Introduction
Most metastatic spinal cord tumors are epidural
metastases caused by the direct invasion of vertebral metastatic
tumors. Intramedullary spinal cord metastasis (ISCM) of malignant
tumors is rare, accounting for 0.9-2.1% of all cases of spinal cord
metastasis. Malignant melanoma accounts for approximately 9% of all
cases of ISCM (1). A literature
review was performed to include studies published in English on
this topic, including our two cases. To the best of our knowledge,
27 cases of ISCMs associated with malignant melanoma have been
reported; 12 cases were examined and 15 cases had no description of
contrast MRI and were excepted. The rim sign was recognized in
33.3% (4/12) of these cases. However, none of the five cases of
ISCMs of malignant melanoma identified in this series showed a rim
sign. This study revealed that the rim sign is observed in ISCMs of
malignant melanoma and in those of other cancers. We believe that
the rim sign in an MRI is a clue for the diagnosis of ISCM of
malignant melanoma.
Case report
Case 1
A 35-year-old woman with no significant medical
history presented with malignant melanoma over the left clavicle.
The tumor was 1.3 mm thick and had ulceration. Therefore, she was
treated by resection of the primary lesion and axillary dissection.
Metastases were detected in two of the 18 lymph nodes.
Four years later, she developed metastasis in the
right lung, subcutaneous metastasis in the scalp, and multiple
metastases in the bone. The tumors were positive for v-Raf murine
sarcoma viral oncogene homolog B1 (BRAF) V600E mutation determined
via a biopsy of metastasis in the scalp. Oral dabrafenib plus
trametinib was initiated. Thereafter, all metastases reduced and
her status was maintained. However, 5 years and 1 month after
surgery, she developed multiple brain metastases and pleural
dissemination and was treated with nivolumab and whole-brain
irradiation. Bone metastases also progressed; therefore, the
sternum and lumbar spine 5 were also irradiated. Four days after
radiation therapy, she developed severe back pain. In addition, she
experienced sensory disturbances caudal to the costal arch. She had
no sensory disturbances or movement disorders in the upper limbs
but had severe movement disorders in both lower limbs.
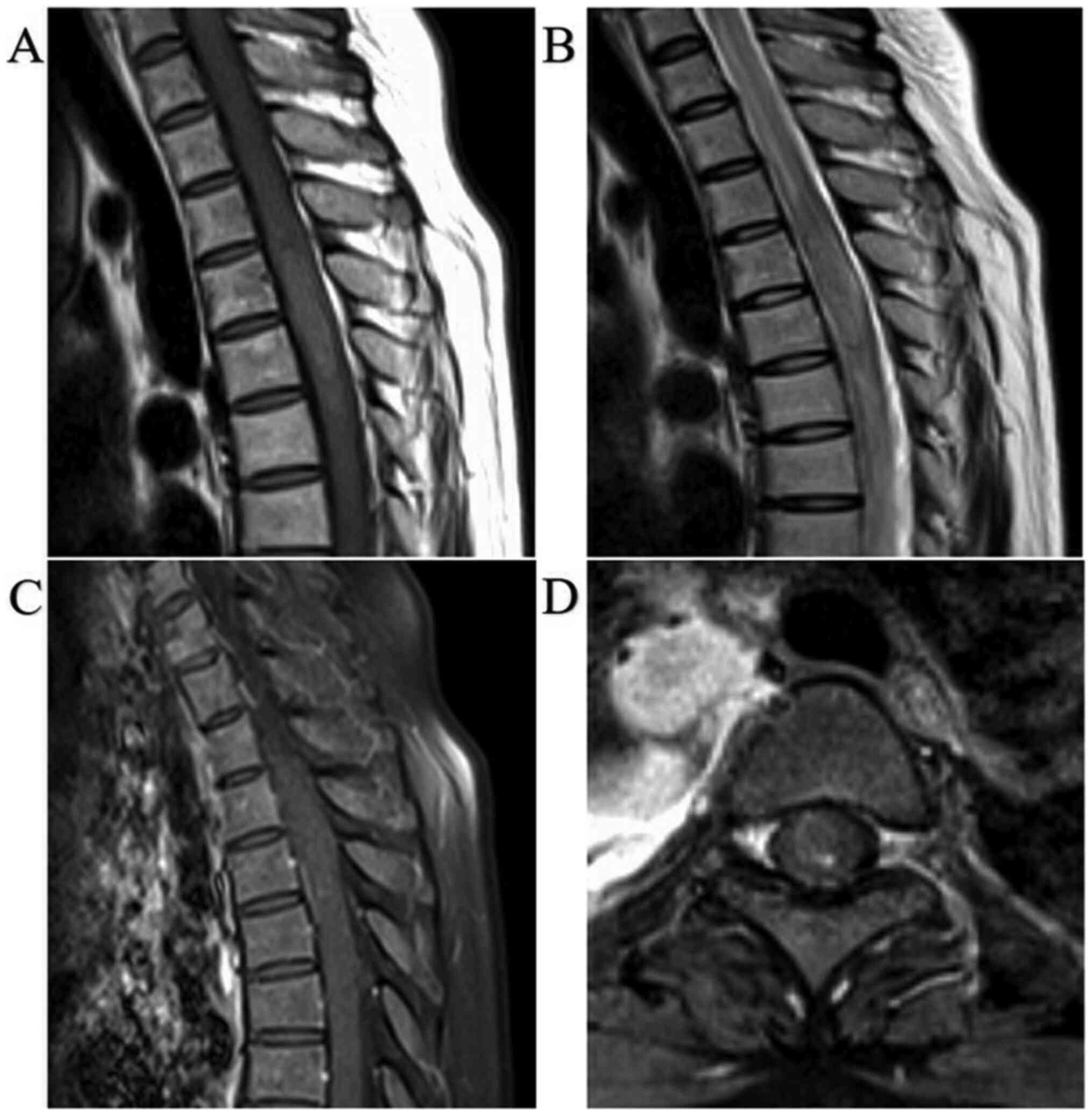
Contrast-enhanced magnetic resonance imaging (MRI)
revealed a low-density mass with gadolinium-based contrast media at
the peripheral ridge of T4-T6 (Fig.
1). Hence, an emergency evacuation was performed on the same
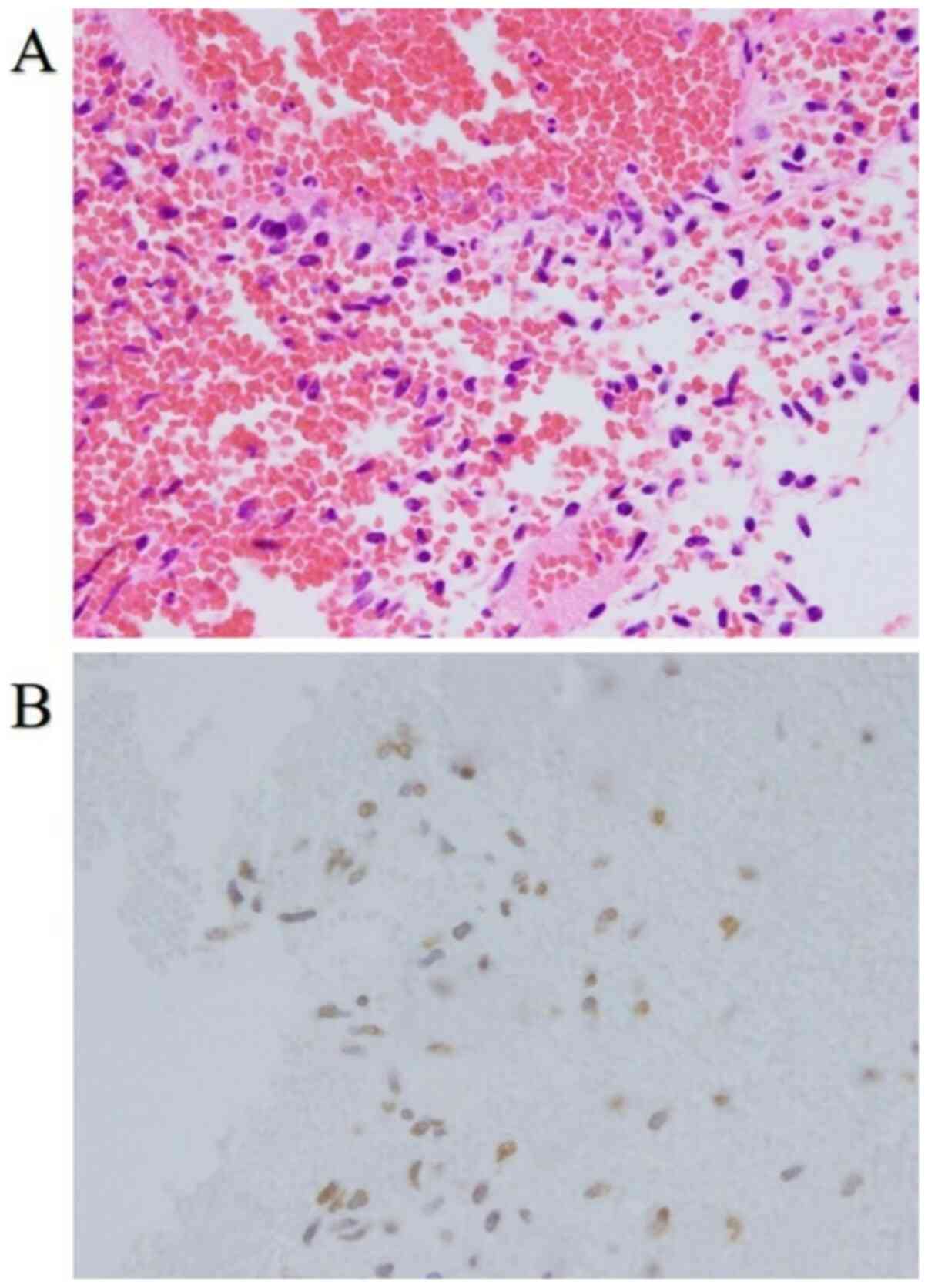
day. Only a hematoma was intraoperatively visualized (Fig. 2A). However, although melanoma cells
were not visualized, histopathological examination revealed that
they were present, as evidenced by SOX-10, S-100, and BRAF-V600E
positive staining and the metastasis in the scalp (Fig. 2B). The patient was diagnosed with
micro-metastases of malignant melanoma.
The day after surgery, a marked improvement in back
pain was reported; however, movement disorders in both her lower
limbs persisted. Fourteen days after surgery, computed tomography
(CT) revealed exacerbation of pleural dissemination. Thereafter,
oral dabrafenib (300 mg per day) plus oral trametinib (2 mg per
day) was resumed; it was highly effective in treating pleural
dissemination. She developed fever; therefore, oral dabrafenib plus
trametinib was continued subsequently. Because the antitumor
effects of the treatment were apparent, cycles of treatment
withdrawal and resumption were intermittently repeated. Six months
after surgery, pleural dissemination increased in size. Therefore,
nivolumab plus ipilimumab combination therapy was initiated.
However, she died soon after.
Case 2
A 48-year-old man with a local recurrence of
malignant melanoma of the left conjunctiva without BRAF mutation
was treated by wide excision and reconstruction by a free flap.
Because the stumps were partially positive, proton therapy was
performed. Two months after the proton therapy, CT revealed right
adrenal and retroperitoneal metastases. Therefore, nivolumab plus
ipilimumab combination therapy was initiated. However, after 1
month, cervical spine and right upper humerus metastases were
detected; thus, radiation therapy was performed. The dose was 20 Gy
at 5 Gy/fraction. Eight months after the combination therapy, he
experienced numbness and weakness in the right upper and lower
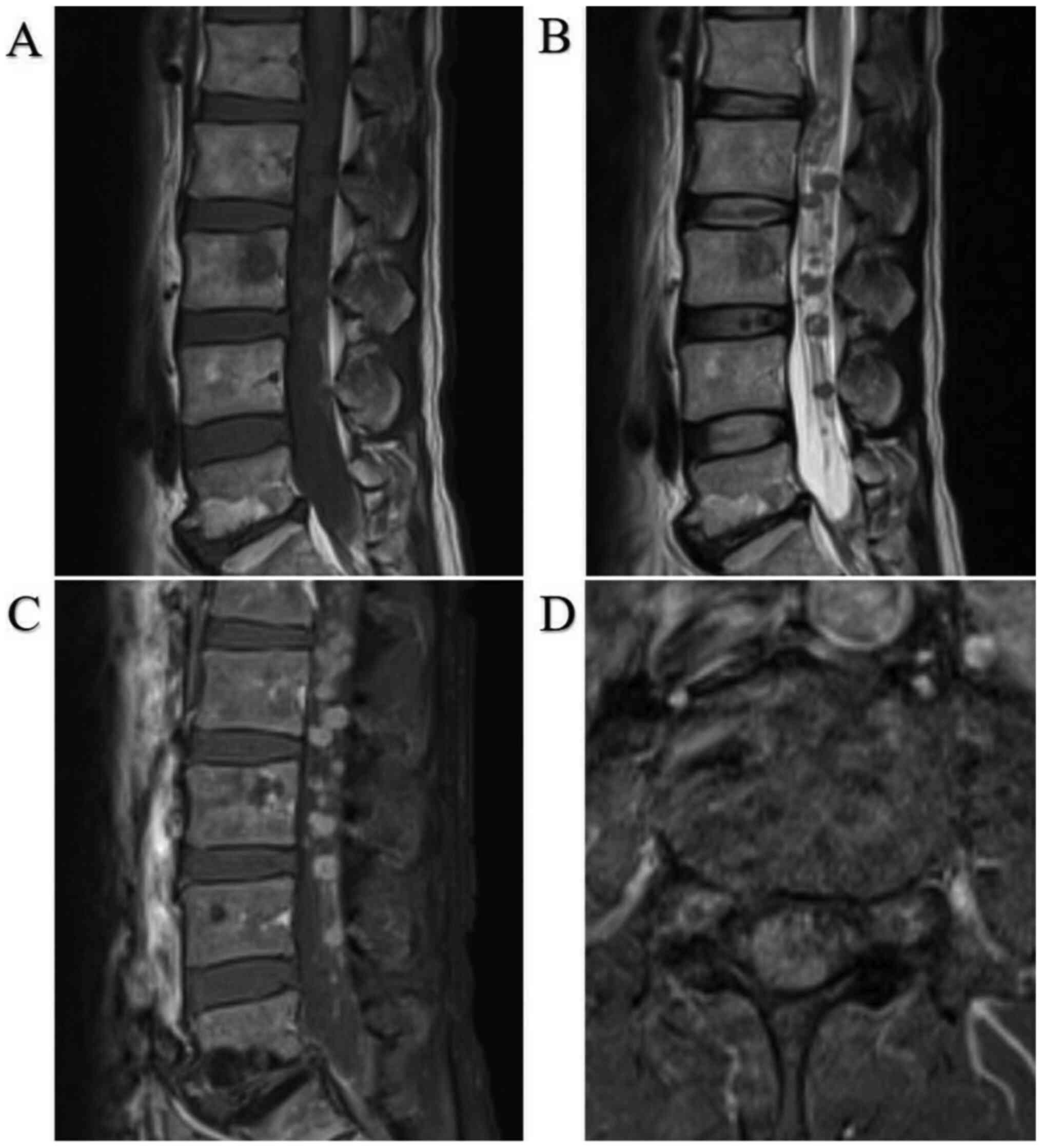
limbs. Contrast-enhanced MRI revealed multiple masses with a
contrast effect at the peripheral ridge of C2, Th1, and most of the
lumbar spine (Fig. 3).
Discussion
Palliative radiation or conservative treatment is
often selected for ISCM because the disease is associated with a
poor prognosis. The main purpose of palliative radiation therapy is
to reduce the pain; therefore, radical cure cannot be achieved with
only radiation therapy.
Patients expected to respond well to therapeutic
intervention are considered candidates for surgical treatment,
which can improve the overall survival and neurological functions
(2). A few patients with ISCMs of
malignant melanoma undergo surgical resection because melanoma
tends to be highly resistant to radiation (3). Therefore, the dose of radiation for
melanoma is often 4 Gy or more/fraction. In case 1, surgical
resection considerably reduced the patient's pain.
Considering the MRI findings, two unique
characteristics of ISCMs, rim and flame signs, have been reported
(4). Rim signs indicate a more
intense thin rim with peripheral enhancement than other tumor areas
and flame signs indicate flame-shaped enhancements at the edge of
the lesion (above or below). These findings have been reported to
be highly specific for ISCMs.
For all ISCMs, the rim sign was detected in 47%
cases and the flame sign in 40% cases. Both signs were found in 27%
cases and neither sign in 40% cases. Either sign was recognized in
60% cases. Melanoma was detected in five cases in this series but
the rim sign was not detected in any (0/5), whereas neither sign
was observed in 60% cases (3/5) (4). The authors of the respective studies
did not mention these results because of the small number of cases
of malignant melanoma.
According to a previous literature review, MRI could
not reveal a specific pattern within the tumor owing to a mixture
of melanin, intra-tumoral hemorrhage, and fat deposition (5). As a result, no characteristics have
been reported so far to aid the diagnosis of ISCM of malignant
melanoma.
To the best of our knowledge, 27 cases of ISCM of
malignant melanoma (including our two cases) have been reported. In
the literature, ISCM of malignant melanoma is described as a small
percentage of the ISCMs of all cancer types; thus, it is sometimes
not reported in detail.
We examined 12 cases, as the 15 other cases were
excepted because there was no description of contrast-enhanced MRI
findings (Table I) (5-7).
One patient underwent MRI without using a contrast agent, but the
characteristics of the lesion was described nonetheless (8).
 | Table IContrast-enhanced MRI findings of
intramedullary spinal cord metastasis of malignant melanoma
reported previously. |
Table I
Contrast-enhanced MRI findings of
intramedullary spinal cord metastasis of malignant melanoma
reported previously.
| First author,
year | Age, years/sex | Primary tumor | Metastatic location
of spinal cord | Brain metastases | Rim sign | Flame sign | Both signs | Either sign | Neither signs | (Refs.) |
|---|
| Connolly et
al, 1996 | 39/F | Left forehead | T8-T9 | - | - | - | - | - | + | (1) |
| Rykken et al,
2013 | NR | NR | NR | NR | - | + | - | + | - | (4) |
| | NR | NR | NR | NR | + | + | + | - | - | |
| | NR | NR | NR | NR | - | - | - | - | + | |
| | NR | NR | NR | NR | - | - | - | - | + | |
| | NR | NR | NR | NR | - | - | - | - | + | |
| Sun et al,
2013 | 67/F | Vulvar | L3-L4 | - | + | - | - | + | - | (5) |
| O'Reilly et
al, 2017 | 30s/M | NR | Multiple (T5/6, T9
and T12–L3, intramedullary and intradural extramedullary) | + | - | - | - | - | + | (6) |
| Śniegocki et
al, 2018 | 49/F | Left forearm | T11 | + | - | - | - | - | + | (3) |
| Ruschel et al,
2018 | 36/M | Left upper limb | C6-C7 | + | - | - | - | - | + | (7) |
| Present study,
2020 | 35/F | Left clavicle | T4-T6 | + | + | - | - | + | - | - |
| Present study,
2020 | 48/M | Left conjunctiva | Multiple (C2, T1,
lumber level broadly) | + | + | - | - | + | - | - |
Moreover, 83.3% (10/12) of these cases showed a
single mass by MRI and multiple masses in only two cases (including
our case 2). Another case was noted to have intramedullary and
intradural extramedullary lesions.
Besides these two cases, a case with multiple masses
discovered at autopsy has been reported (9). However, the lesions were not
identified by an MRI in this case. Therefore, our case 2 is the
first case of ISCM of malignant melanoma with multiple masses
localized within the intramedullary area that was detected by
MRI.
The rim sign was detected in 33.3% (4/12) and the
flame sign was detected in 16.6% patients (2/12). Both signs were
found in 8.3% (1/12), either sign in 33.3% (4/12), and neither in
58.3% of patients (7/12). Although the ISCM of malignant melanoma
was found in 33.3% patients, the rim sign frequency was slightly
lower than that observed in the ISCM of other cancers. Conversely,
the flame sign in ISCMs of malignant melanoma was less than that in
ISCMs of other cancers.
In the cases of ISCMs of malignant melanoma, the
incidence of brain metastasis was as high as 76.4% (13/17). The
response rate of intracranial metastases of BRAF V600
mutation-positive malignant melanoma to combination therapy with
dabrafenib plus trametinib was 58% and that of extracranial
metastases was 55% (10). The
response rate of intracranial lesions to the nivolumab plus
ipilimumab combination therapy for BRAF V600 mutation-negative
malignant melanoma was 57%. Conversely, the response rate of
intracranial metastases to immune-checkpoint inhibitor monotherapy
was almost 22% (11). Hence,
combination therapy was established to be significantly effective.
Our cases are unique as patients with ISCMs of malignant melanoma
are treated by immune-checkpoint inhibitors or molecule-targeted
agents.
In conclusion, the rim sign is detected in some
ISCMs of malignant melanoma and in ISCMs of other cancers. We
believe that the rim sign in MRI is a useful diagnostic clue of
ISCM of malignant melanoma. Although ISCM of malignant melanoma is
difficult to diagnose accurately and is associated with poor
prognosis owing to complications of brain metastases, the prognosis
of malignant melanoma has substantially improved because of
treatment advances. Therefore, more accurate diagnoses and the
development of therapeutic strategies will help improve patients'
quality of life in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Cancer Center Research and Development Fund (grant no.
2020-J-3).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM analyzed and interpreted the patient data and was
a major contributor in writing the manuscript. NY and KNam offered
valuable feedback regarding the study. KNak assisted in the early
stages of this work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
A waiver of informed consent requirement was
obtained from the National Cancer Center Hospital Institutional
Review Board.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Connolly JE Jr, Winfree CJ, McCormick PC,
Cruz M and Stein BM: Intramedullary spinal cord metastasis: Report
of three cases and review of the literature. Surg Neurol.
46:329–338. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Goyal A, Yolcu Y, Kerezoudis P, Alvi MA,
Krauss WE and Bydon M: Intramedullary spinal cord metastases: An
institutional review of survival and outcomes. J Neurooncol.
142:347–354. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Śniegocki M, Nowacka A, Smuczyński W and
Woźniak K: Intramedullary spinal cord metastasis from malignant
melanoma: A case report of a central nervous system secondary
lesion occurred 15 years after the primary skin lesion resection.
Postepy Dermatol Alergol. 35:325–326. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rykken JB, Diehn FE, Hunt CH, Eckel LJ,
Schwartz KM, Kaufmann TJ, Wald JT, Giannini C and Wood CP: Rim and
flame signs: Postgadolinium MRI findings specific for non-CNS
intramedullary spinal cord metastases. AJNR Am J Neuroradiol.
34:908–915. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sun L, Song Y and Gong Q: Easily
misdiagnosed delayed metastatic intraspinal extradural melanoma of
the lumbar spine: A case report and review of the literature. Oncol
Lett. 5:1799–1802. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
O'Reilly MK, Sugrue G, Byrne D and
MacMahon P: Combined intramedullary and intradural extramedullary
spinal metastases in malignant melanoma. BMJ Case Rep.
2017(bcr2017220031)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ruschel LG, Ramina R, da Silva EB Jr,
Cavalcanti MS and Duarte JFS: 5-Aminolevulinic acid
fluorescence-guided surgery for spinal cord melanoma metastasis: A
technical note. Acta Neurochir (Wien). 160:1905–1908.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Conill C, Sánchez M, Puig S, Planas I and
Castel T: Intramedullary spinal cord metastases of melanoma.
Melanoma Res. 14:431–433. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ishii T, Terao T, Komine K and Abe T:
Intramedullary spinal cord metastases of malignant melanoma: An
autopsy case report and review of the literature. Clin Neuropathol.
29:334–340. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Davies MA, Saiag P, Robert C, Grob JJ,
Flaherty KT, Arance A, Chiarion-Sileni V, Thomas L, Lesimple T,
Mortier L, et al: Dabrafenib plus trametinib in patients with
BRAFV600-mutant melanoma brain metastases (COMBI-MB): A
multi-cohort, open-label, phase 2 trial. Lancet Oncol. 18:863–873.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Konstantinou MP, Dutriaux C,
Gaudy-Marqueste C, Mortier L, Bedane C, Girard C, Thellier S,
Jouary T, Grob JJ, Richard MA, et al: Ipilimumab in melanoma
patients with brain metastasis: A retro-spective multicentre
evaluation of thirty-eight patients. Acta Derm Venereol. 94:45–49.
2014.PubMed/NCBI View Article : Google Scholar
|