| Pathogenetic
classification |
|
Type I vs.
type II | 0.29 (0.07-1.14) | 0.077 |
| Vaccine induced
immune response |
|
IgG
response | 0.94 (0.31-2.80) | 0.918 |
|
CTL
response | 1.44 (0.45-5.50) | 0.539 |
| cfDNA integrity (0.01
increase) |
|
Pre-vaccination | 0.69 (0.35-1.05) | 0.097 |
|
Post-vaccination | 0.27 (0.04-0.94) | 0.038 |
|
∆(Post-Pre) | 1.17 (0.77-2.17) | 0.509 |
Discussion
In previous studies, we reported several biomarkers
for peptide vaccine therapy against the advanced stage of various
cancers (12-18).
The vaccines consisted of CTL-epitope peptides, and therefore
vaccine-induced CTL responses were the primary mechanism underlying
the therapeutic effect of the vaccination, and early induction of
the CTL responses to the vaccine peptides was correlated with good
prognosis (12-17).
Some CTL epitope peptides can induce an IgG response in the
presence of helper T cells. Our peptide vaccines are personalized
by each patient's HLA-A locus type and pre-vaccination immunity to
the peptide panel detected by IgG (4-6).
Therefore, early induction or augmentation of IgG responses to the
vaccine peptides is also correlated with good prognosis in patients
treated with the personalized peptide vaccination (12-17).
In contrast, inflammation-related factors such as C-reactive
protein (CRP), interleukin (IL)-6, and serum amyloid A (SAA) were
correlated with poor prognosis of the vaccine-treated patients
(12-17).
High mobility group box-1 (HMGB1), a damage-associated molecular
pattern, is released from both the dead tumor cells and activated
macrophages. Correlation between the plasma levels of HMGB1 and
tumor progression has been reported in various cancers (18). The plasma levels of HMGB1 have also
been correlated with poor prognosis in patients treated with the
vaccines (19).
Dead tumor cells also released DNA fragments into
the plasma, similarly to the case of HMGB1. However, plasma cfDNA
contains not only tumor-derived DNA fragments due to pathogenic
cell death but also normal cell-derived DNA fragments due to
physiologic death (20). The
physiologic death of normal cells is mainly caused by apoptosis and
it generates DNA fragments of <200 bp in length. In contrast,
pathogenic death of tumor cells is mainly due to necrosis and
generates DNA fragments of more random size, including some of
longer length (21). To quantify
the plasma cfDNA fragments derived from dead cells caused by
pathogenic and physiologic cell death, real-time PCR of short
(115-bp) and long (247-bp) fragments of Alu, which is the most
abundant repetitive element in the human genome, has frequently
been used (10,22,23).
The cfDNA integrity, a ratio of the long (Alu-247) versus short
(Alu-115) DNA fragments, is also used to standardize the results
(10,22,23).
In this study, we found that: i) the plasma cfDNA integrity was
decreased during the first cycle of vaccination of patients with
endometrial cancer treated with the personalized peptide vaccines,
and ii) the post-vaccination cfDNA-integrity levels were correlated
with good prognosis. A decrease of the plasma cfDNA integrity
during peptide vaccination has also been observed in patients with
ovarian cancer (9) and non-small
cell lung cancer (NSCLC) (Waki et al, unpublished data). The
relationship between the plasma cfDNA integrity and OS found in
this study was also observed in patients with NSCLC (Waki et
al, unpublished data). Collectively, the decrease of plasma
cfDNA integrity during vaccination and the correlation between
cfDNA integrity and prognosis indicate that cfDNA integrity will be
a critical marker in future cancer vaccines. It is unclear why the
cfDNA integrity was decreased during vaccination. One of the
possibilities is that CTLs induced by the vaccination converted the
necrosis of tumor cells to apoptosis. This possibility was
supported by the negative correlation between the cfDNA integrity
and vaccine-induced immune responses found in ovarian cancer
(9). However, this idea cannot
fully explain the correlation between the cfDNA integrity and
prognosis. Vaccination may induce not only CTL-mediated apoptosis
but also necrosis or other types of cell death by various effector
mechanisms, including antibody-dependent cell lysis. In addition,
most patients had a history of previous chemotherapy and the
carryover of the effect was not neglected. The plasma cfDNA
integrity may reflect the comprehensive effects of these
therapies.
In conclusion, we investigated the circulating cfDNA
integrity of patients with advanced endometrial cancer during
treatment with a personalized peptide vaccination and we found
that: i) plasma cfDNA integrity was decreased during vaccination,
and ii) cfDNA integrity was correlated with prognosis. Some of
these findings have been confirmed in other cancers, and thus the
cfDNA integrity might be an important marker for future cancer
vaccine therapies in general, and might also be applicable for
other immune therapies.
Acknowledgements
The authors would like to thank Ms. M. Ozawa, K.
Yamamoto for technical assistance, and K. Yanaga for secretarial
assistance. All from the Cancer Vaccine Development Division,
Research Center for Innovative Cancer Therapy, Kurume
University.
Funding
The present study was supported by the Kurume
University Research Branding Project.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KW and AY contributed to the study conception and
design. Clinical sample collection was performed by KK and NT.
Material preparation, data collection and analysis were performed
by KW, KY and NK. The first draft of the manuscript was written by
KW and AY, and all authors commented on previous versions of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The clinical studies were approved by the Kurume
University Ethics Committee and registered with the UMIN Clinical
Trial Registry under trial numbers UMIN1482, 10068, 11230 and
14855.
Patient consent for publication
Not applicable.
Competing interests
Akira Yamada is a board member of the Bright Path
Biotherapeutics (Kawasaki, Japan).
References
|
1
|
Yamagami W, Nagase S, Takahashi F, Ino K,
Hachisuga T, Aoki D and Katabuchi H: Clinical statistics of
gynecologic cancers in Japan. J Gynecol Oncol.
28(e32)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McDonald ME and Bender DP: Endometrial
cancer: Obesity, genetics, and targeted agents. Obstet Gynecol Clin
North Am. 46:89–105. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamada A, Sasada T, Noguchi M and Itoh K:
Next-generation peptide vaccines for advanced cancer. Cancer Sci.
104:15–21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sasada T, Yamada A, Noguchi M and Itoh K:
Personalized peptide vaccine for treatment of advanced cancer. Curr
Med Chem. 21:2332–2345. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sakamoto S, Noguchi M, Yamada A, Itoh K
and Sasada T: Prospect and progress of personalized peptide
vaccinations for advanced cancers. Expert Opin Biol Ther.
16:689–698. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kawano K, Tsuda N, Matsueda S, Sasada T,
Watanabe N, Ushijima K, Yamaguchi T, Yokomine M, Itoh K, Yamada A
and Kamura T: Feasibility study of personalized peptide vaccination
for recurrent ovarian cancer patients. Immunopharmacol
Immunotoxicol. 36:224–236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kawano K, Tsuda N, Waki K, Matsueda S,
Hata Y, Ushijima K, Itoh K, Yamada A and Kamura T: Personalized
peptide vaccination for cervical cancer patients who have received
prior platinum-based chemotherapy. Cancer Sci. 106:1111–1117.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Waki K, Yokomizo K, Kawano K, Tsuda N,
Komatsu N and Yamada A: Integrity of plasma DNA is inversely
correlated with vaccine-induced antitumor immunity in ovarian
cancer patients. Cancer Immunol Imunother. 69:2001–2007.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Umetani N, Kim J, Hiramatsu S, Reber HA,
Hines OJ, Bilchik AJ and Hoon DS: Increased integrity of free
circulating DNA in sera of patients with colorectal or
periampullary cancer: Direct quantitative PCR for ALU repeats. Clin
Chem. 52:1062–1069. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Noguchi M, Mine T, Komatsu N, Suekane S,
Moriya F, Matsuoka K, Yutani S, Shichijo S, Yamada A, Toh U, et al:
Assessment of immunological biomarkers in patients with advanced
cancer treated by personalized peptide vaccination. Cancer Biol
Ther. 10:1266–1279. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shirahama T, Muroya D, Matsueda S, Yamada
A, Shichijo S, Naito M, Yamashita T, Sakamoto S, Okuda K, Itoh K,
et al: A randomized phase II trial of personalized peptide vaccine
with low dose cyclophosphamide in biliary tract cancer. Cancer Sci.
108:838–845. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kibe S, Yutani S, Motoyama S, Nomura T,
Tanaka N, Kawahara A, Yamaguchi T, Matsueda S, Komatsu N, Miura M,
et al: Phase II study of personalized peptide vaccination for
previously treated advanced colorectal cancer. Cancer Immunol Res.
2:1154–1162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yutani S, Komatsu N, Yoshitomi M, Matsueda
S, Yonemoto K, Mine T, Noguchi M, Ishihara Y, Yamada A, Itoh K and
Sasada T: A phase II study of a personalized peptide vaccination
for chemotherapy-resistant advanced pancreatic cancer patients.
Oncol Rep. 30:1094–1100. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoshitomi M, Yutani S, Matsueda S, Ioji T,
Komatsu N, Shichijo S, Yamada A, Itoh K, Sasada T and Kinoshita H:
Personalized peptide vaccination for advanced biliary tract cancer:
IL-6, nutritional status and pre-existing antigen-specific immunity
as possible biomarkers for patient prognosis. Exp Ther Med.
3:463–469. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoshiyama K, Terazaki Y, Matsueda S,
Shichijo S, Noguchi M, Yamada A, Mine T, Ioji T, Itoh K, Shirouzu
K, et al: Personalized peptide vaccination in patients with
refractory non-small cell lung cancer. Int J Oncol. 40:1492–1500.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fucikova J, Moserova I, Urbanova L, Bezu
L, Kepp O, Cremer I, Salek C, Strnad P, Kroemer G, Galluzzi L and
Spisek R: Prognostic and predictive value of DAMPs and
DAMP-associated processes in cancer. Front Immunol.
6(402)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Waki K, Kawano K, Tsuda N, Ushijima K,
Itoh K and Yamada A: Plasma levels of high-mobility group box 1
during peptide vaccination in patients with recurrent ovarian
cancer. J Immunol Res. 2017(1423683)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Giacona MB, Ruben GC, Iczkowski KA, Roos
TB, Porter DM and Sorenson GD: Cell-free DNA in human blood plasma:
Length measurements in patients with pancreatic cancer and healthy
controls. Pancreas. 17:89–97. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng J, Tang Q, Cao X and Burwinkel B:
Cell-free circulating DNA integrity based on peripheral blood as a
biomarker for diagnosis of cancer: A systematic review. Cancer
Epidemiol Biomarkers Prev. 26:1595–1602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang R, Pu W, Zhang S, Chen L, Zhu W,
Xiao L, Xing C and Li K: Clinical value of ALU concentration and
integrity index for the early diagnosis of ovarian cancer: A
retrospective cohort trial. PLoS One. 13(e0191756)2018.PubMed/NCBI View Article : Google Scholar
|