Introduction
Two platinum-based regimens are considered to be the
standard treatments for UC. One of these consists of a combination
of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC)
(1). The other consists of a
combination of gemcitabine and cisplatin (GC) (2). A phase III clinical trial of the
regimens demonstrated similar efficacy in terms of the overall
survival and response rates. However, GC had a better safety
profile. In addition, Robinson demonstrated a superior cost-utility
profile for GC (3). Based on these
data, GC is now widely used.
Our previous report demonstrated that outpatient GC
chemotherapy with short hydration was safe for patients with UC and
contributed to improving their quality of life (QOL) (4). The study examined different-day
administration of gemcitabine and cisplatin. Moreover, a same-day
GC regimen with short hydration for lung cancer (5) and bile duct cancer (6) demonstrated its potential to improve
patients' QOL by decreasing the required number of regular hospital
visits.
In the present study, the safety of the same-day GC
regimen with short hydration in patients with UC was assessed
prospectively with the aim of further improving patients' QOL.
Patients and methods
Study design and patients
The present, single-center, phase-1 study
(UMIN000031015) enrolled patients aged 20 years or older with
locally advanced or metastatic UC who were eligible for GC
chemotherapy based on their physician's judgement and had an
Eastern Cooperative Oncology Group performance status of 2 or less.
The patients were screened for adequate hematological and end-organ
function before enrollment. A historical control comprising 61
patients who previously received gemcitabine and cisplatin on
different days (the different-day regimen) with short hydration at
the Tokyo Metropolitan Tama Medical Center between 2010 and 2014
were also enrolled.
Safety evaluation was performed according to
protocol. The severity of adverse events was graded using the
National Cancer Institute Common Terminology Criteria for Adverse
Events (version 5.0) (7).
The present study was conducted in accordance with
the Good Clinical Practice guidelines and conforms to the
Declaration of Helsinki. All the patients provided written informed
consent. The protocol (28-83) was approved by the institutional
review board at Tokyo Metropolitan Tama Medical Center on January
10, 2017.
Treatment
Table I shows the
same-day GC chemotherapy regimen with short hydration. The patients
received gemcitabine 1,000 mg/m2 on days 1, 8, and 15
plus cisplatin 56-70 mg/m2 on day 1. The cisplatin
dosage was determined by the following criteria: Ccr>60 ml/min,
100% dose; 40<Ccr<60 ml/min, 80% dose. Cisplatin was
administered with adequate pre- and post-treatment hydration, and
the cycle was repeated every 28 days. As an anti-emetic,
dexamethasone 9.9 mg and palonosetron 0.75 mg were administered
intravenously, and aprepitant 125 mg was administered orally on day
2. Dexamethasone 8 mg and aprepitant 80 mg were administered orally
on days 3-6 and days 3-4, respectively. Supportive care, including
administration of analgesics, blood transfusions, and antibiotics,
was performed as required. Granulocyte colony stimulating factor
was not used routinely. Same-day GC chemotherapy was first
performed with short hydration at our inpatient center. The
patients chose outpatient or inpatient chemotherapy after the
second round of chemotherapy.
 | Table IRegimen. |
Table I
Regimen.
| Drugs | Fluids | Timing, min |
|---|
| Day 1 | | |
|
Gemcitabine | 100 ml 0.9% NaCl
solution | 30 |
|
Potassium
chloride (10 mEq) + Magnesium sulfate (8 mEq) | 500 ml 0.45% NaCl
solution | 60 |
| | 500 ml 0.45% NaCl
solution | 60 |
|
Dexamethasone
(9.9 mg) + Palonosetron (0.75 mg) | 50 ml 0.9% NaCl
solution | 30 |
| | 200 ml 20%
Mannitol | 30 |
|
Cisplatin | 300 ml 0.9% NaCl
solution | 60 |
|
Potassium
chloride 10 mEq | 500 ml 0.45% NaCl
solution | 60 |
| | 500 ml 0.45% NaCl
solution | 60 |
| Day 8, 15 | | |
|
Gemcitabine | 100 ml 0.9% NaCl
solution | 30 |
To monitor renal function, the serum creatinine (Cr)
level was measured, and the estimated glomerular filtration rate
(eGFR) was calculated on days 3, 8, 15, 22 and 29. Changes in the
serum Cr level of patients receiving short hydration were compared
with those of the consecutive hydration group using a linear mixed
model. Further, to evaluate long-term renal function, the serum Cr
level and eGFR before, and at one year after, chemotherapy in the
surviving patients were assessed [eGFR = 194 x (serum creatinine)
-1.094 x age -0.287 x (0.739 if female)].
Statistical analysis
Categorical variables were compared using unpaired
t-test, Pearson's chi-square test or Fisher's exact test. The
paired t-test was used to assess changes in renal function. All
statistical analyses were carried out with SPSS version 21 (IBM
Corp.) and P<0.05 was considered to indicate statistical
significance.
Results
Patients
Twenty patients received the same-day GC
chemotherapy regimen with short hydration between January 2017 and
March 2018 on an outpatient basis. Table II shows the patient
characteristics. The average age was 69.2 years (range, 53 to 77
years), and the sex distribution was 18 men and two women. Nine
patients underwent a nephrectomy, and two patients had a single
kidney due to hydronephrosis caused by a malignancy. The median
serum Cr value and eGFR value was 0.97 mg/dl (range 0.55-1.35) and
59.5 ml/min (range, 44-87), respectively. There were 11 and seven
patients with an eGFR value <60 ml/min and <50 ml/min,
respectively. The median follow-up was 18.2 months.
 | Table IIPatient characteristics. |
Table II
Patient characteristics.
| Characteristics | Same day | Different day | P-value |
|---|
| Age, years | | | |
|
Average | 69.2 years | 71.3 years | 0.72 |
|
Range | 53-77 years | 40-86 years | |
| Sex, n (%) | | 0.80 | |
|
Male | 18(90) | 56(92) | |
|
Female | 2(10) | 5(8) | |
| Eastern Cooperative
Oncology Group performance status, n (%) | | | 0.48 |
|
0 | 20 | 57 | |
|
1 | 0 | 3 | |
|
2 | 0 | 1 | |
|
3 | 0 | 0 | |
|
4 | 0 | 0 | |
| Disease, n (%) | | | 0.51 |
|
Bladder
cancer | 11(55) | 26(42) | |
|
Upper
urinary cancer | 9(45) | 33(54) | |
|
Others | 0 (0) | 2(4) | |
| Nephrectomy, n
(%) | | | 0.75 |
|
Yes | 9(45) | 25(41) | |
|
No | 11(55) | 36(59) | |
| Hydronephrosis, n
(%) | | | 0.20 |
|
Yes | 2(18) | 14(22) | |
|
No | 9(37) | 22(37) | |
| Serum Creatinine
(mg/dl) before chemotherapy, n (%) | | | 0.65 |
|
≤1.0 | 11(45) | 30(49) | |
|
>1.0 | 9(55) | 31(51) | |
| Estimated glomerular
filtration rate (ml/min) before chemotherapy, n (%) | | | 0.84 |
|
≥60 | 9(45) | 29(48) | |
|
<60 | 11(55) | 32(52) | |
Treatment
In total, 86 courses of the same-day GC chemotherapy
regimen with short hydration were performed on an outpatient basis.
The average number of chemotherapy courses was 4.3 (range, 2-10).
Of all the patients receiving short hydration, nine (45%) received
cisplatin 70 mg/m2 while 11 (55%) received 56
mg/m2 with short hydration at the outpatient
chemotherapy center.
Adverse effects
Three patients had grade 3 neutropenia, one patient
had grade 4 thrombocytopenia, and no patient had anemia (Table III). Only one patient presented
grade 1 nausea. None of the patients experienced dehydration. Two
patients had grade 1 elevation of liver enzymes (Table III). All produced more than 3,000
ml/day of urine during days 2-7. Three patients experienced Grade 1
acute kidney injury according to the National Cancer Institute
Common Terminology Criteria for Adverse Events (NCI CTCAE) version
5.0. In all the cases, renal toxicity was completely reversible
with oral hydration alone.
 | Table IIISafety summary. |
Table III
Safety summary.
| | Grade 1 to 2 | Grade 3 | Grade 4 | Total |
|---|
| CTCAE version
5.0 | No. | % | No. | % | No. | % | No. | % |
|---|
| Non-hematologic |
|
Fatigue | 1 | 5 | 0 | 0 | 0 | 0 | 1 | 5 |
|
Nausea | 1 | 5 | 0 | 0 | 0 | 0 | 1 | 5 |
| Hematologic |
|
Leukopenia | 6 | 30 | 3 | 15 | 0 | 0 | 9 | 45 |
|
Thrombocytopenia | 3 | 15 | 0 | 0 | 1 | 5 | 4 | 20 |
|
Neutropenia | 6 | 30 | 3 | 15 | 0 | 0 | 9 | 45 |
| Hepatic |
|
Increase in
alanine aminotransferase | 2 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Increase in
aspartate aminotransferase | 2 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
Renal function
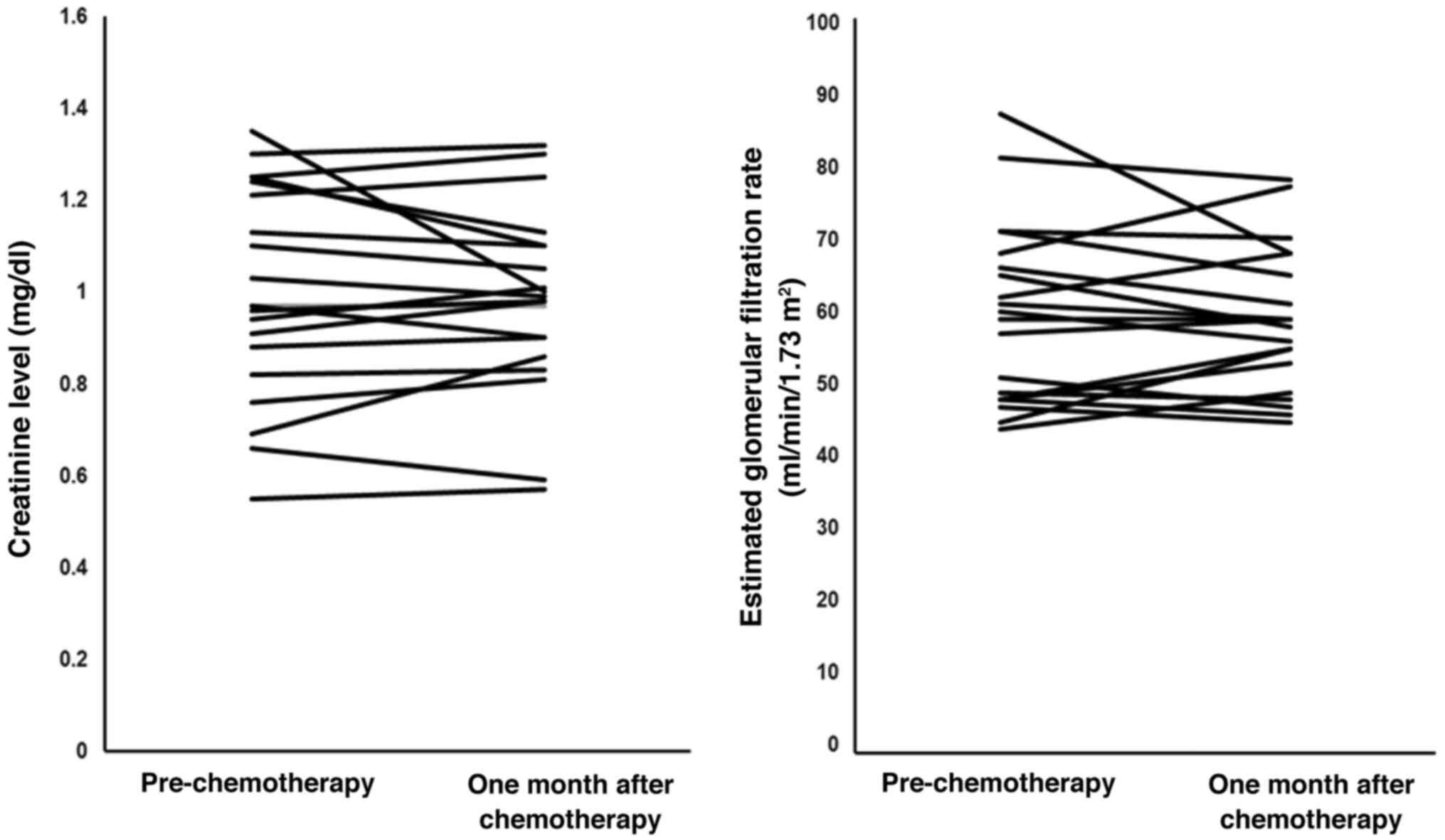
Fig. 1 shows the
changes in serum Cr level and eGFR in each patient receiving the
same-day GC chemotherapy regimen with short hydration. There was no
significant difference in the serum Cr level or eGFR between the
period prior to, and one month after, treatment (P=0.495 and 0.691,
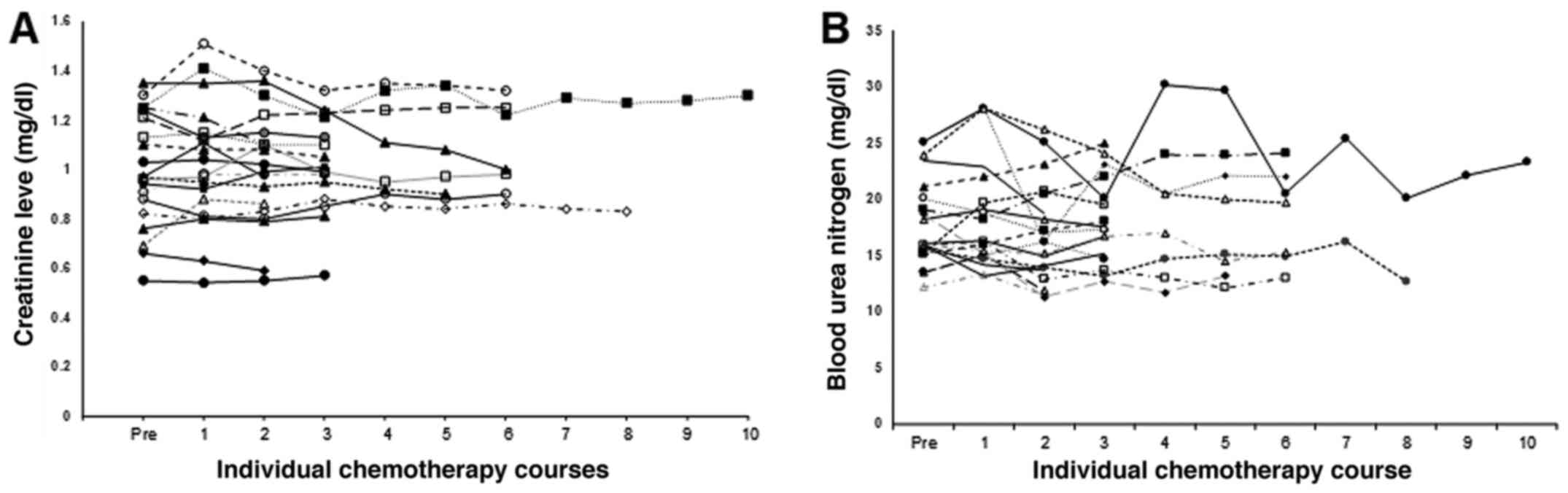
respectively). Fig. 2 shows the
changes in the serum Cr level in each patient receiving the GC same
day regimen with short hydration. The historical control comprised
patients who had received gemcitabine and cisplatin with short
hydration on different days. Table
II shows their characteristics. No significant difference was
found between the characteristics of the groups. Our previous study
demonstrated no change in the serum Cr level in the historical
control. The change in the serum Cr level in the same-day regimen
group, further examined using linear mixed model analysis,
demonstrated no significant change (P=0.513). Next, the serum Cr
level in each group around the time of the chemotherapy was
analyzed using linear mixed model analysis and revealed no
significant difference between the different-day and same-day
regimen groups (P=0.262).
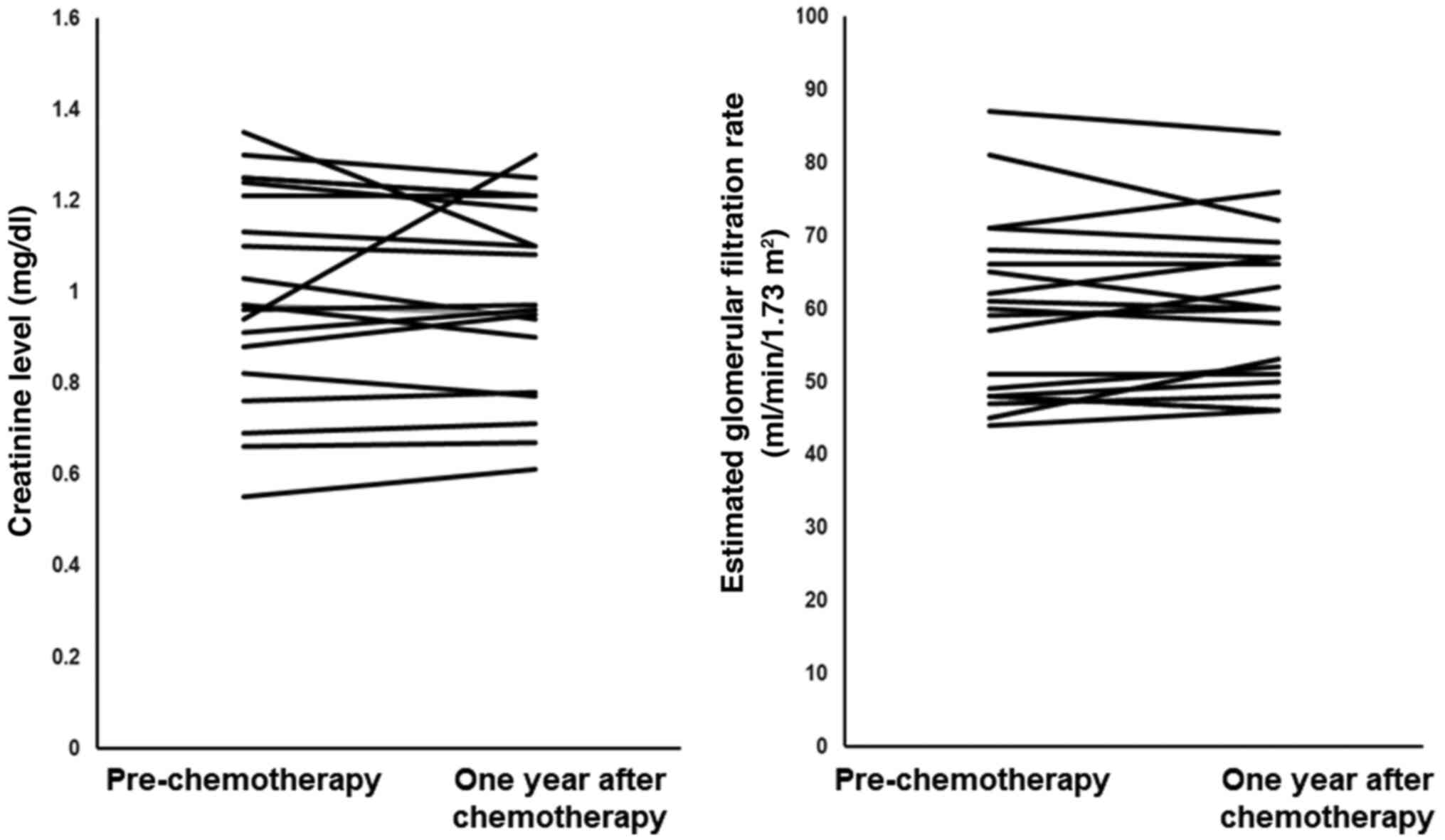
Next, to evaluate the long-term effect of short
hydration, 20 patients who were alive one year after the short
hydration chemotherapy (Fig. 3)
were assessed. Four patients had received adjuvant chemotherapy.
There was no significant difference in the serum Cr level or eGFR
between the period prior to, and one year after, the administration
of the same-day GC chemotherapy regimen with short hydration
(P=0.810 and 0.610, respectively).
Discussion
Our previous study demonstrated the safety of
outpatient GC chemotherapy with short hydration and its potential
to improve patients' quality of life (QOL) (4). To improve patients' QOL further, the
safety and effectiveness of same-day GC regimen with short
hydration in UC patients were assessed prospectively.
The same-day GC chemotherapy with short hydration is
the standard treatment for metastatic lung cancer (5) or bile duct cancer (6) while the different-day regimen is the
standard treatment for UC (2).
Patients with UC are usually older and have a solitary functional
kidney due to a nephrectomy or hydronephrosis (8,9). These
factors can sometimes induce severe adverse responses to treatment.
In the present cohort, four patients were older than 75 years and
11 patients had a solitary functional kidney. The serum Cr values
never worsened after the same-day GC chemotherapy with short
hydration. Patients with the different-day chemotherapy with short
hydration were enrolled as the historical control, but no
significant difference between the groups was found.
The frequency and the severity of adverse events
were similar between the groups. Chemotherapy-induced nausea and
vomiting, which can reduce oral intake and sometimes induce
dehydration, were observed in patients in both treatment groups. In
the current study, none of the patients with short hydration
experienced vomiting thanks to the use of anti-emetics (serotonin
and neurokinin 1 receptor antagonists) (10) although some patients experienced
mild nausea. Decrease in appetite was denied. There were also no
severe hematological toxicities in patients receiving the same-day
GC chemotherapy regimen with short hydration. Three patients had
grade 3 neutropenia, and one patient had grade 4 thrombopenia, but
none of the patients had febrile neutropenia or required a blood
transfusion.
Naiki et al (11) demonstrated the safety of the
same-day GC regimen with short hydration in patients with UC. The
present study differs from the latter study in two respects. First,
because the present study was based on actual clinical situations,
the regimen was administered on an outpatient basis. In our
previous study, we demonstrated that the different-day GC
chemotherapy with short hydration could be performed safely on an
outpatient basis and improve patients' QOL. The same-day GC regimen
with short hydration was able further to improve patients' QOL.
Second, the present study (70%) included more patients with
solitary kidney than a previous study (30%), enabling renal
function to be analyzed in greater detail. We used the linear mixed
model, which is superior to repeated measures ANOVA for analyzing
data collected multiple times. Our data showed that there was no
significant change in the slope of the incline for the time course
of the serum creatinine level. In addition, renal function was
analyzed one year after chemotherapy because of reports of late
nephrotoxicity induced by cisplatin.
In this study, QOL data were not collected because
the control data were unable to be established. However, the new
regimen described herein demonstrated the ability to decrease the
number of regular hospital visits and improve patients' QOL.
Further, the decrease in hospital visits may also help to reduce
medical expenses and conserve medical resources.
In the present study, six partial responses (38%),
three stable diseases (19%), and seven progressive diseases (43%)
according to the Response Evaluation Criteria in Solid Tumors
(RECIST), version 1.1. were observed, in line with the findings of
previous reports. However, the small sample size limited our
assessment of the effect of the same-day GC chemotherapy
regimen.
The present study demonstrated that the same-day GC
chemotherapy regimen with short hydration was safe for older
patients with urological cancer with compromised renal function as
well as for outpatients with UC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TA designed the present study and critically revised
the manuscript. IT, FN and SH performed data collection and
analyzed the data. TA wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The protocol (28-83) was approved by the
Institutional Review Board at Tokyo Metropolitan Tama Medical
Center. All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Ahmed T, Weiselberg LR, Geller N, Hollander PS, Herr HW, Sogani
PC, et al: Preliminary results of M-VAC (methotrexate, vinblastine,
doxorubicin and cisplatin) for transitional cell carcinoma of the
urothelium. J Urol. 133:403–407. 1985.PubMed/NCBI View Article : Google Scholar
|
|
2
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Robinson P, Maase H, Bhalla S, Kielhorn A,
Aristides M, Brown A and Tilden D: Cost-utility analysis of the GC
versus MVAC regimens for the treatment of locally advanced or
metastatic bladder cancer. Expert Rev Pharmacoecon Outcomes Res.
4:27–38. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Azuma T, Matayoshi Y, Sato Y, Sato Y,
Nagase Y and Oshi M: The safety and effect of chemotherapy with
short hydration for urothelial cancer on patients' quality of life.
Jpn J Clin Oncol. 46:958–963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tiseo M, Martelli O, Mancuso A, Sormani
MP, Bruzzi P, Di Salvia R, De Marinis F and Ardizzoni A: Short
hydration regimen and nephrotoxicity of intermediate to high-dose
cisplatin-based chemotherapy for outpatient treatment in lung
cancer and mesothelioma. Tumori. 93:138–144. 2007.PubMed/NCBI
|
|
6
|
Abdel-Rahman O, Elsayed Z and Elhalawani
H: Gemcitabine-based chemotherapy for advanced biliary tract
carcinomas. Cochrane Database Syst Rev. 4(CD011746)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
Accessed Sep 12, 2020.
|
|
8
|
Dash A, Galsky MD, Vickers AJ, Serio AM,
Koppie TM, Dalbagni G and Bochner BH: Impact of renal impairment on
eligibility for adjuvant cisplatin-based chemotherapy in patients
with urothelial carcinoma of the bladder. Cancer. 107:506–513.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kaag MG, O'Malley RL, O'Malley P, Godoy G,
Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G,
et al: Changes in renal function following nephroureterectomy may
affect the use of perioperative chemotherapy. Eur Urol. 58:581–587.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Basch E, Prestrud AA, Hesketh PJ, Kris MG,
Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM,
Freundlich B, et al: American Society of Clinical Oncology.
Antiemetics: American Society of Clinical Oncology clinical
practice guideline update. J Clin Oncol. 29:4189–4198.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Naiki T, Sugiyama Y, Tasaki Y, Iida K,
Etani T, Hamamoto S, Nagai T, Nozaki S, Ando R, Kawai N, et al:
Efficacy of a newly modified short hydration method for gemcitabine
and cisplatin combination chemotherapy in patients with urothelial
carcinoma. Oncology. 98:612–620. 2020.PubMed/NCBI View Article : Google Scholar
|