Introduction
The incidence of pancreatic cancer, which is one of
the most aggressive cancers and has a short overall survival (OS),
is increasing year by year (1-3).
There have been substantial advances in the therapeutic modalities
for advanced pancreatic cancer, including carbon beam ion
radiotherapy, systemic chemotherapies using gemcitabine (GEM),
tegaful-gimeracil-oteracil potassium (S-1) and oxaliplatin,
irinotecan, fluorouracil, leucovorin (Folfirinox), as well as an
EGFR-inhibitor erlotinib. Immune checkpoint inhibitors have been
approved only for pancreatic cancer patients with a high burden of
microsatellite instability (MSI-high), who make up a very small
subset (~1%) of the total cases (4). Therefore, the development of new
approaches is needed. The field of cancer immunotherapy has
drastically moved forward during these three decades since Boon and
his colleagues reported for the first time a tumor-associated
antigen, MAGE-A1, recognized by cytotoxic T lymphocyte (CTL) in
1991. Cancer vaccines, including peptide-based cancer vaccines, may
be a promising approach. However, early trials of these vaccines
did not realize sufficient clinical benefits to warrant approval
for advanced cancer patients, including PC patients (5-7).
We therefore developed a novel immunotherapeutic approach, the
personalized peptide vaccination (PPV), in which human leukocyte
antigen (HLA)-matched peptides are selected and administered based
on the pre-existing immunity before vaccination (8,9).
Randomized clinical trials of PPV for patients with
chemotherapy-naïve prostatic cancer or chemotherapy-resistant
bladder cancer showed clinical benefits (10,11).
The PPV also showed clinical benefits for some recurrent
glioblastoma patients (12).
However, PPV trials in patients with advanced pancreatic cancers
failed to provide a clear clinical benefit (13-16).
In the present study, we attempted to identify factors such as
biomarkers, disease stage, the number of previously conducted
chemotherapy regimens, associated with the lower clinical benefits
in these previous phase II clinical trials of PPV, which
collectively enrolled 309 pancreatic cancer patients.
Materials and methods
Peptides and protocols of clinical
study
Thirty-one candidate peptides were available for
PPV. All 31 peptides were cytotoxic T lymphocyte (CTL) epitopes
restricted to the HLA-A2, -A24, or -A3 supertypes (A3, A11, A31, or
A33), or HLA-A26 of HLA-class Ⅰmolecules (12.13). Twelve peptides
were for the HLA-A2, 14 for the HLA-A24, and 9 for the HLA-A3
supertypes, and 4 were matched for HLA-A26 of cancer patients as
reported previously (Table SI).
The peptides were prepared under conditions of Good Manufacturing
Practice using a Multiple Peptide System (San Diego, CA). Patients
were vaccinated with 2 to 4 peptides based on human leukocyte
antigen (HLA) type and pre-existing immunity by measuring
peptide-specific immunoglobulin G (IgG) levels. Each of the
selected peptides was mixed with incomplete Freund's adjuvant
(Montanide ISA-51VG; Seppic, Paris, France) and injected
subcutaneously into the inguinal, abdominal, or lateral thigh areas
as 1.5 ml emulsion (3 mg/each peptide).
All protocols were approved by the ethical committee
of Kurume University at first followed by the regional ethical
committee (Fukuoka clinical research board <Number 718004>,
and then registered in the UMIN Clinical Trials Registry of
Japanese government. Detailed protocols are presented online only
(https://upload.umin.ac.jp/cgi-open-bin/ctr/index.cgi?function=02)
where the protocols were written by Japanese with brief translation
to English. In brief, there were 3 different protocols with regard
to the vaccination intervals. The patients who became resistant to
the standard systemic therapies (n=180) received 6 injections of
PPV at 1-week intervals (the 1st cycle) followed by 6 injections at
2-week intervals (the 2nd cycle) (protocol PRT1; UMIN registration
numbers: 000001482, 000001881, 000006295, 000019390). Cancer
patients in the early or any other stages except those who became
resistant to the standard systemic therapies (n=109) received 4
injections of PPV at 1-week intervals and then 4 injections at
2-week intervals (the 1st cycle), followed by 4 injections at
2-week intervals and then 4 injections at 4-week intervals (the 2nd
cycle) (protocol PRT2; UMIN registration numbers: 000006297,
000011593, 000029789). In these two protocols, patients received
1.5 ml emulsion (3 mg/each peptide) of 2 to 4 peptides at each
visit for the vaccination. The third protocol was used for cancer
patients who lived in the northern parts of Japan or in other
countries (n=20) and thus both the patients and family needed to
stay in Kurume at least two days; they received 4 injections of PPV
at 4-week intervals (the 1st cycle) followed by the same schedule
for the 2nd cycle (protocol PRT3; UMIN registration numbers:
000006927, 000011230). In this third protocol, patients received
3.0 ml emulsion (6 mg/each peptide) of 2 to 4 peptides at each
visit for the vaccination, with half the dose injected into either
side of the body. In all three protocols, patients who wished to
continue the PPV after the 2nd cycle received the vaccination at 2-
to 12-week intervals until withdrawal of consent or unacceptable
toxicity. All these studies were in accordance with the Declaration
of Helsinki and the International Conference on Harmonization of
Good Clinical Practice guidelines, and the studies were conducted
in an outpatient setting. Written-informed consent to participate
in the clinical trial and to use their data for research and
publication purposes was obtained from all individual participants
before their inclusion in the study.
Patients
The phase 2 clinical trials of PPV were conducted
from November 2008 to March 2018 at the Cancer Vaccine Center of
Kurume University and Kurume University Hospital. A total of 309
patients with pancreatic cancer were enrolled, 301 patients with
advanced-stage disease (258 with stage IV and 43 with recurrent
disease) and 8 with non-advanced stage disease (stages I-III). The
clinical outcomes for some of these patients have been partly
reported previously (15,16). Eligibility criteria at the time of
entry were a pathologically confirmed diagnosis of pancreatic
cancer for those patients for whom a tumor sample was available
(n=145) or clinically diagnosed pancreatic cancer for those without
a tumor sample (n=160), positive IgG responses specific to at least
2 of the HLA-class-IA matched peptides in pre-vaccination plasma,
positive status for the HLA-A2, -A24, or -A3 supertypes (A3, A11,
A31, or A33) or positive status for HLA-A26, age of ≥20 years,
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
of 0 or 1, life expectancy of at least 12 weeks, and adequate bone
marrow function, hepatic function, and renal function. Exclusion
criteria were acute infection, a history of severe allergic
reactions, or other systemic diseases. The histological diagnoses
for the 145 patients with available tumor samples consisted of
adenocarcinoma (n=126), invasive ductal carcinoma (10), intra-ductal papillary mucinous
carcinoma (IDMC) (5), mucinous
cystadenocarcinoma (3), or
neuroendocrine tumor (1). In all
the protocols, concomitant chemotherapy regimens were permitted,
including but not limited to gemcitabine (GEM), TS-1 (an oral
fluoropyrimidine derivative), GEM+ nab-paclitaxel (nabPTX),
GEM+TS-1, and leucovorin+5-fluorouracil+irinotecan+oxaliplatin
(FOLFIRINOX). All patients provided written informed consent for
study participation and data collection.
Peptide-specific immune responses
Peripheral blood (30 ml) was taken from patients
before and after each cycle of vaccinations. Plasma was separated
after centrifugation and frozen until use. The IgG responses
specific to the vaccine peptides were measured in plasma by a
Luminex system as reported previously (8-11).
Statistical analysis
The Kaplan-Meier method, log-rank test, Cox
proportional hazards analysis, Student's t-test, chi-square test,
and Fisher's exact test were used for the statistical analyses. OS
was calculated as the time in months from the date of study
enrollment to death or to the date of last contact. The data-cutoff
for OS was June 2018. Time-to-event endpoints were analyzed using
the Kaplan-Meier method, and between-group comparisons for OS were
conducted using the log-rank test. All reported P-values were
two-sided, and P-values of <0.05 were considered significant.
JMP version 12 or SAS version 9.4 software (SAS Institute Inc.) was
used to perform all analyses.
Results
Baseline characteristics
The baseline characteristics of the 309 pancreatic
cancer patients are shown in Table
I. There were 171 males and 138 females, and the median age was
64 years. HLA-status was HLA-A24 in 180, HLA-A2 in 130, and HLA-A3
supertypes (A3, A11, A31, A33) in 161 patients, and HLA-A26 in 58
patients. Performance status (PS) was 0 in 241 and 1 in 68
patients. Clinical stages were stage I in 3, II in 2, III in 3, and
IV in 258 patients, and there were 43 recurrent cases. The number
of chemotherapeutic courses prior to the vaccination was 0, 1, 2,
3, or 4 in 28, 136, 108, 29, or 8 patients, respectively.
Chemotherapy regimens administered during the 1st to 2nd courses of
PPV were none (n=59 patients), gemcitabine (GEM) (59), S-1(65),
GEM+S-1(40), GEM+ nab-paclitaxel (14), and others (71), respectively. The
median number of vaccinations was 9, with a range of 1 to 60.
Informed consent was obtained from all patients prior to entering
the study.
 | Table ICharacteristics of the enrolled
patients (n=309). |
Table I
Characteristics of the enrolled
patients (n=309).
| Variable | Characteristics of
patients (n=309) | Patients who
completed the first cycle of PPV (n=228) | Patients who did not
complete the first cycle of PPV (n=81) | P-value |
|---|
| Median age (range),
years | 64 (33-83) | 64 (33-83) | 64 (39-79) | 0.40a |
| Sex | | | | 0.24b |
|
Male | 171 | 97 | 40 | |
|
Female | 138 | 131 | 41 | |
| HLA status | | | | |
|
HLA-A24 | 180 | 132 | 48 | 0.90b |
|
HLA-A2 | 130 | 96 | 34 | 1.00b |
|
HLA-A3
family | 161 | 120 | 41 | 0.80b |
|
HLA-A26 | 58 | 40 | 18 | 0.41b |
| Clinical stage
(surgery) | | | | 0.67b |
|
I | 3 | 3 | 0 | |
|
II | 2 | 2 | 0 | |
|
III | 3 | 4 | 0 | |
|
IV | 258 | 190 | 68 | |
|
Recurrence | 43 | 29 | 13 | |
| Performance
status | | | | 0.01b |
|
0 | 241 | 185 | 56 | |
|
1 | 66 | 43 | 23 | |
|
2 | 2 | 0 | 2 | |
| Numbers of
chemotherapy prior to PPV | | | |
<0.01b |
|
0 | 28 | 23 | 5 | |
|
1 | 136 | 108 | 28 | |
|
2 | 108 | 78 | 30 | |
|
3 | 29 | 18 | 11 | |
|
4 | 8 | 1 | 7 | |
| Combination
treatment (up to the 2nd cycle) | | | |
<0.01b |
|
None | 59 | 34 | 25 | |
|
GEM | 59 | 45 | 14 | |
|
GEM+TS-1 | 40 | 28 | 12 | |
|
TS-1 | 65 | 48 | 17 | |
|
GEM+nabPTX | 14 | 7 | 7 | |
|
Other
chemotherapies | 72 | 66 | 6 | |
| Number of
vaccinations | | | |
<0.01a |
|
Median
(range) | 9 (1-60) | 12 (4-60) | 3 (1-7) | |
| Median OS
(months) | | | | |
|
From
diagnosis | 17.6 | 19.5 | 13.6 |
<0.01c |
|
From
PPV | 5.8 | 8.4 | 2.1 |
<0.01c |
Eighty-one of 309 patients (26%) failed to complete
the 1st cycle of PPV due to rapid disease progression. All 81 of
these patients were in the advanced disease stages. Significant
differences were seen between the 81 patients who did not complete
the 1st cycle and the remaining 228 patients who did complete the
1st cycle in regard to PS (worse in the former group), number of
chemotherapy courses prior to PPV (larger in the former), and the
percentage of patients receiving chemotherapy combined with PPV
(lower in the former) (Table I). In
addition, the white blood cell and neutrophil numbers along with
c-reactive protein (CRP), a typical inflammatory protein, prior to
study entry were significantly higher in the 81 patients who did
not complete the 1st cycle, whereas the red cell and lymphocyte
numbers were significantly higher in the 228 patients who did
complete the 1st cycle (Table
II).
 | Table IILaboratory markers of the enrolled
patients (n=309). |
Table II
Laboratory markers of the enrolled
patients (n=309).
| Variable | Characteristics of
all patients (n=309) | Patients who
completed the first cycle of PPV (n=228) | Patients who did
not complete the first cycle of PPV (n=81) | P-value |
|---|
| Pre-vaccination
cell counts | | | | |
|
White blood
cells | 5,570 | 5,115 | 6,520 | <0.01 |
|
Red blood
cells | 354 | 358 | 332 | <0.01 |
|
Lymphocytes | 1,346 | 1,390 | 1,190 | <0.01 |
|
Platelets | 22 | 21.5 | 22.2 | 0.16 |
|
Neutrophils | 3,369 | 3,138 | 4,443 | <0.01 |
|
%
neutrophils | 64 | 62 | 69 | <0.01 |
| Pre-vaccination CRP
(mg/dl) | 0.33 | 0.21 | 1.20 | <0.01 |
| Pre-vaccination IgG
(FIU) | | | | |
|
To 31
peptides | 603 | 616 | 504 | 0.18 |
|
To
vaccinated peptides | 2,339 | 2,235 | 2,545 | 0.56 |
| Post-vaccination
IgG (FIU) | | | | |
|
To 31
peptides | - | 1,584 | - | - |
|
To
vaccinated peptides | - | 4,612 | - | - |
| Post-vaccination
immune boosting (%) | - | 92.1 | - | - |
| Median OS
(months) | | | | |
|
Immune
boosting + | - | 9.2 (n=208) | - | <0.01 |
|
Immune
boosting - | - | 4.9 (n=20) | - | - |
Adverse events (AEs)
The numbers of grade 1, 2, 3, 4 or 5 AEs were 1,018,
555, 190, 14 and 86, respectively (Table SII). The most frequent adverse
event was injection site reaction (grade 1: n=403; grade 2: n=55;
grade 3: n=8). Frequently observed grade 3 AEs were elevation of
GGT (n=24), anemia (21),
lymphopenia (20), injection site
reaction (8), ascites (7), glucose intolerance (7), and anorexia (7). Grade 4 AEs were anemia (n=4),
hepatobiliary disorders (n=3), lymphopenia (2), leukocytopenia (1), neutropenia (1), colonic obstruction (1), duodenal stenosis (1), and hypercalcemia (1). Frequently observed grade 5 AEs were
neoplasms (44), multi-organ failure (17), hepatic failure (4), and respiratory failures (3). According to the assessment by an
independent safety evaluation committee in this trial, all of these
severe AEs, except injection site reaction (8), were related to the cancer progression
or the combination chemotherapies.
Immune responses
Pre-vaccination peptide-specific IgG titers to each
of the 31 peptides were measured using a Luminex system with a
cut-off level of 10 fluorescence intensity units (FIU) taken as a
detectable level of IgG as reported previously (8-12).
The positive rate of antibody in the patients' plasma (n=309) was
largely different among the 31 peptides, ranging from 12 to 90%
with a median positive rate of 56%; Table III lists the peptides in order
from highest IgG positivity to lowest. The magnitudes of IgG titers
for the 31 peptides among the patients showing detectable levels
were also different from each other, with the median FIU being 35
(range: 23-132) among the 31 peptides (Table III).
 | Table IIIPre- and post-vaccination IgG
responses to each of the 31 peptides and their correlation to
patient OS. |
Table III
Pre- and post-vaccination IgG
responses to each of the 31 peptides and their correlation to
patient OS.
| | Pre-vaccination
IgG | | Post-vaccination
IgG responses (at the end of 1 or 2 cycles) | Correlation between
vaccination and OS (at the end of 1 or 2 cycles) |
|---|
| Peptides | Positive cases, n
(%) | Negative cases, n
(%) | Median FIU of
positive cases | Vaccinated cases, n
(%) | Dropped cases, n
(%) | Positive/ Negative,
n (%) | Median FIU of
Positive cases | Positive cases
(median OS) | Negaive cases
(median OS) | P-value |
|---|
| SART2-93 | 279(90) | 30(10) | 54 | 125(72) | 35(28) | 30/60(33) | 1,500 | 30 (13.1 M) | 60 (6.4 M) | <0.01 |
| Lck-486 | 258(83) | 51(17) | 44 | 86(79) | 18(21) | 46/22(68) | 11,301 | 46 (12.0 M) | 22 (4.4 M) | <0.01 |
| Lck-488 | 267(86) | 42(14) | 58 | 121(72) | 34(28) | 51/36(59) | 13,342 | 51 (12.3 M) | 36 (4.9 M) | <0.01 |
| Lck-90 | 254(82) | 55(18) | 44 | 77(78) | 17(22) | 27/33(45) | 1,499 | 27 (14.3 M) | 33 (5.0 M) | <0.01 |
| SART3-734 | 257(83) | 52(17) | 132 | 97(77) | 22(23) | 31/44(41) | 7,928 | 31 (10.1 M) | 44 (6.2 M) | 0.04 |
| PSA-248 | 242(78) | 67(22) | 42 | 18(78) | 4(22) | 11/3(79) | 13,221 | 11 (13.6 M) | 3 (4.2 M) | <0.01 |
| SART3-511 | 228(74) | 81(26) | 39 | 50(82) | 9(18) | 14/27(34) | 537 | 14 (14.3 M) | 27 (7.2 M) | 0.02 |
| SART3-309 | 235(76) | 74(24) | 31 | 40(77) | 9(23) | 18/13(58) | 4,306 | 18 (12.4 M) | 13 (5.2 M) | <0.01 |
| WHSC2-141 | 224(72) | 85(28) | 37 | 42(79) | 9(21) | 18/15(55) | 14,760 | 18 (12.5M) | 15 (5.8 M) | <0.01 |
| CypB-129 | 223(72) | 86(28) | 29 | 59(80) | 12(20) | 22/25(47) | 1,523 | 22 (9.3 M) | 25 (5.5 M) | <0.01 |
| Lck-246 | 217(70) | 92(30) | 45 | 42(79) | 9(21) | 18/15(55) | 3,467 | 18 (12.0 M) | 15 (4.9 M) | <0.01 |
| WHSC2-103 | 212(69) | 97(31) | 35 | 94(77) | 22(23) | 26/46(36) | 647 | 26 (11.9 M) | 46 (6.7 M) | <0.01 |
| SART3-302 | 210(68) | 99(32) | 80 | 53(75) | 13(25) | 31/9(78) | 20,126 | 31 (10.9 M) | 9 (7.2 M) | 0.26 |
| MRP3-1293 | 187(61) | 122(39) | 30 | 38(79) | 8(21) | 18/12(60) | 8,963 | 18 (12.5 M) | 12 (5.0 M) | <0.01 |
| EGF-R-800 | 179(58) | 130(42) | 28 | 21(71) | 6(29) | 9/6(60) | 391 | 9 (13.1 M) | 6 (7.0 M) | 0.12 |
| PAP-213 | 169(55) | 140(45) | 31 | 25(76) | 6(24) | 11/8(58) | 2,772 | 11 (12.3 M) | 8 (4.6 M) | 0.10 |
| Lck-449 | 166(54) | 143(46) | 23 | 16(94) | 1(6) | 5/10(33) | 18,286 | 5 (11.9 M) | 10 (4.9 M) | 0.03 |
| ppMAPkkk-432 | 150(49) | 159(51) | 42 | 39(62) | 15(38) | 6/18(25) | 497 | 6 (12.0 M) | 18 (7.9 M) | 0.10 |
| HNRPL-140 | 152(49) | 157(51) | 38 | 29(83) | 5(17) | 14/10(58) | 1,965 | 14 (16.6 M) | 10 (9.8 M) | <0.01 |
| PAP-248 | 174(56) | 135(44) | 43 | 33(85) | 5(15) | 14/14(50) | 2,485 | 14 (10.1 M) | 14 (7.4 M) | 0.56 |
| UBE2V-43 | 152(49) | 157(51) | 36 | 30(77) | 7(23) | 17/6(74) | 28,486 | 17 (15.9 M) | 6 (5.3 M) | <0.01 |
| SART3-109 | 142(46) | 167(54) | 30 | 51(78) | 11(22) | 19/21(48) | 1,896 | 19 (10.1 M) | 21 (5.4 M) | 0.08 |
| HNRPL-501 | 140(45) | 169(55) | 37 | 47(72) | 13(28) | 21/13(62) | 5,696 | 21 (10.3 M) | 13 (3.6 M) | <0.01 |
| SART2-161 | 118(38) | 191(62) | 31 | 18(72) | 5(28) | 5/8(38) | 3,278 | 5 (17.2 M) | 8 (6.0 M) | 0.26 |
| PSMA-624 | 111(36) | 198(64) | 23 | 8(62) | 3(38) | 2/3(40) | 12,213 | 2 (22.5 M) | 3 (2.3 M) | 0.28 |
| PTHrP-102 | 102(33) | 207(67) | 24 | 22(82) | 4(18) | 8/10(44) | 397 | 8 (5.1 M) | 10 (9.2 M) | 0.62 |
| Lck-208 | 82(27) | 227(73) | 23 | 13(69) | 4(31) | 1/8(11) | 2,016 | 1 (5.1 M) | 8 (7.9 M) | 0.28 |
| EZH2-735 | 67(22) | 242(78) | 22 | 5(80) | 1(20) | 2/2(50) | 20,059 | 2 (-M) | 2 (6.2 M) | 0.43 |
| MRP3-503 | 58(19) | 251(81) | 35 | 10(80) | 2(20) | 3/5(38) | 8,487 | 3 (25.4 M) | 5 (8.1 M) | 0.50 |
| UBE2V-85 | 46(15) | 263(85) | 27 | 6(100) | 0 (0) | 4/2(67) | 3,026 | 4 (48.7 M) | 2 (14.2 M) | 0.11 |
| Lck-422 | 36(12) | 273(88) | 28 | 9(89) | 1(11) | 1/7(13) | 78 | 1 (12.0 M) | 7 (9.6 M) | 0.89 |
Post-vaccination peptide-specific lgG levels were
measured at the end of both the 1st cycle and 2nd cycle in plasma
from the 228 patients who completed at least the 1st cycle of
vaccination. It was considered to be a positive immune response
when the post-vaccination IgG titer at the end of either the 1st or
2nd cycle was two times higher than the pre-vaccination titer
(8-10).
Six peptides (PSMA-624, Lck-208, EZH2-735, MRP3-503, UBE2V-85 and
Lck-422) with <10 cases of evaluable patients were excluded in
the following analysis to avoid a possible bias. Under these
circumstances, the percentage of patients showing positive IgG
responses among the 228 patients who completed at least the 1st
cycle differed greatly for the 25 peptides, ranging from 25 to 79%,
with a median rate of 55% (Table
III). The magnitudes of IgG titers of the 25 peptides among the
patients showing the positive responses were also very different,
with a median FIU of 3,278 among the 25 peptides, which was 89-fold
greater than the pre-vaccination level (37 FIU, as shown
above).
Effect of chemotherapy on the
PPV-induced immune boosting
We also investigated whether chemotherapy suppressed
the vaccination-induced immune boosting by comparing the rate and
magnitude of IgG boosting between the PPV patients with and those
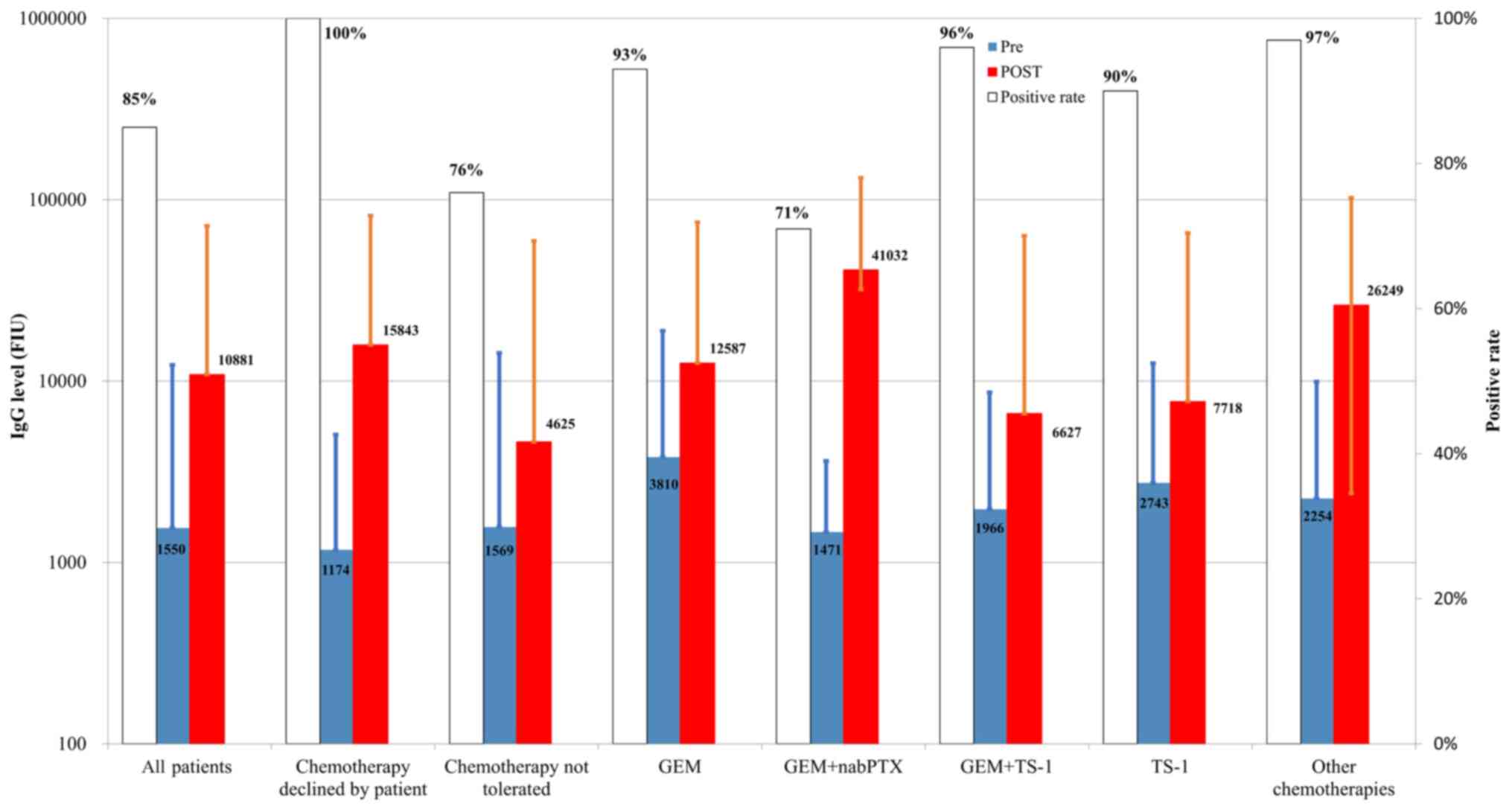
without chemotherapy (Fig. 1). IgG
boosting was observed in post-vaccination plasma in 23 of 27 (85%)
PPV patients without chemotherapy (10 of 10 patients (100%) who
declined chemotherapy of their own will and 13 of 17 patients (76%)
who could not tolerate chemotherapy), 42 of 45 (93%) with GEM, 5 of
7 (71%) with GEM + nabPTX, 27 of 28 (96%) with GEM+TS-1, 43 of 48
(90%) with TS-1, and 63 of 65 (97%) with the other chemotherapies
(Fig. 1). We further investigated
the n-fold increase of IgG boosting from the pre-vaccination to the
post-vaccination IgG titers using the IgG levels shown in Fig. 1. The increase was 7.0-fold in 27 PPV
patients without chemotherapy (13.5-fold in the 10 patients who
declined chemotherapy of their own will, and 2.9-fold in the 17
patients who could not tolerate chemotherapy), 3.3-fold in the 45
patients with GEM, 27.9-fold in the 7 patients with GEM+nabPTX,
2.9-fold in the 29 patients with GEM+TS-1, 2.8-fold in the 48
patients with TS-1, and 11.6-fold in the 65 patients who received
other chemotherapies. These results suggested that the combined
chemotherapy did not suppress PPV-induced boosting of
peptide-specific IgG from the viewpoint of either the positive rate
or the magnitude of IgG boosting.
Overall survival (OS)
The median OS of 309 patients was 5.8 months (M)
with a 95% confidence interval (CI) of 5.2-6.7 M from the 1st
vaccination, while it was 17.6 M with a 95% CI of 16.0-19.5 M from
the initial diagnosis (Table I).
The median time from the 1st vaccination of the 81 patients who
failed to complete the 1st cycle of PPV due to rapid disease
progression was much shorter than that of the remaining 228
patients who completed the 1st cycle (2.1 M, 95% CI: 1.8-2.7 vs.
8.4 M, 95% CI: 8.4-9.9; P<0.01), although that from the 1st
diagnosis was not different between the 81 patients who failed to
complete the 1st cycle and the remaining 228 patients (11 vs. 11 M)
(Table I).
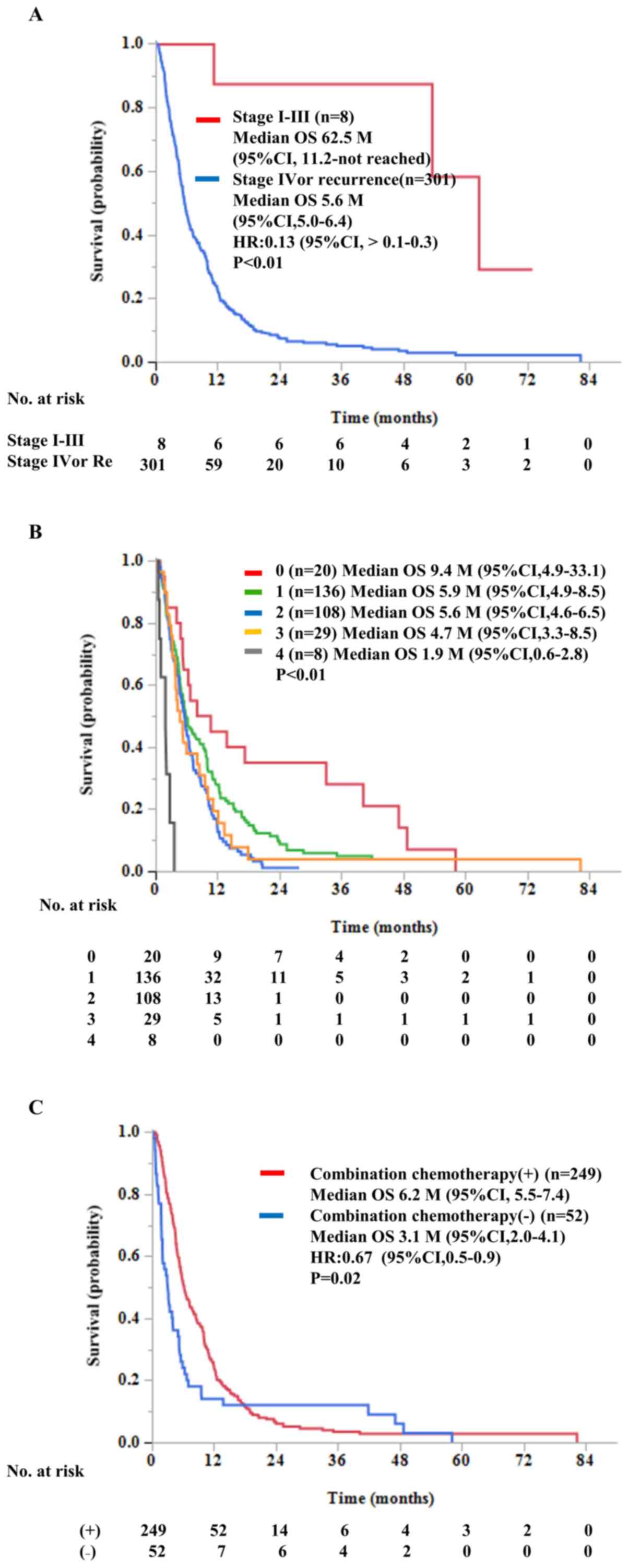
The 309 patients were also divided into those with
non-advanced (stages I-III) and those with advanced (stage IV or
recurrence) stage disease to better understand the role of
prophylactic or therapeutic PPV for pancreatic cancer,
respectively. The median OS of the 8 patients with stages I to III
disease from the 1st vaccination or from the initial diagnosis was
62.6 M (95% CI: 11.2-not reached) (Fig.
2A) or 72.3 M, (95% CI: 16.1-not reached) (data not shown),
respectively. All 8 of these patients were over 60 years old
(range: 61 to 83 years). Six and two patients were histologically
diagnosed as having adenocarcinoma and IDMC, respectively. Seven
patients and one patient received RO or R1 surgery, respectively.
Recurrence was observed in 4 patients, and progression free
survival and OS from the 1st vaccination were 42 and 63 M in the
stage I adenocarcinoma case, 38 and >39 M in the stage III IDMC
case, 6 and 53 M in the stage III adenocarcinoma case, and 6 and 11
M in the stage III adenocarcinoma case, respectively. The remaining
4 patients with PPV alone were free from recurrence.
The median OS of the 301 patients with
advanced-stage disease (stage IV or recurrence) was 5.6 M (95% CI:
5.6-6.4) (Fig. 2A) from the 1st
vaccination. Among them, no significant difference in the median OS
was seen between the stage IV (n=258) and recurrent cases (n=43)
(5.6 M, 95% CI: 5.0-6.3 vs. 7.2 M, 95% CI: 4.0-9.8; P=0.33) (data
not shown). No significant difference in the median OS was found
among the different types of HLA-class I (180A24+ patients: 5.7 M,
95% CI: 5.0-6.7 M; 130A2+ patients: 5.8 M, 95% CI: 5.1-7.7; 161A3
supertypes+ patients: 5.8 M, 95% CI: 4.8-6.7; 43 A26+ patients: 4.8
M, 95% CI: 3.9-10.0) (data not shown). In contrast, the median OS
in the patients under PTR1 (n=180, 5.3 M, 95% CI: 4.6-6.1 M) was
shorter than that of the patients under PRT2 (n=109, 7.7 M, 95% CI:
5.6-10.0) or PRT3 (n=20, 6.5 M, 95% CI: 3.7-12.1) (P<0.001),
primarily since the vast majority of the patients under PRT1 failed
to respond to all the available standard chemotherapies prior to
entry into the PPV study (data not shown).
Effect of chemotherapy on OS
We investigated the effect of pre-vaccination
chemotherapy status on OS. The median OS values of the 301 patients
with advanced cancer were inversely correlated with the number of
chemotherapy regimens conducted prior to the vaccination (Fig. 2B). The median OS values of the
patients with 0 (n=20), 1(136), 2(108), 3(29), or 4(8) courses of
chemotherapy prior to the vaccination were 9.4 M (95% CI:
4.9-33.1), 5.9 M (95% CI: 4.9-8.5), 5.6 M (95% CI: 4.6-6.5), 4.7 M
(95% CI: 3.3-8.5), and 1.9 M (0.6-2.8), respectively
(P<0.001).
We then investigated the effect of combination
chemotherapies on the OS of the 301 patients with advanced cancer.
The median OS values of patients who refused (n=19) or could not
tolerate (n=33) combination chemotherapy were 5.4 M (95% CI:
1.9-9.6) and 2.9 M (95% CI: 1.8-3.4) (P=0.03), respectively. Those
for the patients receiving the various chemotherapies were as
follows: GEM (n=59) (6.1 M, 95% CI: 4.6-7.3), S-1 (n=65) (4.9 M,
95% CI: 4.0-7.8), GEM and TS-1 (n=40) (5.4 M, 95% CI: 4.6-9.4), GEM
and nab-paclitaxel (n=14) (17.3 M, 95% CI: 3.4-24.1), and other
chemotherapy regimens, including 6 cases of FORIFIRI (n=71) (7.9 M,
95% CI: 5.8-11.5) (data not shown). The median OS of the patients
who received PPV alone for at least the 1st to 2nd cycles of PPV
either because they refused the treatment of their own will or
because they could not tolerate chemotherapy (n=52) (3.1 M, 95% CI:
2.0-4.1 M) was significantly shorter than that of the patients who
received PPV in combination with chemotherapy (n=249) (6.2 M, 95%
CI: 5.5-7.4 M) (P=0.01) (Fig. 2C).
PPV combined with chemotherapy might be more appropriate for
patients who had previously undergone a smaller number of
chemotherapy regimens.
Correlation between the immune
boosting and OS
The median OS of the 208 patients who exhibited IgG
boosting (9.2 M, 95% CI: 7.3-10.1) was significantly (P<0.01)
longer than that of the 20 patients who did not show IgG boosting
(4.9 M, 95% CI: 2.8-5.6) (Table
II), confirming the results reported previously (8-14).
We also examined whether this was also the case for each of the 31
peptides in the 309 patients with pancreatic cancer. Six (PSMA-624,
Lck288, EZH2-735, MRP3-503, UBE2v-43, and Lck422) of the 31
peptides that were used in only a few cases (<10 tested cases)
were excluded from this analysis to avoid a possible bias (Table III). The median OS of the patients
that exhibited IgG boosting against each of the 17 of 25 peptides
(65.4%) that were used for >10 cases was significantly longer
(P<0.05) than that of the patients with no IgG boosting
(Table III). No significant
difference was found in the remaining 7 peptides.
Biomarkers
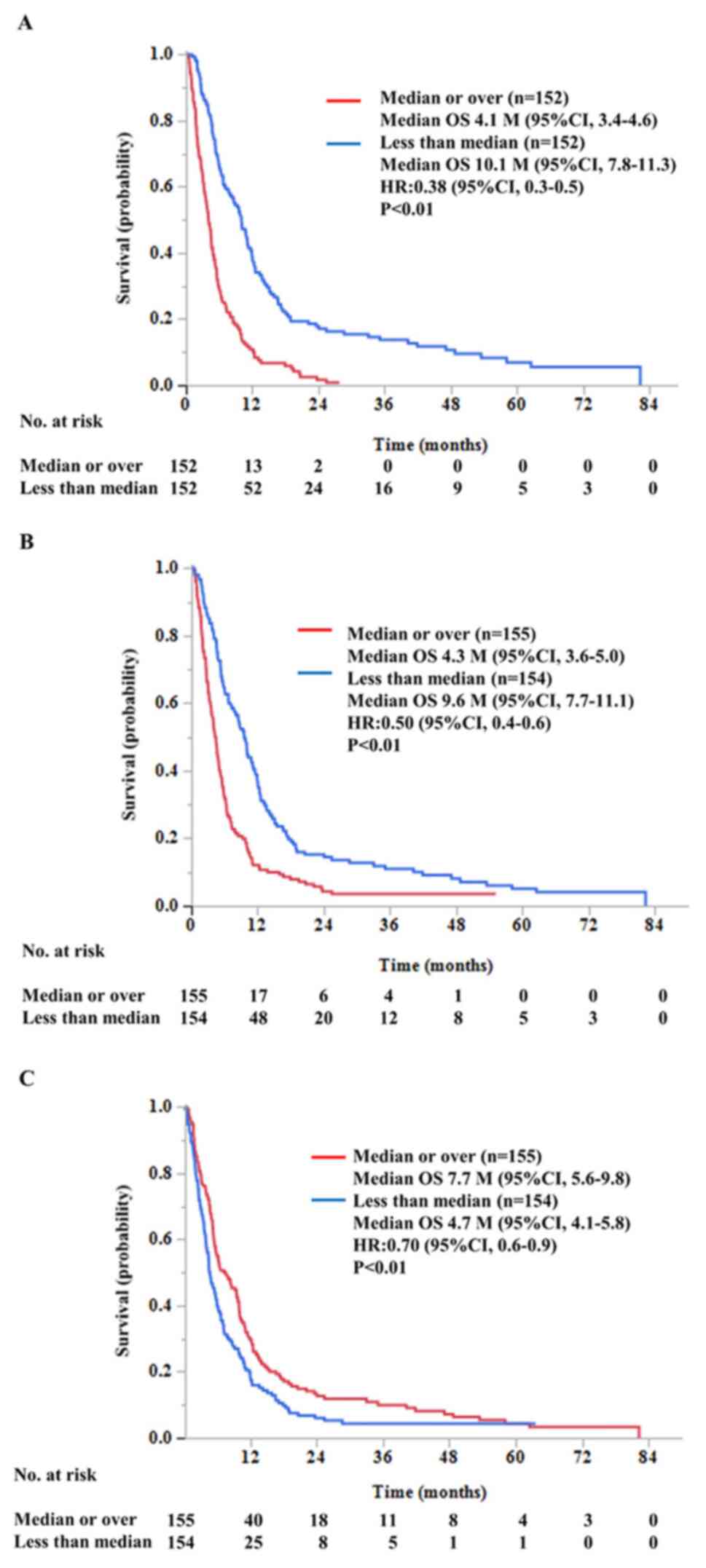
Finally, we investigated the correlation between the
OS and pre-vaccination CRP level or neutrophil numbers in each of
the 309 patients (Fig. S1). The
higher CRP levels or neutrophil numbers seemed to be associated
with shorter OS. Indeed, the median OS of the patients having
higher CRP levels or higher neutrophil numbers (median or higher
than median value) was significantly shorter than that of the
patients with lower values for these parameters (Fig. 3A and B), respectively. The opposite was true in
the case of lymphocyte numbers (Fig.
3C). Similar results were obtained in the 228 patients who
completed at least the 1st cycle of the vaccination (data not
shown). In contrast, only a higher CRP level, but not either higher
neutrophil numbers or lower lymphocyte numbers, was an unfavorable
biomarker at the statistically significant level for the 81
patients who failed to complete the 1st cycle of the vaccination
(data no shown).
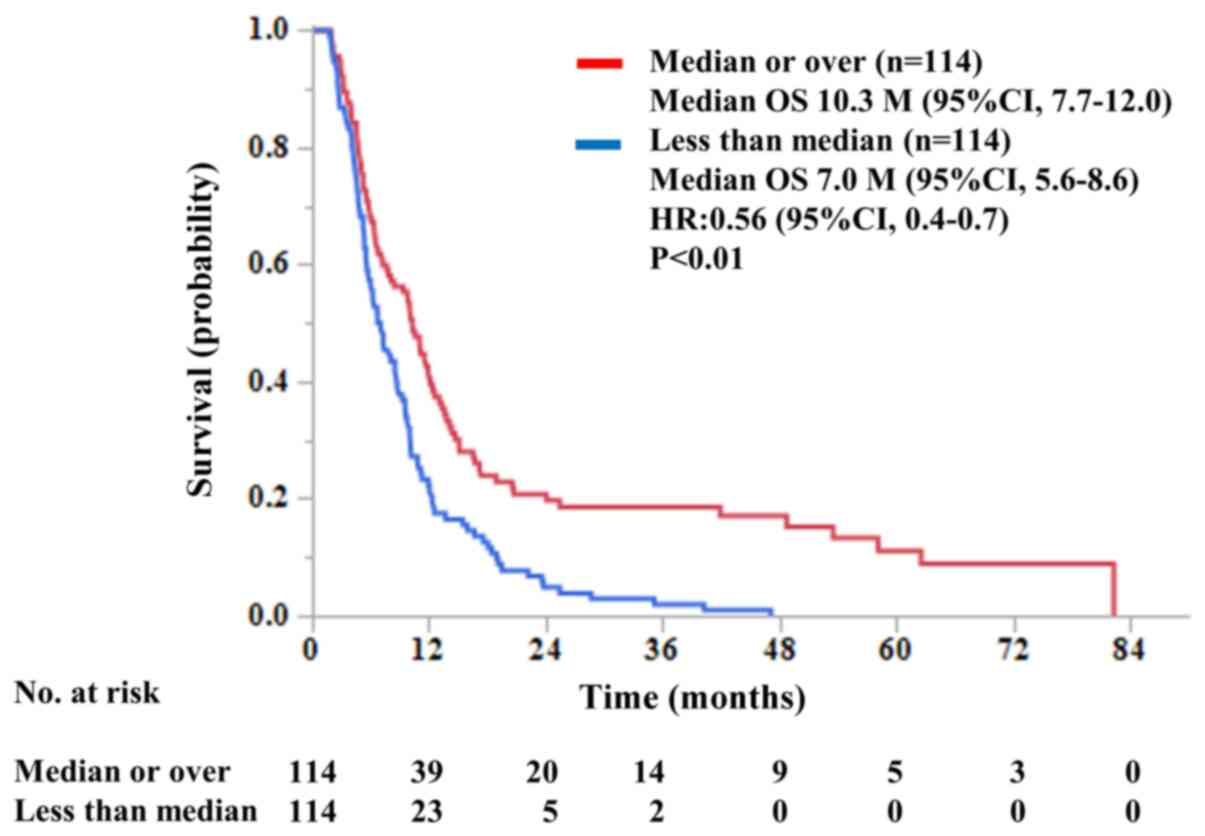
We also investigated the correlation between the OS
and either pre- or post-vaccination IgG levels against the
vaccinated peptides in all 309 patients, but significant levels of
correlation were not observed (data not shown). Then, we examined
the correlation between the OS and the IgG levels for each of the
vaccinated peptides in each of the 228 patients who completed at
least the 1st cycle of the vaccination (Fig. S2). The higher levels of the
increased IgG seemed to be associated with longer OS. Indeed, the
median OS of the patients with higher increased IgG levels (median
or higher than the median value) was significantly longer than that
of their counterparts with lower IgG levels (10.3 M, 95% CI:
7.7-12.0 vs. 7.0 M, 95% CI: 5.6-8.6) (Fig. 4).
Discussion
One of the unexpected results of this study was that
as many as 81 of the 301 patients with advanced-stage disease
failed to complete the 1st cycle of PPV due to a rapid disease
progression, regardless of the fact that the median OS of these 81
patients from the 1st diagnosis was not different from that of the
remaining 228 patients (11 vs. 11 M, respectively). Pre-vaccination
biomarkers to discriminate the former group from the latter group
were PS (worse in the former group), chemotherapy regimens prior to
PPV (larger in the former), neutrophil or lymphocyte numbers
(higher or lower in the former group), and CRP (higher in the
former group). We previously reported that these factors were
unfavorable for the OS of cancer patients receiving PPV (11-16).
In addition, we reported that only the CRP level, but not either
neutrophil or lymphocyte numbers, was a unfavorable biomarker in
both the 228 patients who completed at least the 1st cycle of the
vaccination and the 81 patients who failed to complete the 1st
cycle. Therefore, a higher pre-vaccination CRP level might be a
more useful factor associated with lower clinical benefits of PPV
for pancreatic cancer. A suitable cut-off level to discriminate
non-responders from responders might be <0.2 mg/dl, the baseline
range at our institution, since the median OS of patients with
higher CRP (≥0.2 mg/dl) (n=194) levels was significantly shorter
than that of the 115 patients within the normal range (4.3 M, 95%
CI: 3.7-4.9 vs. 11.1 M, 95% CI: 9.6-12.5; P<0.01, HR: 2.89)
(data not shown).
Both the positive rate of antibody and the magnitude
of IgG titers in pre-vaccination samples were largely different
among the 31 peptides. These diversities among the peptides were
also observed in the post-vaccination samples (the positive IgG
responses and magnitude of IgG titers). However, it was commonly
observed that the patients showing immune boosting to each of the
vaccinated peptides showed longer survival than those without such
boosting, suggesting that the vast majority of these 31 peptides
maintained their ability to prolong clinical benefits through
immune boosting. Therefore, the diversities among the peptides
might not be a risk factor associated with lower clinical benefits
of PPV.
IgG boosting was observed in the post-vaccination
plasma from the majority of the patients who completed the 1st
cycle of the vaccination irrespective of whether their PPV regimen
was combined with a chemotherapy regimen. We previously reported
that neither GEM nor TS-1 suppressed the PPV-induced immune
boosting in patients with advanced pancreatic cancer (13,14) or
other advanced cancers (17-20),
respectively. The previous results showed that the rate of increase
of IgG levels were higher in patients not receiving chemotherapy
than in patients receiving chemotherapy. This result seems to be
contradictory, but we think that these seemingly contradictory data
could be at least due to that the immunity of 10 patients who
declined chemotherapy was relatively kept enough to be activated by
PPV, but that of 17 patients who could not tolerate chemotherapy
was too suppressed or exhausted to be activated by PPV. All these
results suggested that the addition of chemotherapy did not
suppress PPV-induced boosting of peptide-specific IgG from the
viewpoint of the positive IgG response rate or the magnitude of IgG
boosting as compared to those in patients with PPV alone. This
issue, however, needs to be confirmed by means of a clinical trial
with a large number of patients.
Taken together, our results revealed that the
following six pre-vaccination factors were associated with lower
clinical benefits of PPV for pancreatic cancer patients: Higher
levels of CRP, higher numbers of neutrophils, lower numbers of
lymphocytes and red blood cells, advanced disease stages, and
larger numbers of pre-vaccination chemotherapy regimens. The sole
post-vaccination unfavorable factor was PPV monotherapy. The
concomitant administration of various regimens of chemotherapy did
not suppress either the PPV-induced immune boosting or clinical
benefits.
The vast majority of the 309 pancreatic cancer
patients enrolled in this trial were in the advanced stages, and
only 8 of them (2.7%) were in the early stages and entered the PPV
trial in order to prevent recurrence. Among these 8 patients, the 4
patients who underwent PPV alone were free from recurrence. The
magnitude of PPV-induced IgG boosting for these 8 patients was
significantly higher than that of the 301 patients with
advanced-stage cancer (P<0.01) by Fisher's exact test (data not
shown). It is generally recognized from an immunological point of
view that cancer vaccines are more appropriate for prevention of
recurrence than for treatment of advanced cancers. However, none of
the previously conducted prophylactic cancer vaccine trials,
including the MAGRIT study using MAGE3 antigen, demonstrated clear
clinical benefits (6,21,22).
In addition, recent advances in surgical and chemotherapeutic
approaches have led to considerable increases in survival in
patients with pancreatic cancer, particularly in those with
non-advanced stages (23-25).
Our results also showed that PPV might be more affordable for
patients with early stages of pancreatic cancer than those with
advanced stages, although this issue shall be addressed in the next
stage of clinical study of PPV with a large number of patients with
stage I-III disease.
Our results showed that pre-vaccination inflammatory
signatures, rather than post-vaccination immunological signatures,
were associated with lower clinical benefits of personalized
peptide vaccination (PPV) for pancreatic cancer. The major
limitation of the present study, however, is the retrospective
nature of the analysis of the 309 pancreatic cancer patients under
PPV treatment. Thus, the present results, while informative, are
far from definitive.
Supplementary Material
Correlation between the overall
survival and pre-vaccination (A) c-reactive protein levels, (B)
neutrophil numbers or (C) lymphocyte numbers in each of the 309
patients. The OS is individually presented for the group that
completed the first cycle of PPV (blue line) and the group that did
not (red line). PPV, personalized peptide vaccination.
Correlation between the overall
survival and the IgG levels of the vaccinated peptides in each of
the 228 patients who completed at least the 1st cycle of the
vaccination. PPV, personalized peptide vaccination; FIU,
fluorescence intensity unit.
Peptide candidates used for
personalized peptide vaccination.
Adverse events during PPV.
Acknowledgements
Not applicable.
Funding
The present study was supported by BrightPath
Biotherapeutics Co. and Taiho Pharmaceutical Company.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YU, TY, KI and SY treated the patients at the Cancer
Vaccine Center, Kurume University. YU designed six studies (UMIN
registration nos. 000001881, 000006297, 000006295, 000019390,
000029789 and 000011593). MY designed three studies (UMIN
registration nos. 000001482, 000006927 and 000011230). YU, DM, MU,
SS, AY TS and KO acquired patient samples. YU, SS, AY, TS and SY
analyzed patient data. YU, KO and KI drafted the manuscript and
confirmed the authenticity of all the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All studies were conducted in an outpatient setting
in accordance with the Declaration of Helsinki and the
International Conference on Harmonization of Good Clinical Practice
guidelines. Written-informed consent to participate in the clinical
trial and for application of the data to research and publication
purposes was obtained from all individual participants before their
inclusion in the study.
Patient consent for publication
All patients consented to the publication of their
research results.
Competing interests
Tetsuro Sasada received a grant from BrightPath
Biotherapeutics Co. Akira Yamada is a part-time executive of
BrightPath Biotherapeutics Co. Kyogo Itoh received research funding
from Taiho Pharmaceutical Company. The remaining authors declare
that they have no competing interests.
References
|
1
|
Kleeff J, Korc M, Apte M, La vecchia C,
Jhonson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2(16022)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hingorani SR, Zheng L, Bullock AJ, Seery
TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary
N, Oberstein PE, et al: Randomized Phase II Study of PEGPH20 plus
nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in
patients with untreated, metastatic pancreatic ductal
adenocarcinoma. J Clin Oncol. 36:359–366. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Johansson H, Andersson R, Bauden M, Hammes
S, Holdenrieder S and Ansari D: Immune checkpoint therapy for
pancreatic cancer. World J Gastroenterol. 22:9457–9476.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Morrison AH, Byrne KT and Vonderheide RH:
Immunotherapy and prevention of pancreatic cancers. Trends Cancers.
4:418–428. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bezu J, Kepp O, Cerrato G, Pol J, Fucikova
J, Spisek R, Zitvogel L, Kroemer G and Galluzzi L: Trial watch:
Peptide-based cancer vaccines in anticancer therapy.
Oncoimmunology. 7(e1511506)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamaue H, Tsunoda T, Tani M, Miyazawa M,
Yamao K, Mizuno N, Okusaka T, Ueno H, Boku N, Fukutomi A, et al:
Randomized phase II/III clinical trial of elpamotide for patients
with advanced pancreatic cancer: PEGASUS-PC study. Cancer Sci.
106:883–890. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sasada T, Yamada A, Noguchi M and Itoh K:
Personalized peptide vaccine for treatment of advanced cancer. Curr
Med Chem. 21:2332–2345. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: A new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yoshimura K, Minami T, Nozawa M, Kimura T,
Egawa S, Fujimoto H, Yamada A, Itoh K and Uemura H: A Phase 2
randomized controlled trial of personalized peptide vaccine
immunotherapy with low-dose dexamethasone versus dexamethasone
alone in chemotherapy-naive castration-resistant prostate cancer.
Eur Urol. 70:35–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Noguchi M, Matsumoto K, Uemura H, Arai G,
Eto M, Naito S, Ohyama C, Nasu Y, Tanaka M, Moriya F, et al: An
open-label, randomized phase ii trial of personalized peptide
vaccination in patients with bladder cancer that progressed after
platinum-based chemotherapy. Clin Cancer Res. 22:54–60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Narita Y, Arakawa Y, Yamasaki F, Nishikawa
R, Aoki T, Kanamori M, Nagane M, Kumabe T, Hirose Y, Ichikawa T, et
al: A randomized, double-blind, phase III trial of personalized
peptide vaccination for recurrent glioblastoma. Neuro Oncol.
21:348–359. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yanagimoto H, Mine T, Yamamoto K, Satoi S,
Terakawa N, Takahashi K, Nakamura K, Honma S, Tanaka M, Mizoguchi
J, et al: Immunological evaluation of personalized peptide
vaccination with gemcitabine for pancreatic cancer. Cancer Sci.
98:605–611. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yanagimoto H, Shiomi H, Satoi S, Mine T,
Toyokawa H, Yamamoto T, Tani T, Yamada A, Kwon A, Komatsu N, et al:
A phase II study of personalized peptide vaccination combined with
gemcitabine for non-resectable pancreatic cancer patients. Oncol
Rep. 24:795–801. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yutani S, Komatsu N, Yoshitomi M, Matsueda
S, Yonemoto K, Mine T, Noguchi M, Ishihara M, Yamada A, Itoh K and
Sasada T: A phase II study of a personalized peptide vaccination
for chemotherapy-resistant advanced pancreatic cancer patients.
Oncol Rep. 30:1094–1100. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yutani S, Komatsu N, Matsueda S, Yoshitomi
M, Shirahama T, Yamada A, Itoh K and Sasada T: Juzentaihoto failed
to augment antigen-specific immunity but prevented deterioration of
patients' conditions in advanced pancreatic cancer under
personalized peptide vaccine. Evid Based Complement Alternat Med.
2013(981717)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sato Y, Fujiwara T, Mine T, Shomura H,
Homma S, Maeda Y, Tokunaga N, Ikeda Y, Ishihara Y, Yamada A, et al:
Immunological evaluation of personalized peptide vaccination in
combination with a 5-fluorouracil derivative (TS-1) for advanced
gastric or colorectal carcinoma patients. Cancer Sci. 98:1113–1119.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takahashi R, Toh U, Iwakuma N, Takenaka M,
Otsuka H, Furukawa M, Fujii T, Seki N, Kawahara A, Kage M, et al:
Feasibility study of personalized peptide vaccination for
metastatic recurrent triple-negative breast cancer patients. Breast
Cancer Rese. 16(R70)2014.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Kawano K, Tsuda N, Matsueda S, Sasada T,
Watanabe N, Ushijima K, Yamaguchi T, Yokomine M, Itoh K, Yamada A
and Kamura T: Feasibility study of personalized peptide vaccination
for recurrent ovarian cancer patients. Immunopharmacol
Immunotoxicol. 36:224–236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kibe S, Yutani S, Motoyama S, Nomura T,
Tanaka N, Kawahara A, Yamaguchi T, Matsueda S, Komatsu N, Miura M,
et al: phase ii study of personalized peptide vaccination for
previously treated advanced colorectal cancer. Cancer Immunol Res.
2:1154–1162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takayama K, Sugawara S, Saijo Y, Maemondo
M, Sato A, Takamori S, Harada T, Sasada T, Kakuma T, Kishimoto J,
et al: Randomized phase II study of docetaxel plus personalized
peptide vaccination versus docetaxel plus placebo for patients with
previously treated advanced wild type EGFR non-small-cell lung
cancer. J Immunol Res. 2116(1745108)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vansteenkiste JF, Cho BC, Vanakesa T, De
Pas T, Zielinski M, Kim MS, Jassem J, Yoshimura M, Dahabreh J,
Nakayama H, et al: Effect of the MAGE3-cancer immunotherapeutic as
adjuvant therapy in patients with MAGE-A3-positive non-small-cell
lung cancer (MAGRIT): A randomized, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 17:822–835.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Satoi S, Yamamoto T, Motoi F, Matsumoto I,
Yoshitomi H, Amano R, Tahara M, Murakami Y, Arimitsu H, Hirono S,
et al: Clinical impact of developing better practices at the
institutional level on surgical outcomes after distal
pancreatectomy in 1515 patients: Domestic audit of the Japanese
Society of Pancreatic Surgery. Ann Gastroenterol Surg. 2:212–219.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hajatdoost L, Sedaghat K, Walker EJ,
Thomas J and Kosari S: Chemotherapy in pancreatic cancer: A
systematic review. Medicina (Kaunas). 54(48)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Neoptolemos JP, Kleeff J, Michl P,
Costello E, Greenhalf W and Palmer DH: Therapeutic developments in
pancreatic cancer: Current and future perspectives. Nat Rev
Gastroenterol Hepato. 15:333–348. 2018.PubMed/NCBI View Article : Google Scholar
|