Introduction
Germ cell tumor with somatic-type malignancy
(GCTSTM) is a very rare disease with an incidence of 2% among all
germ cell tumors (GCTs) in males. A primary GCTSTM is most likely
to occur in the mediastinum, accounting for 25-30% of cases. Almost
all patients with GCTSTM are male, with a highest incidence between
20 and 40 years of age. The prognosis of GCTSTM is extremely poor,
with a median survival of nine months (1). Studies have shown concomitant
malignancies with sarcomatous components, usually rhabdomyosarcoma,
followed by angiosarcoma, leiomyosarcoma, liposarcoma, and
undifferentiated sarcoma (2). Early
detection is difficult, and almost every GCTSTM diagnosis is based
on histopathology from the site of resected relapsed tumor after
some type of chemotherapy, such as treatment for lung metastasis
(3). Moreover, GCTSTM is mostly
resistant to standard cisplatin-based chemotherapy; consequently,
physicians are often cautious about choosing this treatment
method.
Herein we report a case of GCTSTM with a durable
response to Pazopanib, a multityrosine kinase inhibitor, which was
extremely effective after cisplatin-based chemotherapy for GCT.
Case report
A 21-year-old Asian man presenting with a right-side
chest pain visited our hospital. A contrast-enhanced computed
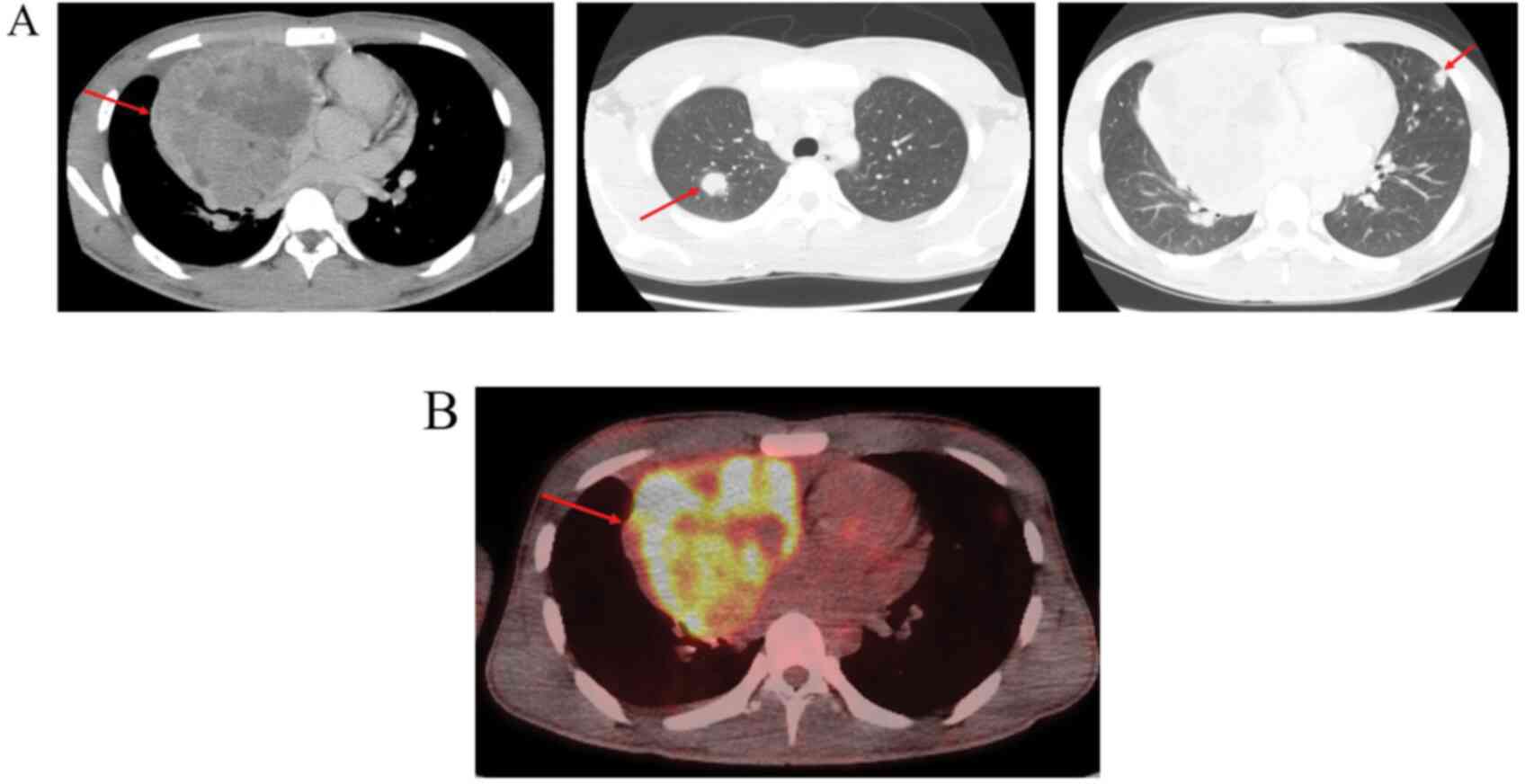
tomography (CT) showed a 9-cm tumor in the anterior mediastinum
with multiple lung metastases (Fig.
1A). Laboratory studies showed elevated serum α-fetoprotein at
395 ng/ml and human chorionic gonadotropin (HCG) at 1,720 mIU/ml.
The tumor showed high uptake on 18F-FDG positron emission
tomography; suspicious for GCT (Fig.
1B). No definite bone metastasis was detected on 99 m
Tc-methyldiphosphonate bone scintigraphy. Because CT-guided biopsy
of the anterior mediastinal tumor revealed nonseminomatous mixed
GCT, the patient was referred to the respiratory surgery
department. No abnormality was found on bilateral examination of
the testes. The patient began four cycles of chemotherapy with
bleomycin, etoposide, and cisplatin (BEP), based on the poor
prognosis according to the International Germ Cell Consensus
Classification. Due to a new lung metastatic lesion immediately
after BEP chemotherapy, vinblastine, ifosfamide, and cisplatin were
administered as second-line chemotherapy; however, the HCG tumor
marker was elevated. A third-line chemotherapy was initiated
consisting of two cycles of paclitaxel, ifosfamide, and cisplatin
(TIP). After a confirmation of normal HCG values, the patient
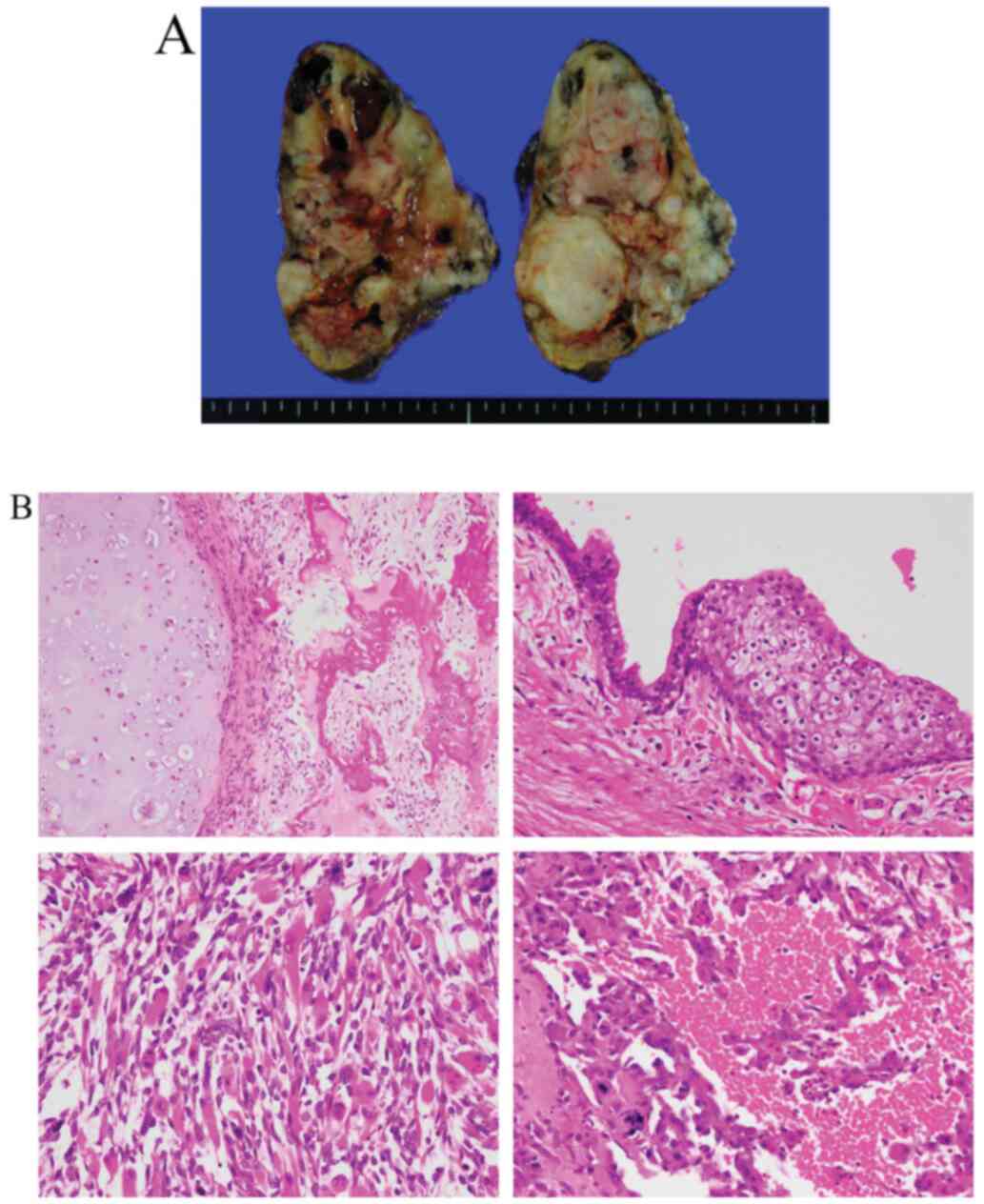
underwent a resection of the mediastinal tumor and a partial
lobectomy (Fig. 2A and B). Given that histopathological findings
showed viable cells, the patient also received one cycle of TIP as
adjuvant chemotherapy and was discharged with regular
follow-up.
A follow-up chest CT revealed progression of lung
metastases even though the tumor markers remained within normal
ranges. Consequently, the patient underwent lung diagnostic
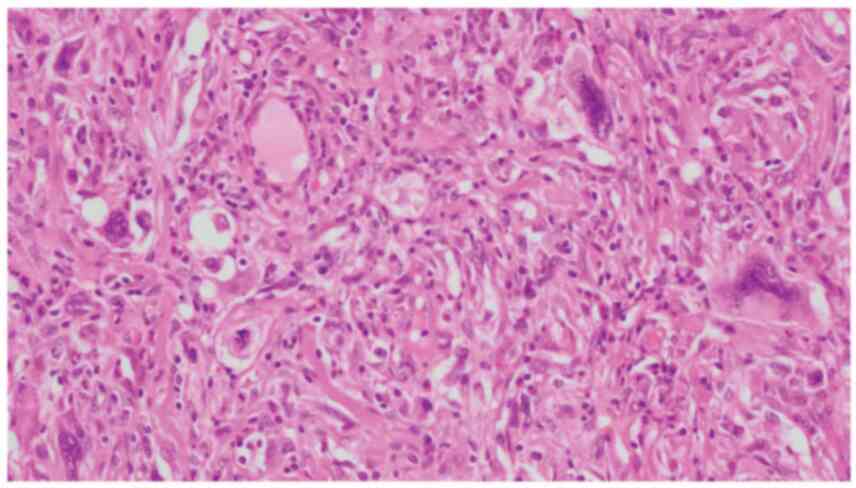
metastasectomy. Histopathologic findings were highly suspicious for
undifferentiated sarcomatous components and GCTSTM (Fig. 3). Immunohistochemical staining was
positive for S-100 (focal) and αSMA (focal) and negative for
AE1/AE3, CAM5.2, CD30, CD34, placental alkaline phosphatase, and
HCG. Multiple lung metastases appeared despite the persistence of
normalized HCG during the follow-up period. Therefore, the patient
was placed on Pazopanib (800 mg/day), a multityrosine kinase
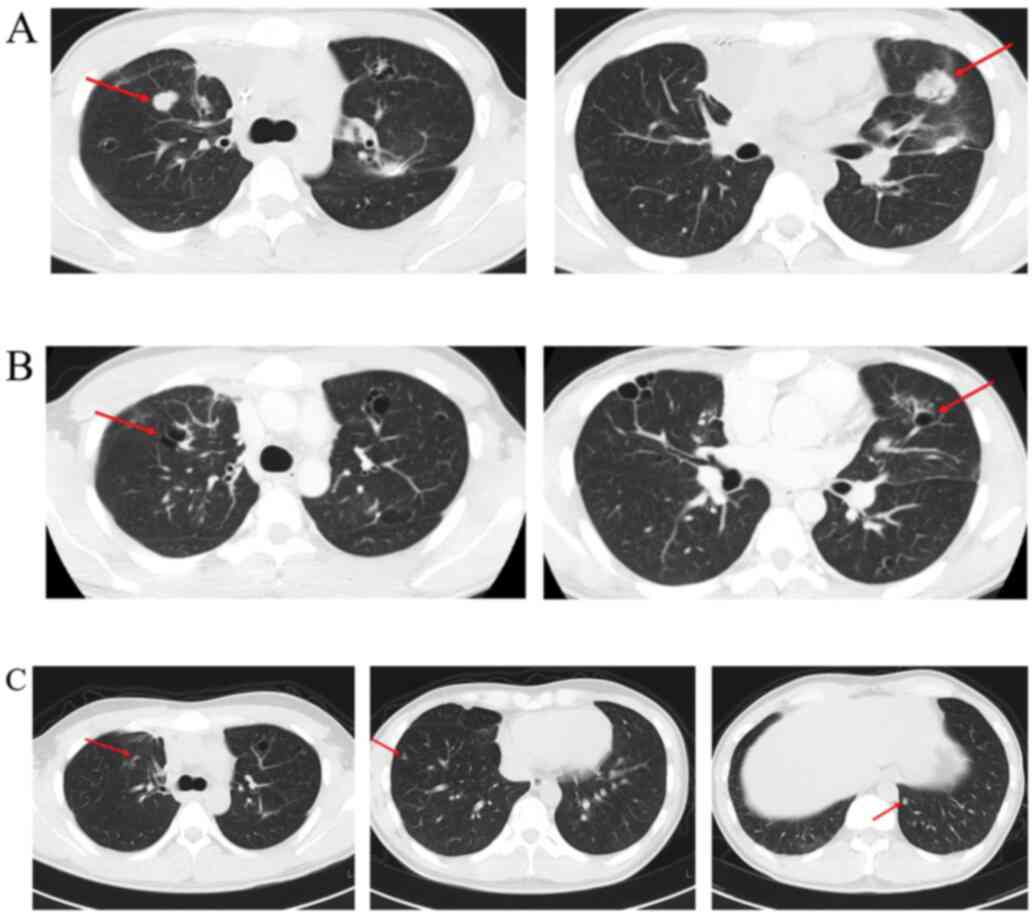
inhibitor. The patient was able to continue Pazopanib, showing a
dramatic therapeutic effect with no adverse events (Fig. 4A and B). The patient was maintained on Pazopanib
for two years; he discontinued this therapy owing to noncompliance
four years ago and has had stable disease since then (Fig. 4C).
Discussion
As highly treatable malignancies, treatment outcomes
are successful for GCTs, with complete resolution in more than 90%
of newly diagnosed patients, even though 70-80% of patients have
metastatic disease at first presentation (4). Conversely, mediastinal nonseminomatous
GCTs are well known for their poor prognoses (1,5).
Favorable prognostic factors for these GCTs include younger age
(5), localized disease at diagnosis
(6,7), the feasibility of complete resection
(8), absence of somatic-type
malignancy (3,6), and the response to standard
chemotherapeutic regimens (9).
Concerning prognostic factors, the patient, in this case, had
metastases at diagnosis. Moreover, complete resection was
unsuccessful because of lung metastases that occurred despite tumor
markers within the normal range. This case was increasingly
complicated because of histopathologic findings that were highly
suspicious for GCTSTM.
Pazopanib is a multityrosine kinase inhibitor of
vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2,
VEGFR-3, platelet-derived growth factor receptor (PDGFR)-α and -β,
fibroblast growth factor (FGF) receptor-1 and -3, and KIT, as well
as other emerging targets, including B-RAF proteins (10,11).
Preclinical models of pazopanib showed it inhibited ligand-induced
autophosphorylation of VEGFR-2, KIT and PDGFR-β receptors. This
drug equally impairs FGF and VEGF-mediated angiogenesis as well as
xenograft growth in multiple human cancers (12). Pazopanib is the first and only
tyrosine kinase inhibitor currently approved for treating multiple
histologic subtypes of soft tissue sarcoma (13). Plasma levels of VEGF and basic FGF
were elevated 10-13-fold in patients with sarcoma in comparison to
controls. Microarray gene expression data showed a markedly
elevated expression of matrix metalloproteinase-2 and PDGFR-α in
sarcoma tissue compared with non-malignant tissue (14). The mechanism of the therapeutic
effect of multityrosine kinase inhibitor for GCTSTM remains
unknown; however, Pazopanib may be effective for GCTSTM because 67%
of GCTSTM cases are rhabdomyosarcoma (2).
In the present case report, the histopathologic
findings of lung metastasis revealed undifferentiated sarcomatous
components, which complicated the diagnosis pathologically.
However, the patient's clinical course and responsiveness to
chemotherapy, along with the transition of tumor markers and
radiologic findings, supported a high degree of suspicion for a
diagnosis. Furthermore, although this patient had several poor
prognostic factors, Pazopanib achieved a durable response.
A single-arm, phase 2 clinical study that used
Pazopanib for 43 patients with refractory GCTs after ≥2
platinum-based regimens failed to achieve a favorable response.
After four weeks of treatment, 70.3% of the patients showed
decreased serum tumor markers, two (4.7%) had confirmed partial
remission, 19 (44.2%) had stable disease, and 16 (37.2%)
experienced disease progression. The three-month progression-free
survival was 12.8%, and the six-month overall survival was 42.7%.
Although Pazopanib therapy did not show increased progression-free
survival, there was confirmed early antitumor activity in
refractory GCTs. Moreover, it was possible to identify new missense
mutations in the tissue of three patients who responded well to the
therapy (15). These findings may
correlate with a durable response, as documented in the present
case.
In the present case, tumor markers did not invert
even after induction chemotherapy, and primary tumor resection was
performed after confirmation of marker inversion after salvage
chemotherapy. Eventually, the tumor contained a teratomatous
component and somatic mutations were suspected.
Donadio et al indicated that teratoma with
malignant transformation is typically found incidentally at the
time of surgery (4). Therefore, it
was possible to confirm that Pazopanib could be successful only
after primary excision following chemotherapy and metastatic
excision for lung metastasis.
In conclusion, this report documents a case of
metastatic GCTSTM for which treatment with Pazopanib was effective.
To the best knowledge of these authors, this case is the first in
which Pazopanib shows a durable response for GCTSTM. Further
investigation is warranted to clarify the molecular mechanisms of
GCTSTM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT, HY, and KI designed the study and drafted the
manuscript. MI, SK and KS made substantial contributions to the
study conception and design. YT also retrieved pathology images. HI
and AM contributed to the interpretation of histopathological
findings. YT, HY, TN, and YK reviewed the patient's history,
clinical and imaging data. AM supervised the entire project. YT and
HY critically revised the manuscript. All the authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the current case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC (eds): World Health Organization Classification of
Tumors, Pathology and Genetics of Tumors of the Lung, Pleura,
Thymus and Heart. 1st edition. IARC Press, Lyon, 2004.
|
|
2
|
Motzer RJ, Amsterdam A, Prieto V,
Sheinfeld J, Murty VV, Mazumdar M, Bosl GJ, Chaganti RS and Reuter
VE: Teratoma with malignant transformation: Diverse malignant
histologies arising in men with germ cell tumors. J Urol.
159:133–138. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Malagón HD, Valdez AM, Moran CA and Suster
S: Germ cell tumors with sarcomatous components: A
clinicopathologic and immunohistochemical study of 46 cases. Am J
Surg Pathol. 31:1356–1362. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Donadio AC, Motzer RJ, Bajorin DF, Kantoff
PW, Sheinfeld J, Houldsworth J, Chaganti RS and Bosl GJ:
Chemotherapy for teratoma with malignant transformation. J Clin
Oncol. 21:4285–4291. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marina N, London WB, Frazier AL, Lauer S,
Rescorla F, Cushing B, Malogolowkin MH, Castleberry RP, Womer RB
and Olson T: Prognostic factors in children with extragonadal
malignant germ cell tumors: A pediatric intergroup study. J Clin
Oncol. 24:2544–2548. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Michael H: Somatic neoplasms arising in
germ cell tumors. Pathol Case Rev. 10:181–185. 2005.
|
|
7
|
Mikuz G and Colecchia M: Teratoma with
somatic-type malignant components of the testis. A review and an
update. Virchows Arch. 461:27–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Arai K, Ohta S, Suzuki M and Suzuki H:
Primary immature mediastinal teratoma in adulthood. Eur J Surg
Oncol. 23:64–67. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kesler KA, Rieger KM, Ganjoo KN, Sharma M,
Fineberg NS, Einhorn LH and Brown JW: Primary mediastinal
nonseminomatous germ cell tumors: The influence of postchemotherapy
pathology on long-term survival after surgery. J Thorac Cardiovasc
Surg. 118:692–700. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
GlaxoSmithKline: Votrient Prescribing
Information [cited Jun 1, 2020]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022465lbl.pdf.
2009.
|
|
11
|
Gril B, Palmieri D, Qian Y, Anwar T, Ileva
L, Bernardo M, Choyke P, Liewehr DJ, Steinberg SM and Steeg PS: The
B-Raf status of tumor cells may be a significant determinant of
both antitumor and anti-angiogenic effects of pazopanib in
xenograft tumor models. PLoS One. 6(e25625)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kumar R, Knick VB, Rudolph SK, Johnson JH,
Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE,
Onori JA, et al: Pharmacokinetic-pharmacodynamic correlation from
mouse to human with pazopanib, a multikinase angiogenesis inhibitor
with potent antitumor and antiangiogenic activity. Mol Cancer Ther.
6:2012–2021. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yoon SS, Segal NH, Park PJ, Detwiller KY,
Fernando NT, Ryeom SW, Brennan MF and Singer S: Angiogenic profile
of soft tissue sarcomas based on analysis of circulating factors
and microarray gene expression. J Surg Res. 135:282–290.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Necchi A, Lo Vullo S, Giannatempo P, Raggi
D, Calareso G, Togliardi E, Crippa F, Pennati M, Zaffaroni N,
Perrone F, et al: Pazopanib in advanced germ cell tumors after
chemotherapy failure: Results of the open-label, single-arm, phase
2 Pazotest trial. Ann Oncol. 28:1346–1351. 2017.PubMed/NCBI View Article : Google Scholar
|