Introduction
Endometrial cancer (EC) is the leading gynecological
malignancy in the Western world, and one of the top ten most common
cancers among women (1). Obesity,
polycystic ovarian syndrome and nulliparity are risk factors for EC
(1-3).
The risk of developing EC increases with age and more than 90% of
cases present in peri- and postmenopausal women, with a peak
incidence in the sixth decade (4).
Based on clinical and molecular characteristics, EC is classified
into two subgroups. Type 1, also known as endometrioid type, is the
more common one, representing ~80% of uterine cancer cases, and is
typically hormone sensitive, linked to an excess of estrogen, and
has a better prognosis. Type 2, or non-endometrioid type, accounts
for 20%, and is typically estrogen independent and comprises
sarcomas, clear cell carcinomas and others. Type 2 is more common
in senior women and clinically presents as a more aggressive
disease with a higher recurrence rate than type 1 (1,5,6).
Since symptoms such as abnormal vaginal bleedings
present early, EC is often diagnosed at an early stage, which
likely contributes to the favorable prognosis, with overall 5-year
survival higher than 80% (6).
However, metastasis, chemoresistance and recurrence remain a
challenge.
Tumour progression, notably metastasis and
chemoresistance, involves changes in cellular metabolism that
benefit tumour cell growth and survival; of these changes, the
Warburg effect, or high rates of aerobic glycolysis, is perhaps the
most well-known (7,8). Alterations in the levels of PKM2,
GAPDH and ATP5B, i.e., enzymes related to cellular metabolism, were
shown to be associated with the shorter survival in patients with
ovarian carcinomas (9). Alterations
in mitochondrial functions are instrumental to the metabolic
plasticity of tumour cells (10,11),
but also to metastasis, as upregulation of mitochondrial oxidative
phosphorylation by metastatic cells was linked to superoxide
production and subsequent regulation of cell adhesion processes
(12).
The transcriptional coactivator peroxisome
proliferator-activated receptor gamma (PPARγ) coactivator 1 (PGC1α)
is important in regulating mitochondrial biogenesis and function,
and lower levels of PGC1α expression have been noted in various
cancers, such as breast, colon (13), and ovarian cancers (14). Of note here, PGC1α has been shown to
be influenced by the presence of estrogen. Other mechanisms are
possible; thus, type 1 EC is reported to harbor mutations in
mitochondrial DNA (mtDNA) which affect Complex I, and which might
thereby lead to the observed upregulation of mitochondrial
biogenesis and PGC1α (15,16). In line with differential regulatory
pathways, both increased and decreased levels of PGC1α have been
associated with more aggressive cancer and poor prognosis (17).
The voltage-dependent anion channel type 1 (VDAC1)
protein located in the outer mitochondrial membrane regulates
mitochondrial import and export of ions and metabolites, including
Ca2+, ATP and NADH, and thereby regulates oxidative
phosphorylation (18). VDAC1 is
also involved in regulating cell death through interactions with
various proteins including hexokinase (HK), Bcl-2 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (19). VDAC1 is often used as a marker of
mitochondrial content of cells (20).
In the present study, we assessed protein expression
of PGC1α and VDAC1 in type I and II ECs and paired non-cancerous
tissue, in order to examine a putative correlation between them and
clinical data such as subtype, stage and grade, clinical resistance
and overall survival.
Materials and methods
Patient material
Tumour samples were obtained from 148 patients
diagnosed with EC between January 2008 and March 2012 at Karolinska
University Hospital, Stockholm. Upon resection of the uterus,
approximately 1 cm3 of the tumour was collected for
analysis, along with a sample of normal endometrium. In all, the
study included 126 (85%) patients with type I and 22 (15%) patients
with type II endometrial adenocarcinoma. In addition, paired benign
non-cancerous tissue was obtained from 135 (91%) of these women.
Cases were classified into type I and II according to
histopathological assessment and further characterized using the
International Federation of Gynecology and Obstetrics (FIGO) system
into stage, extent of myometrium invasion and grade of endometrial
carcinoma. Cases were assessed by the hospital pathology laboratory
at the time of diagnosis for hormone receptor status, p53 and
ploidy. Tumour characteristics of the total cohort and
characteristics, according to subtype, are described in Table I.
 | Table ITumour characteristics (n=148). |
Table I
Tumour characteristics (n=148).
| Characteristics | No. (%) |
|---|
| Histology | |
|
Endometrial
only | 125 (84.5) |
|
Serous or
mixed | 15 (10.1) |
|
Clear
cell | 7 (4.7) |
| Stage | |
|
1 | 103 (69.6) |
|
2 | 25 (16.9) |
|
3 | 17 (11.1) |
|
4 | 3 (2.0) |
| Grade | |
|
1 | 39 (26.4) |
|
2 | 60 (40.5) |
|
3 | 49 (33.1) |
| Depth of myometrial
invasion | |
|
None | 10 (6.8) |
|
<50% | 72 (49.0) |
|
≥50% | 63 (42.9) |
|
Through the
serosa | 2 (1.4) |
| Relapse | 25 (16.3) |
Immunohistochemistry and
antibodies
Immunohistochemical staining was performed on
formalin-fixed, paraffin-embedded tumour blocks, as described
previously (9). Tumour sections (4
µm) were stained using the Vectastain Elite ABC kit (Vector
Laboratories). For antigen retrieval, sections were heated in a
microwave oven in citrate buffer for 20 min. Primary antibodies
were anti-Ki-67 (M7240, Dako; Agilent Technologies, Inc.; 1:400),
anti-PGC1α (ab54481, Abcam; 1:200) and anti-VDAC1 targeting the
N-terminus (1-19 amino acids) and the central region (150-250 amino
acids) (529532 from Calbiochem, and ab15895 from Abcam,
respectively). Slides were incubated with primary antibody for 30
min at room temperature and then with secondary antibody before
addition of the avidin-biotinylated peroxidase complex.
Evaluation of
immunohistochemistry
Two observers (OCW and LL), blinded for clinical
outcome, independently evaluated all slides by assessing the whole
tumour area or, in the normal tissues, epithelial cells. Positive
PGC1α and VDAC1 immunoreactivities were observed, and the
percentages of positively stained cells were categorized
semiquantitatively from 0 to 3 (0, <1; 1, 1-25; 2, 25-50 and 3,
>50%). On the same scale, the maximum staining intensity was
scored 0-3 (negative, weak, moderate and strong). Immunoreactivity
scores represent the products of the two parameters. The ratio of
tumour (T) immunoreactivity score to normal (N) was then
calculated. As only 135 (91%) paired normal tissues were available
for analysis, the T/N immunoreactivity was trichotomized as T/N
<1, T/N=1 or T/N >1. Ki-67 staining was evaluated as
percentage of tumour cells with a positive nucleus. Five separate
sets of 100 cells were counted, and the average number of positives
given as the reported value.
Statistical analysis
We compared the expression of PGC1α and VDAC1 in
malignant and benign paired tissue using the Wilcoxon signed rank
test. The Wilcoxon test was used on the stratified cohorts in order
to compare PGC1α expression at different stages. A P-value <0.05
was set to indicate a statistically significant difference. To
assess survival was significantly different between patients with
different levels of PGC1α or VDAC1 expression, we used Kaplan-Meier
and log rank test. Spearman's rank correlation coefficient was used
in order to estimate the correlation between PGC1α, TFAM, p53,
respectively, and tumour characteristics. The Kruskal-Wallis test
was used for comparing several groups, and the Mann-Whitney U test
when there were two groups, since there were non-parametric data.
Analyses and figures were made using IBM SPSS 25.0 Mac OS.
Ethics
The study obtained ethics approval from the Regional
Ethics Committee Stockholm, Sweden, which includes approval of the
patient consent process. Registration no. 2010/1536-31/2.
Results
Clinicopathological features of the
cohort
Using immunohistochemistry, we analyzed the
expression of PGC1α and VDAC in 148 cases of EC, including 126 type
I (85%), and 22 type II (15%). Patients' median age at the time of
diagnosis was 70.0 (IQR 65.3-77.0), BMI=26.3 (23.7-30.1) and
parity=2 (1-3).
44.9% (67/148) of the women were previously prescribed hormone
replacement therapy. No significant difference in median age was
observed between the subtypes. Tumour characteristics are described
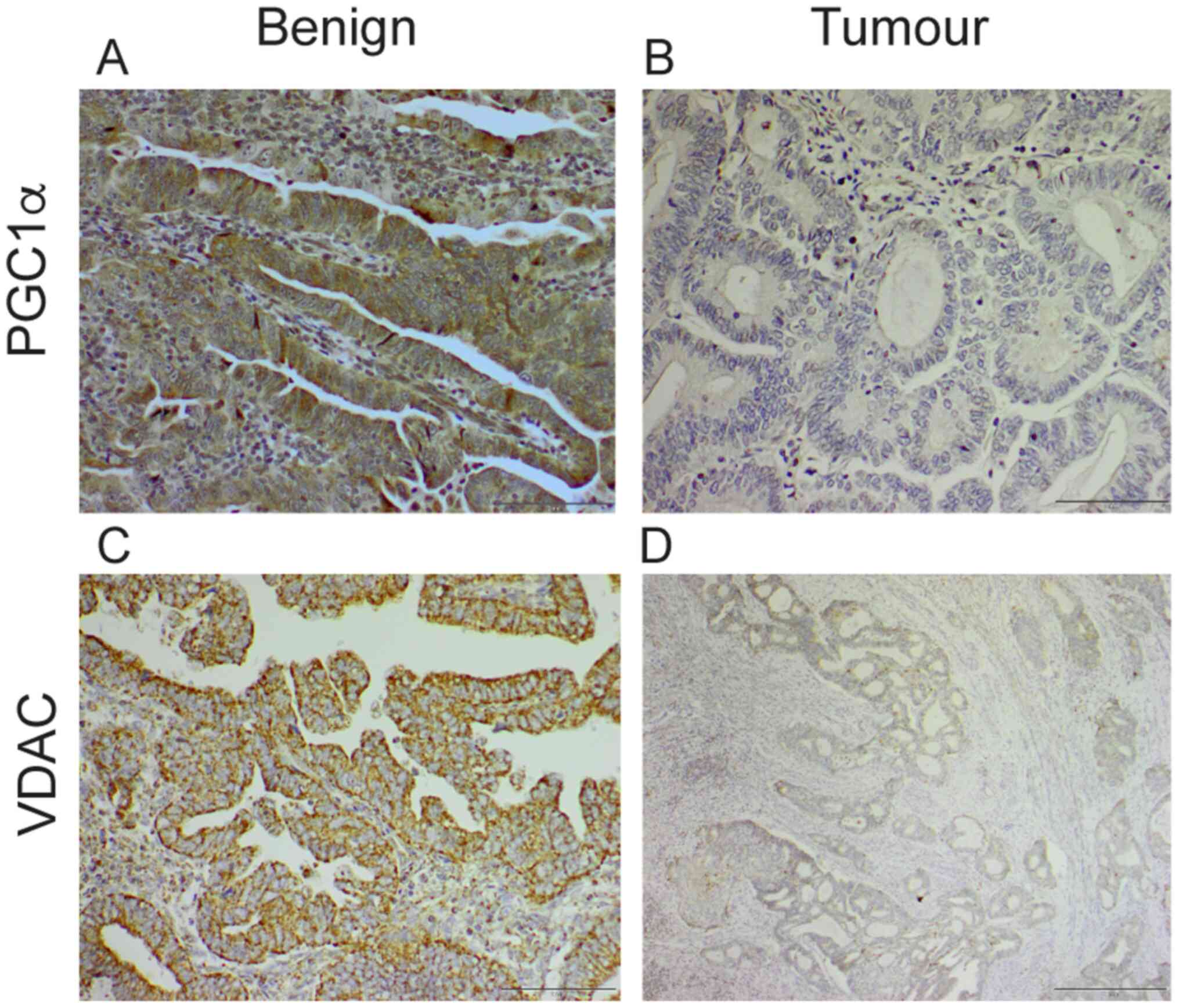
in I. Representative images of immunohistochemical staining for
PGC1α and VDAC are shown in Fig. 1.
As expected, significant differences between subtypes were found
for the biomarkers ERα and progesterone. The percentage of
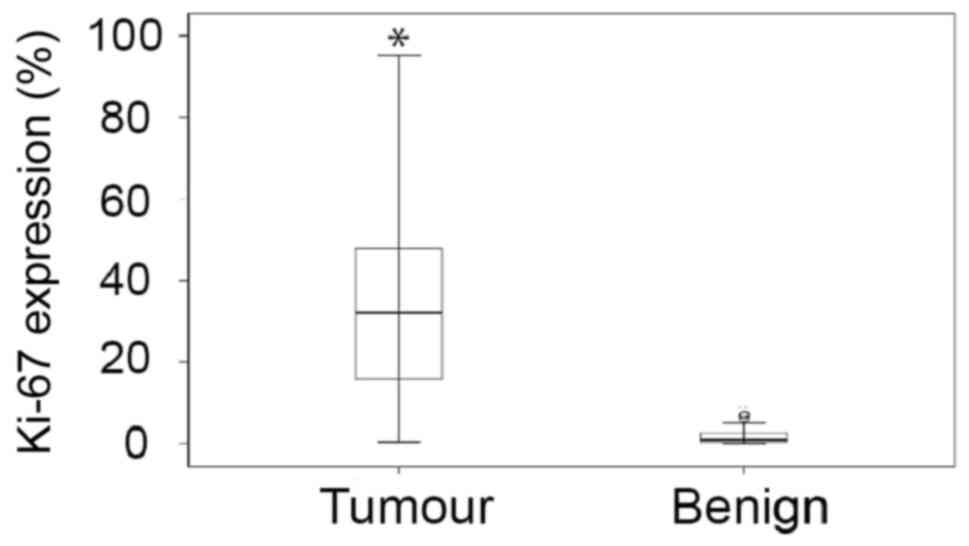
Ki67-positive cells, a well-established marker of proliferation
(21), was over 20-fold higher in
malignant tissue compared to the benign (Fig. 2). Ki67 expression in tumour tissue
correlated with a shorter time to relapse (P<0.001).
PGC1α expression in EC
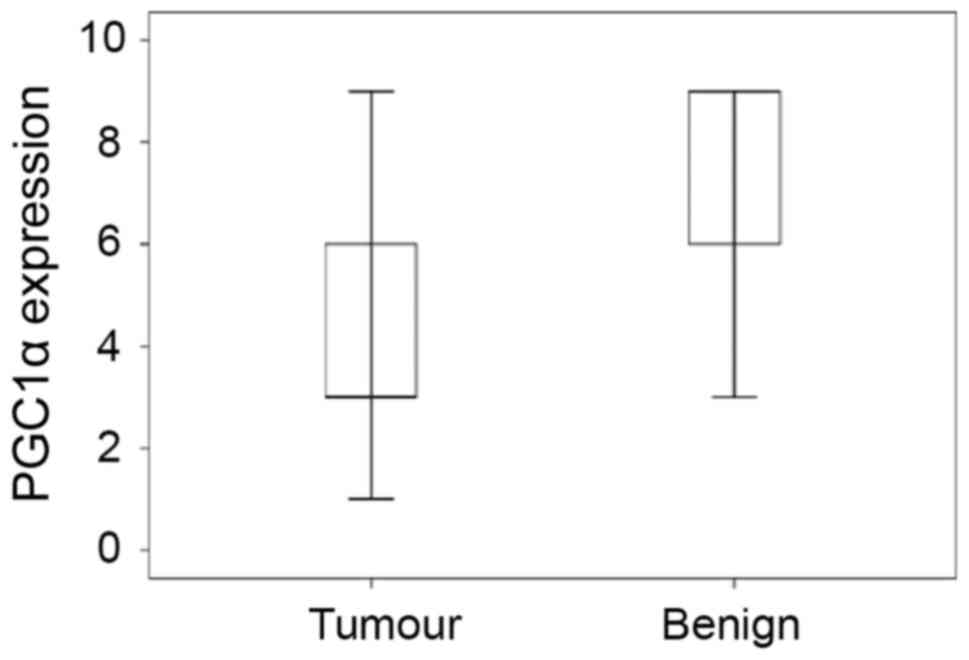
PGC1α expression was found to be significantly
decreased in tumour tissue (P=9.2E-19) compared to paired benign
tissue (Fig. 3). We also examined
one of its downstream effectors, the mitochondrial transcription
factor TFAM, and noted a weak, positive correlation between TFAM
and PGC1α expression within the malignant tissue, which was
statistically significant (rs=0.378, P=0.016; 2-tailed
sig.) (Fig. S1). There was also a
positive correlation with VDAC expression (rs=0.310;
P<0.0001) (Fig. S2), and a weak
one with p53 (rs=0.2, P=0.016; data not shown).
No association between tumour characteristics
(invasivity, stage and grade) and PGC1α expression was found
(Table II). There was no
significant difference in expression between tumours larger or
smaller than 30 mm (P=0.09; Table
II). Nor was there any significance with a cutoff of 40 mm
(P=0.192). There was no significant difference in expression
between type I and II tumours (P=0.113; Mann-Whitney U test).
Neither was there any association between expression and relapse or
mortality (P=0.345 and 0.758, respectively; Log-rank test).
However, we did observe a tendency towards shorter time to death
with lower PGC1α expression in grade 1 FIGO patients. Although
interesting, this finding was not significant according to ANOVA,
probably due to the low number of observations in the groups.
 | Table IIAssociations between tumour
characteristics and PGC1α expression in tumour tissue. |
Table II
Associations between tumour
characteristics and PGC1α expression in tumour tissue.
| Characteristic | Number | PGC1α score, median
(IQR 25, 75) | P-value |
|---|
| Invasion | | | |
|
0 | 9 | 4.00 (3.00,
6.00) | 0.294 |
|
1 | 73 | 4.00 (3.00,
6.00) | |
|
2-3 | 65 | 3.00 (3.00,
6.00) | |
| Stage | | | |
|
1 | 103 | 3.00 (3.00,
6.00) | 0.773 |
|
2 | 25 | 6.00 (3.00,
6.00) | |
|
3-4 | 20 | 6.00 (3.00,
6.00) | |
| Grade | | | |
|
1 | 39 | 3.00 (3.00,
6.00) | 0.903 |
|
2 | 60 | 3.50 (3.00,
6.00) | |
|
3 | 49 | 6.00 (3.00,
6.00) | |
| Tumour
sizea, mm | | | |
|
≤30 | 47 | 3.50 (3.00,
6.00) | 0.090 |
|
>30 | 43 | 3.00 (3.00,
6.00) | |
| Histology | | | |
|
Type I | 125 | 3.00 (3.00,
6.00) | 0.113 |
|
Type II | 22 | 6.00 (3.00,
6.00) | |
VDAC expression in EC
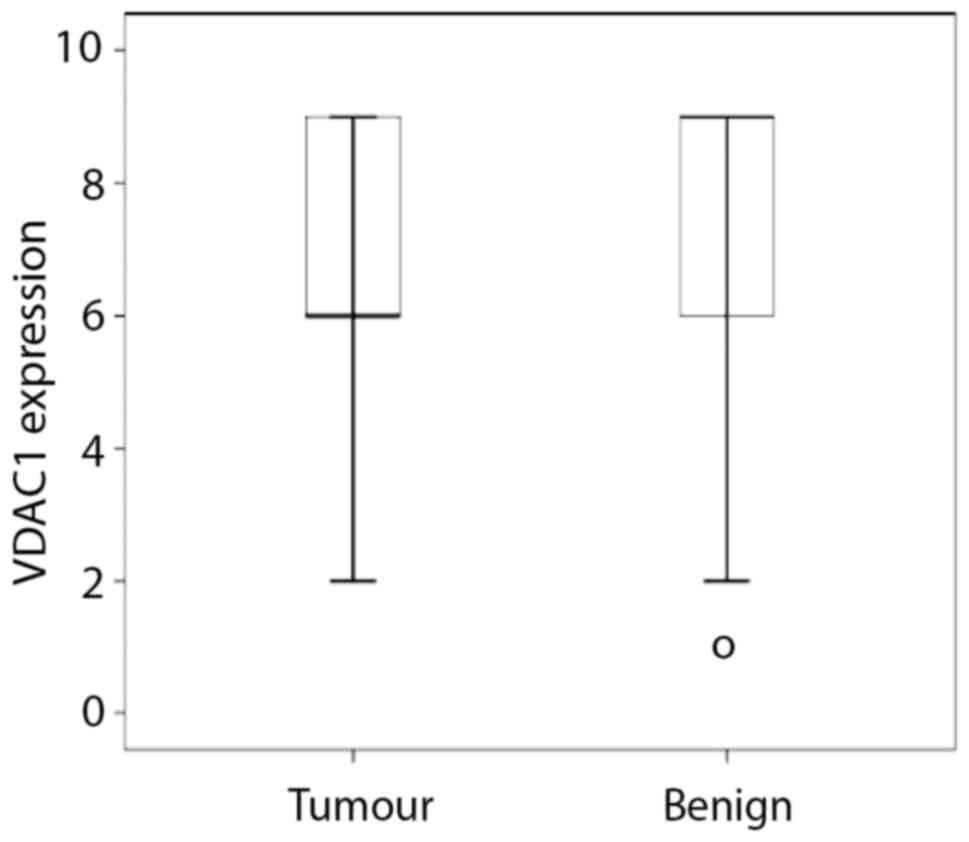
Expression of VDAC was significantly lower in tumour
tissue compared to benign tissue (Fig.
4). Although there was no correlation between VDAC1 and
mortality (data not shown), there was a weak correlation between
intermediate VDAC1 expression and shorter time to relapse
(chi2=6.81; P=0.03; Log-rank test) (Fig. S3). However, it was non-significant
after adjustment for age (data not shown).
Discussion
Tumour pathogenesis and progression go hand in hand
with major metabolic alterations, notably altered mitochondrial
function(s) (22). The
transcriptional coactivator PGC1α is well-studied, particularly in
normal tissue, as a major regulator of mitochondrial biogenesis and
function, and is generally perceived to promote an oxidative
metabolism (13). Regarding its
role in cancer and as a prognostic marker, reports vary greatly, as
both high and low levels have been found to correlate with worse
outcome (17). Here, we studied the
expression of PGC1α in EC. The main findings were that its
expression was lower in cancer tissue than in benign tissue from
the same patient and that there was no correlation between the
expression of PGC1α and aggressive course of the disease. We also
examined the expression of VDAC located in the mitochondrial outer
membrane and which regulates mitochondrial import and export of
ions and metabolites. VDAC expression was lower in the tumour
tissue than in benign tissue. In agreement with the current
understanding of Ki67 as a cellular marker for proliferation, we
observed significantly higher Ki67 expression in tumour tissue than
in adjacent benign tissue, and an association with shorter time to
relapse. Others have shown that survival in EC patients is
independently influenced by Ki67 expression (23).
That the decreased expression of PGC1α in EC tissue
was similar in the two subtypes of EC was perhaps unexpected
considering that type I is estrogen sensitive and that a model for
how hyperestrogenism promotes EC progression involving PGC1α has
been proposed (24). Moreover, the
results contradict those of Ren et al (25) who reported increased PGC1α
expression in a small (n=15) cohort of EC type I tumours; however,
these were compared to benign tissue from healthy controls, and
data were on mRNA rather than the actual protein. In a larger study
comparing EC tissue to benign, Cormio et al (16) reported doubled levels of PGC1α and
of the mitochondrial transcription factor TFAM which is known to be
in part regulated by PGC1α (13).
An important difference between their study and the present one is
that they assessed protein expression levels by western blotting,
i.e., in heterogeneous extracts, whereas we evaluated it
specifically in cancer cells.
Similar to our results, others have reported
decreased expression of PGC1α in, for instance, colon (26), breast (27) and clear-cell ovarian cancer
(14). Furthermore, other studies
have associated a decrease in the expression of PGC1α with poor
prognosis in human breast cancer and hepatocellular carcinoma
(13). By contrast, we could not
find any overall correlation in EC between PGC1α expression and
prognosis. However, there was a tendency that among grade 1
patients, the lower expression could be associated with a shorter
time to death. This tendency, which needs to be confirmed, supports
the notion of a context-dependent protective function of PGC1α
(17). A recent example of the same
is the finding that decreased PGC1α expression may contribute to
tumour invasion and metastasis (28).
VDAC expression is often used as a marker of
mitochondrial content. In line with downregulation of PGC1α and a
putative downregulation of mitobiogenesis, VDAC expression was also
decreased in both type I and II EC tumour cells compared to benign
tissue. However, its expression has been reported to vary in cancer
cells (20,29). Likewise, we have noted VDAC
upregulation also in the absence of PGC1α expression in ovarian
clear cell carcinoma (14), a
subtype that is notoriously resistant to treatment. Altogether,
this supports the idea that VDAC is not necessarily a
‘housekeeping’ indicator of mitochondrial content, and we therefore
suggest that the roles and functions of VDAC in tumour cells depend
on cellular context.
Our data are based on a large and representative
group of patients. Importantly, each patient is her own control, as
we used paired tumour and benign tissue samples from the same
patients, and thus did not have to create a matching control group.
On the other hand, it is impossible to exclude the risk that the
healthy tissue adjacent to cancer tissue does not harbour some
pre-cancerous molecular changes.
Further investigation of the correlation between
lower PGC1α expression and shorter time-to-death, in particular in
the FIGO Grade 1 group, could be of clinical significance. If
expression of PGC1α at early cancer stages is correlated with a
higher risk of recurrence it might thus signal that these patients
be treated more aggressively than is generally the case today.
In summary, we have shown downregulation of PGC1α as
well as VDAC protein levels in EC of both types, indicating altered
mitochondrial functions in EC compared to benign tissue. The
results also indicate that PGC1α and VDAC levels are not of major
prognostic value in EC.
Supplementary Material
Correlation between PGC1α and TFAM
scores. y-axis, TFAM scores 3.9; x-axis, PGC1α scores 2-9. Numbers
next to each dot indicate the number of observations represented in
the dot. PGC1α, peroxisome proliferator-activated receptor γ
coactivator 1; TFAM, transcription factor A mitochondrial.
Correlation between PGC1α and VDAC
scores. y-axis, VDAC scores 2-9; x-axis, PGC1α scores 0-10. Numbers
next to each dot indicate the number of observations represented in
the dot. PGC1α, peroxisome proliferator-activated receptor γ
coactivator 1; VDAC1, voltage-dependent anion channel type 1.
Survival analysis for three levels of
VDAC expression in tumour tissue. Blue: Low expression;
immunohistochemistry score 3. Red: Intermediate expression;
immunohistochemistry score 6. Green: High expression;
immunohistochemistry score 9.
Acknowledgements
Ms. Inger Bodin (Department of Oncology-Pathology,
Karolinska Institute, Stockholm, Sweden) is gratefully acknowledged
for immunohistochemistry expertise.
Funding
The present study was supported by grants from the
Swedish Cancer Society (grant no. 140373) and Stockholm Regional
Council (grant no. SLL-562083).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
OCW and LL scored the stained slides, structured all
data and wrote manuscript drafts. IG performed statistical analyses
and contributed to the final manuscript. MM wrote the ethical
approval application and collected the tissue samples and clinical
data. MG organized lab work and immunohistochemistry and
contributed to the overall design. MS conceived the study and
finalized the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Regional Ethics
Committee of Stockholm, Sweden, and this includes approval of the
patient consent process. Both oral and written consent was received
in conjunction with consultation with a clinician. Registration
number 2010/1536-31/2.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arora V and Quinn MA: Endometrial cancer.
Best Pract Res Clin Obstet Gynaecol. 26:311–324. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harris HR and Terry KL: Polycystic ovary
syndrome and risk of endometrial, ovarian, and breast cancer: A
systematic review. Fertil Res Pract. 2(14)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raglan O, Kalliala I, Markozannes G,
Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E,
Martin-Hirsch P, Tsilidis KK and Kyrgiou M: Risk factors for
endometrial cancer: An umbrella review of the literature. Int J
Cancer. 145:1719–1730. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C: ESMO Guidelines Working Group.
Endometrial cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 (Suppl
6):vi35–vi39. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yeramian A, Moreno-Bueno G, Dolcet X,
Catasus L, Abal M, Colas E, Reventos J, Palacios J, Prat J and
Matias-Guiu X: Endometrial carcinoma: Molecular alterations
involved in tumor development and progression. Oncogene.
32:403–413. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Porporato PE, Payen VL, Baselet B and
Sonveaux P: Metabolic changes associated with tumor metastasis,
part 2: Mitochondria, lipid and amino acid metabolism. Cell Mol
Life Sci. 73:1349–1363. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
DeBerardinis RJ and Chandel NS:
Fundamentals of cancer metabolism. Sci Adv.
2(e1600200)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hjerpe E, Egyhazi Brage S, Carlson J,
Frostvik Stolt M, Schedvins K, Johansson H, Shoshan M and
Avall-Lundqvist E: Metabolic markers GAPDH, PKM2, ATP5B and
BEC-index in advanced serous ovarian cancer. BMC Clin Pathol.
13(30)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guaragnella N, Giannattasio S and Moro L:
Mitochondrial dysfunction in cancer chemoresistance. Biochem
Pharmacol. 92:62–72. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guerra F, Arbini AA and Moro L:
Mitochondria and cancer chemoresistance. Biochim Biophys Acta
Bioenerg. 1858:686–699. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Porporato PE, Payen VL, Perez-Escuredo J,
De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T,
Bouzin C, et al: A mitochondrial switch promotes tumor metastasis.
Cell Rep. 8:754–766. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Villena JA: New insights into PGC-1
coactivators: Redefining their role in the regulation of
mitochondrial function and beyond. FEBS J. 282:647–672.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gabrielson M, Bjorklund M, Carlson J and
Shoshan M: Expression of mitochondrial regulators PGC1α and TFAM as
putative markers of subtype and chemoresistance in epithelial
ovarian carcinoma. PLoS One. 9(e107109)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guerra F, Kurelac I, Cormio A, Zuntini R,
Amato LB, Ceccarelli C, Santini D, Cormio G, Fracasso F, Selvaggi
L, et al: Placing mitochondrial DNA mutations within the
progression model of type I endometrial carcinoma. Hum Mol Genet.
20:2394–2405. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cormio A, Guerra F, Cormio G, Pesce V,
Fracasso F, Loizzi V, Cantatore P, Selvaggi L and Gadaleta MN: The
PGC-1alpha-dependent pathway of mitochondrial biogenesis is
upregulated in type I endometrial cancer. Biochem Biophys Res
Commun. 390:1182–1185. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mastropasqua F, Girolimetti G and Shoshan
M: PGC1α: Friend or Foe in. cancer? Genes (Basel).
9(48)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shoshan-Barmatz V and De S: Mitochondrial
VDAC, the Na+/Ca2+ Exchanger, and the
Ca2+ Uniporter in Ca2+ dynamics and
signaling. Adv Exp Med Biol. 981:323–347. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shoshan-Barmatz V and Mizrachi D: VDAC1:
From structure to cancer therapy. Front Oncol.
2(164)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shoshan-Barmatz V and Ben-Hail D: VDAC, a
multi-functional mitochondrial protein as a pharmacological target.
Mitochondrion. 12:24–34. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Teicher BA, Linehan WM and Helman LJ:
Targeting cancer metabolism. Clin Cancer Res. 18:5537–5545.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Salvesen HB, Iversen OE and Akslen LA:
Identification of high-risk patients by assessment of nuclear Ki-67
expression in a prospective study of endometrial carcinomas. Clin
Cancer Res. 4:2779–2785. 1998.PubMed/NCBI
|
|
24
|
Cormio A, Cormio G, Musicco C, Sardanelli
AM, Gasparre G and Gadaleta MN: Mitochondrial changes in
endometrial carcinoma: Possible role in tumor diagnosis and
prognosis (review). Oncol Rep. 33:1011–1018. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ren Z, Yang H, Wang C and Ma X: The
effects of PGC-1α on the proliferation and energy metabolism of
malignant endometrial cancer cells. Onco Targets Ther. 8:769–774.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Feilchenfeldt J, Brundler MA, Soravia C,
Totsch M and Meier CA: Peroxisome proliferator-activated receptors
(PPARs) and associated transcription factors in colon cancer:
Reduced expression of PPARgamma-coactivator 1 (PGC-1). Cancer Lett.
203:25–33. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Watkins G, Douglas-Jones A, Mansel RE and
Jiang WG: The localisation and reduction of nuclear staining of
PPARgamma and PGC-1 in human breast cancer. Oncol Rep. 12:483–488.
2004.PubMed/NCBI
|
|
28
|
Luo C, Lim JH, Lee Y, Granter SR, Thomas
A, Vazquez F, Widlund HR and Puigserver P: A PGC1α-mediated
transcriptional axis suppresses melanoma metastasis. Nature.
537:422–426. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shoshan-Barmatz V, Ben-Hail D, Admoni L,
Krelin Y and Tripathi SS: The mitochondrial voltage-dependent anion
channel 1 in tumor cells. Biochim Biophys Acta. 1848:2547–2575.
2015.PubMed/NCBI View Article : Google Scholar
|