Introduction
Kaposi sarcoma (KS) is a rare angioproliferative
disorder associated with the human herpes virus-8 (HHV-8)
infection. The latter is a necessary but not sufficient condition
in the multifactorial pathogenesis of the disease. Four
epidemiological forms have been identified as follows: Classic,
endemic (in Africa), iatrogenic (in patients undergoing
immuno-suppressive therapies), and epidemic (in HIV-patients)
(1). KS frequently manifests itself
with skin lesions (macules, nodules, plaques) and can lead to the
development of visceral metastases. In the advanced stage of the
disease, KS is associated with a more unfavourable prognosis
(1,2). Moreover, KS can be indolent or may
have a rapidly progressive and life-threatening behaviour (3).
Standard treatment options for KS are limited.
Particularly, skin lesions can be treated with several local
therapies, such as surgery, radiotherapy, cryosurgery,
CO2-laser, or intra-lesional chemotherapy (3). Moreover, antiretroviral drugs are used
in the HIV-related form of KS, while metastatic KSs are generally
treated with chemotherapy, which is based on pegylated liposomal
doxorubicin and paclitaxel (3).
Electrochemotherapy (ECT) is an option used in the
treatment of KS skin lesions due to its high response rate in
neoplastic lesions of different histological types (4). ECT is the combination of
electroporation and systemic administration of chemotherapeutic
agents. According to the ESOPE guidelines (4), the only absolute contraindications to
ECT are pregnancy, lactation, and allergy or hypersensitivity to
the used drugs. Electroporation can produce transient pores in the
cell membrane allowing the entry of hydrophilic molecules into the
cytoplasm (5). The two drugs that
have been successfully tested for this purpose are bleomycin and
cisplatin. Their cytotoxicity has increased to 1,000 and 80 times
by electroporation, respectively (6). Furthermore, preclinical and in
vivo studies have shown a similar efficacy of ECT in neoplastic
lesions from different primary tumors (7). Moreover, if necessary, patients can
also undergo repeated ECT treatments, as long as the lifetime
bleomycin dose does not exceed 400,000 IU, without precluding any
subsequent local or systemic treatments.
ECT was initially introduced for the treatment of
primary and metastatic skin tumors (4). However, ECT has been suggested to be
particularly effective in treating KS skin lesions. In addition to
the chemosensitizing effect, electroporation produces a ‘vascular
lock’ in the treated volume that is potentially able to control an
angio-proliferative neoplasm, such as KS (8).
However, the existing evidence on the efficacy of
ECT in KS skin lesions is limited. In particular, randomized
studies and literature reviews are lacking. Therefore, in the
present study, a systematic review was performed to assess tumor
response and toxicity of ECT. Moreover, the aim of the present
study was to evaluate the long-term local control and the impact of
ECT on the quality of life (QoL).
Materials and methods
Research methodology
The protocol of the present systematic review was
submitted for registration in the PROSPERO international
prospective register of systematic reviews on October 2,
2019(9). The analysis was performed
based on the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines (10). The primary endpoint of the analysis
was the tumor response to treatment. The secondary endpoints were
the adverse events, local control, and impact on QoL.
Bibliographic search
The systematic analysis was carried out using the
PubMed, Scopus, and Cochrane databases from the earliest date to
October 18, 2019. The search strategies used in the different
libraries included were the following: PubMed[((electroporation) OR
electrochemotherapy) AND Kaposi sarcoma], Scopus[(TITLE-ABS-KEY
(electroporation) OR TITLE-ABS-KEY (electrochemotherapy) AND
TITLE-ABS-KEY (Kaposi AND sarcoma))], Cochrane [‘electroporation’
in Title Abstract Keyword OR ‘electrochemotherapy’ in Title
Abstract Keyword AND ‘Kaposi sarcoma’ in Title Abstract Keyword].
The reference lists of the selected papers were confirmed to
identify additional publications.
Inclusion criteria
The studies with available full text, published in
English, reporting on patients with KS skin lesions treated with
ECT were included in this systematic review. Both retrospective
studies and prospective trials were included. The studies that did
not report tumor response or toxicity or those that did not report
these outcomes separately from other primary tumors, the case
reports, the systematic or narrative reviews, the meta-analyses,
the letter-commentaries-editorials, the planning and imaging
studies, the surveys, the guideline-recommendations and/or those
that reported duplicate data were excluded. In case of inclusion of
the same patients in subsequent publications, the most complete or
recent article was selected.
Study selection
Following removal of duplicate publications based on
their title/abstract, MF and SCa independently reviewed titles,
abstracts, and keywords to perform a preliminary selection. In case
of differences in the selection, the final decision was obtained
through a discussion with a third author (AGM). The full text of
all articles potentially suitable for the purposes of this analysis
was examined independently by MF and SCa. In the event of
discrepancies, the analysis proceeded as described above.
Data extraction
From the papers selected for inclusion in the
review, the data required for the analysis were independently
extracted by MF and SCa using a predefined data collection form.
The following parameters were included: ECT treatment
characteristics (drug, route of administration, number of
treatments, number and dimensions of treated lesions), previous and
concomitant treatments and their characteristics, tumor response,
adverse events, local control and impact on QoL.
The collected data were subsequently confirmed by
AGM to identify possible discrepancies among the extracted data. In
the event of conflicting data, the final decision was taken by
discussion.
Quality assessment
The quality of the papers selected was considered in
terms of clear definition of the study population, treatment
modality, clear level of the reported outcomes (per lesion or per
patient) and missing data.
Results
Search results
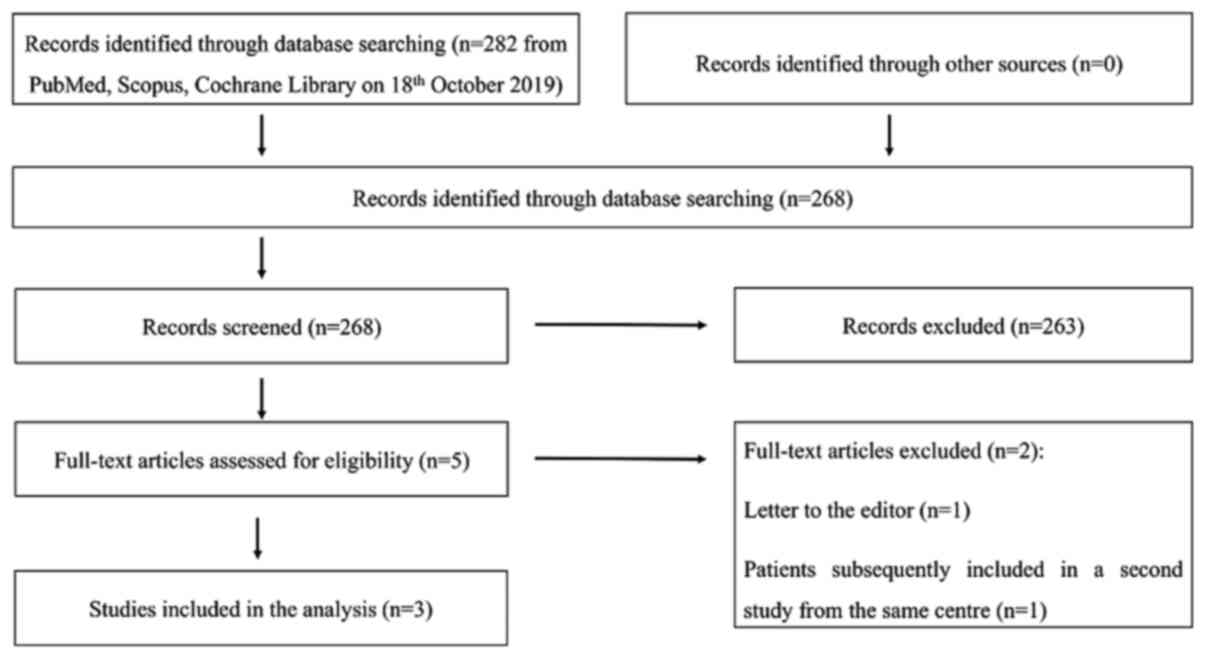
Fig. 1 indicates the
PRISMA flowchart of the study selection. The initial search led to
the identification of 282 studies (268 following duplicate
removal). Five full texts were examined following screening of the
titles and abstracts. Following assessment of the latter, three
studies were included in the analysis (11-13).
One record, which was a letter to the editor, was excluded and the
secondary title partially reported on patients was subsequently
included in another study from the same centre. All studies were
defined as prospective trials by the authors.
Patients and treatment
characteristics
The characteristics and results of the analyzed
studies are summarized in Table I.
Overall, the analyzed papers included 68 patients with KS skin
lesions treated with ECT. The number of patients in the studies
ranged between 18 and 27 (11-13),
while the total number of treated lesions, reported in only two
studies was 72(12) and
532(11), respectively. Tumor size
was not reported in any study.
 | Table IStudy characteristics. |
Table I
Study characteristics.
| Study
characteristics | Patients
characteristics | Treatment | Results |
|---|
| Author/year | Study design | Median follow-up
(range), months | n̊ patients | n̊ lesions | Median age (range),
years | Male/Female | Anesthesia | n̊ sessions | CR (%) | PR (%) | ORR (%) | Toxicity | (Refs.) |
|---|
| Curatolo et al
2012 | Phase II | 18 (2-50) | 23 | 532 | 77 (43-86) | 13/10 | Mild general
spinal | 1-3 | 60.9a | 35 | 100 | Cutaneous infection
8.7% local pain 8.7% | (11) |
| Latini et al
2012 | Prospective | NR (6-48) | 18 | 72 | 67 (48-90) | 14/4 | General | 1-3 | 88.9 | 11 | 100 | Ulceration 5.5% | (12) |
| Starita et al
2017 | Prospective | NR (24-68) | 27 | NR | 74 (43-88) | 24/3 | General-local-loco
regional | 1-6 |
74.1+ | 0 | 100 | NR | (13) |
ECT was performed using intravenous bleomycin in all
studies with the number of sessions ranging between one and six.
Intravenous bleomycin was administered as intravenous bolus (15,000
IU/m2) based on the ESOPE guidelines (4). Different types of anaesthesia were
used as follows: Local, spinal, local-regional and general. The
follow-up period was reported as range in all papers. The exact
range values were 2.0-50.4 months (11), 6-48 months (12) and 24-68 months (13), respectively. The median follow-up
was reported in only one series (18 months) (11). Previous treatments were not reported
in one study (13). However,
Curatolo et al (11)
described previous local or systemic treatments as follows:
Systemic chemotherapy (26.1%), radiotherapy (21.7%), intralesional
chemotherapy (4.3%) and interferon (13.0%). All patients in the
series of Latini et al (12)
were treated on locally recurrent lesions following
cryotherapy.
Two papers reported changes in HHV-8 parameters
following ECT. In both series, the viral load and viral blood titre
correlated with ECT response. More specifically, the levels of the
viral-related parameters declined markedly in patients with
complete response (CR) (12,13).
Tumor response and follow-up
In all studies, tumor response was reported on a per
patient basis. The CR ranged between 65 and 100% with an overall
response rate (ORR) (CR plus partial response) of 100% in all
studies (11-13).
Patients with stable or progressive disease were not reported. The
reported CR rate after a single ECT session was 60.9% as reported
by Curatolo et al (11) and
74.1% in the study by Starita et al (13). Moreover, Latini et al
(12) reported 88.9% CR rate after
the first ECT session.
All authors evaluated tumor response at four weeks
following the last ECT session. However different criteria were
used in the present study. In fact, two studies (11,13)
used the Response Evaluation Criteria in Solid Tumors and one study
used (12) the World Health
Organization guidelines.
Toxicity
The incidence of adverse events was low (5.5-8.7%)
in the two studies reporting toxicity (11,12).
More specifically, 5.5% of the subjects exhibited a local
ulceration in the study by Latini et al (12), while Curatolo et al (11) reported 8.7% of subjects with
cutaneous infection and 8.7% with local pain in their series. No
cases of systemic toxicity were recorded. One paper (11) used the World Health Organization
toxicity scoring system.
Other outcomes
Long term outcome data were analyzed only in one
study reporting 2-year local control and overall survival (OS)
rates of 76.2 and 74.4%, respectively (11). Moreover, in the same series, a QoL
improvement, assessed by means of the Patient Global Assessment
(PGA) (14), was recorded in 95% of
patients.
Quality assessment
All studies presented an intermediate risk of bias
with regard to patients and treatment description. In fact, only
two studies reported the number of treated lesions (11,12),
whereas the parameter tumor size was not reported. All studies
clearly reported the level of response evaluation and the scoring
criteria (11-13).
Finally, with regard to the missing data, toxicity was described in
two studies (11,12), while only one of them reported the
grading criteria (11).
Discussion
A systematic literature review was performed on ECT
in KS skin lesions. The limited number of analyzed studies suggests
that this treatment is safe and that it is associated with a very
high tumor response rate.
However, the present analysis has one clear
limitation. The selection of the studies led to the analysis of
only three studies (11-13).
Although several series included KS among ECT-treated tumors, in
the majority of the cases, the results on KS alone were not
reported separately and therefore they were excluded.
It should be noted that the analyzed studies
exhibited a small sample size, a wide variability in terms of
lesions/patient ratio and did not report the size of the treated
lesions. The latter limitation prevented the assessment of a
possible correlation between outcomes and tumor dimension. It is
important to note that none of the analyzed papers presented a
comparison of the clinical response of the patients with regard to
the following parameters: Age, sex, previous treatment history,
number of lesions, tumor size and body site. Moreover, although all
studies were defined as prospective, none of them described the
method of sample size calculation. Furthermore, another limitation
is that only one study reported the results in terms of local
control and OS (11). Therefore,
the investigation of the long-term control of the disease has not
been performed thoroughly. Finally, the number of sessions varied
widely between the different reports. However, it should be noted
that the use of multiple sessions in the same patient was due to
several reasons. Firstly, certain patients exhibited a high number
of lesions that had to be treated in the same session, whereas
other patients required several courses to achieve a satisfactory
tumor response after the first one. In addition, patients who
developed new lesions between courses were reported.
The results of the analysis suggested a high
response rate, which was estimated at 100% of the ORR rate in all
studies. The CR rate, although generally satisfactory, indicated a
rather significant variability (65-100%). These differences may be
due to the ‘per patient basis’ response evaluation combined with
the high variability of the lesions/patient ratio noted in the
different studies. The response rate ‘per patients’ was
significantly lower in cases of high number of lesions. The
probability of response in all lesions was reduced as the number of
the latter increased. Therefore, the lower CR rate reported by
Curatolo et al (11) (65%)
compared with that of Starita et al (13) (100%)could probably be explained by
the higher lesion/patient ratio of the first series. Furthermore,
the differences in terms of the response rate may be due to the
different evaluation criteria used in the different studies. It is
interesting to note that Curatolo et al reported QoL
improvement in 95% of patients, despite the lower CR rate (65%). In
addition, in this case it was hypothesized that the large number of
lesions per patient reduced the response rate ‘per patient’ but
still produced the reduction in the size in a number of lesions,
which was sufficient to improve the QoL (11).
Both studies reported data on toxicity and indicated
a relatively low incidence of adverse events (11,12).
In particular, the incidence of toxicity was 5.5-8.7% with regard
to the adverse event of each patient. However, based on the
incidence of toxicity regarding events per lesion, the results were
even more encouraging (overall: 0.8%). These results confirmed the
safety of ECT in the treatment of skin lesions (5). However, the patients did not undergo
combined modality treatments including ECT in the analyzed studies.
Therefore, it is impossible to define both tolerability and
potential synergistic effects of integrated treatments including
ECT in KS skin lesions.
The comparison of ECT with other local therapies in
KS demonstrated that radiotherapy was also well-tolerated and
associated with favourable results in terms of response (47-99%)
(15-17)
and local disease control (18).
However, ECT is a repeatable method, as demonstrated by several
studies on the treatment of skin neoplasms (7). KS is in the 30th place worldwide with
regard to the incidence and in the 29th with regard to mortality.
However, there are regions where incidence and mortality of this
disease are much higher. For example, 32,446 out of the 41,799 new
cases registered in 2018, have been diagnosed in Africa. In
addition, 17,659 out of the 19,902 deaths from KS recorded in the
same year, were recorded in Africa (19). It is well known that in the African
continent the chronic shortage of radiotherapy centres and
equipment is associated with the requirement for significant
initial investments and with the difficulties in maintaining the
equipment. Therefore, the possibility of treating other neoplasms
(skin and vulva) with ECT represents a useful integration to the
scarce radiotherapy resources available. In this regard, it should
be emphasized that the initial costs and maintenance of the ECT are
much lower than those of radiotherapy.
Intralesional chemotherapy is also associated with a
high response rate (70.0-98.7%) (20,21),
although this may be lower compared with the results uniformly
recorded in our analysis (100%).
Another modality of electroporation, is
‘irreversible electroporation’, a non-thermal tissue ablation
technology, which uses short pulses of a high voltage current,
without concurrent delivery of chemotherapy, leading to cell
membrane disruption and cell death as a result of loss of
homeostasis. However, this technique is used only in the treatment
of deep tumors and its application on KS has not been reported
previously.
The comparison of ECT with systemic chemotherapy is
generally used in advanced disease and is based on pegylated
liposomal doxorubicin plus paclitaxel or interferon α-2a or 2b. The
data demonstrate that the latter is associated with a relatively
high response rate (71-100%) particularly considering this specific
advanced disease setting (3). It is
important to note that these response rates cannot be compared with
those of ECT, since the use of systemic chemotherapy is justified
only in case of metastatic KS. However, it is speculated that in
metastatic diseases, ECT may exert a potential role in
oligo-persistent or oligo-progressive skin lesions, notably in
symptomatic cases.
In conclusion, the present analysis suggested that
ECT may be considered a therapeutic option in KS skin lesions.
Further studies are required to improve the existing knowledge on
this field. These studies may concern the following topics: i) The
comparison between ECT and other local therapies of KS, ii) the
role of ECT in skin recurrences following other local therapies,
iii) the combination of chemotherapy and palliative ECT in patients
with advanced disease and the optimal timing among the two
treatments, iv) a long-term evaluation of outcomes, v) the design
of clinical trials on KS subjects using ECT in areas with high
incidence and mortality from this disease, vi) the development of
predictive models of local control in order to favour tailored
treatment in patients with different presentations of KS skin
lesions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MF, GM, MB and AGM conceived and designed the study.
MF, SCa, AZ and AG extracted data from previous studies. MF, AMP,
FDT, FD, SCi, LT, PDI and AGM analysed and interpreted the data.
All authors wrote the manuscript. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
FDT is an employee at IGEA. AGM and PDI received
grants from IGEA. All other authors declare no competing
interests.
References
|
1
|
Ruocco E, Ruocco V, Tornesello ML,
Gambardella A, Wolf R and Buonaguro FM: Kaposi's sarcoma: Etiology
and pathogenesis, inducing factors, causal associations, and
treatments: Facts and controversies. Clin Dermatol. 31:413–422.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schneider JW and Dittmer DP: Diagnosis and
treatment of Kaposi sarcoma. Am J Clin Dermatol. 18:529–539.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lebbe C, Garbe C, Stratigos AJ, Harwood C,
Peris K, Marmol VD, Malvehy J, Zalaudek I, Hoeller C, Dummer R, et
al: Diagnosis and treatment of Kaposi's sarcoma: European
consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur J
Cancer. 114:117–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marty M, Sersa G, Garbay JR, Gehl J,
Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D,
et al: Electrochemothrapy, an easy, highly effective and safe
treatment of cutaneous and subcutaneous metastases: Results of
ESOPE (European standard operating procedures of
electrochemotherapy) study. Eur J Cancer Suppl. 4:3–13. 2006.
|
|
5
|
Cadossi R, Ronchetti M and Cadossi M:
Locally enhanced chemotherapy by electroporation: Clinical
experiences and perspective of use of electrochemotherapy. Future
Oncol. 10:877–890. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sersa G, Miklavcic D, Cemazar M, Rudolf Z,
Pucihar G and Snoj M: Electrochemotherapy in treatment of tumours.
Eur J Surg Oncol. 34:232–240. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Esmaeili N and Friebe M:
Electrochemotherapy: A review of current status, alternative IGP
approaches, and future perspectives. J Healthc Eng.
2019(2784516)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cemazar M, Parkins CS, Holder AL, Chaplin
DJ, Tozer GM and Sersa G: Electroporation of human microvascular
endothelial cells: Evidence for an anti-vascular mechanism of
electrochemotherapy. Br J Cancer. 84:565–570. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Centre for Reviews and Dissemination,
University of York. PROSPERO: International Prospective Register of
Systematic Reviews. https://www.crd.york.ac.uk/prospero. Accessed October
1, 2019.
|
|
10
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. J Clin Epidemiol.
62:1006–1012. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Curatolo P, Quaglino P, Marenco F, Mancini
M, Nardò T, Mortera C, Rotunno R, Calvieri S and Bernengo MG:
Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous
lesions: A two-center prospective phase II trial. Ann Surg Oncol.
19:192–198. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Latini A, Bonadies A, Trento E, Bultrini
S, Cota C, Solivetti FM, Ferraro C, Ardigò M, Amorosi B, Palamara
G, et al: Effective treatment of Kaposi's sarcoma by
electrochemotherapy and intravenous bleomycin administration.
Dermatol Ther. 25:214–218. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Starita N, Di Monta G, Cerasuolo A, Marone
U, Anniciello AM, Botti G, Buonaguro L, Buonaguro FM and Tornesello
ML: Effect of electrochemotherapy on human herpesvirus 8 kinetics
in classic Kaposi sarcoma. Infect Agent Cancer.
12(35)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pincus T, Bergman M, Sokka T, Roth J,
Swearingen C and Yazici Y: Visual analog scales in formats other
than a 10 centimeter horizontal line to assess pain and other
clinical data. J Rheumatol. 35:1550–1558. 2008.PubMed/NCBI
|
|
15
|
Stelzer KJ and Griffin TW: A randomized
prospective trial of radiation therapy for AIDS-associated Kaposi's
sarcoma. Int J Radiat Oncol Biol Phys. 27:1057–1061.
1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Caccialanza M, Marca S, Piccinno R and
Eulisse G: Radiotherapy of classic and human immunodeficiency
virus-related Kaposi's sarcoma: Results in 1482 lesions. J Eur Acad
Dermatol Venereol. 22:297–302. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hamilton CR, Cumming BJ and Harwood AR:
Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys.
12:1931–1935. 1986.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fort M, Guet S, Colson-Durand L, Auzolle C
and Belkacemi Y: Role of radiation therapy in non-melanoma cancers,
lymphomas and sarcomas of the skin: Systematic review and best
practice in 2016. Crit Rev Oncol Hematol. 99:200–213.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
GLOBOCAN. 2018, Cancer Incidence and
Mortality Worldwide: IARC CancerBase. Lyon, France: International
Agency for Research on Cancer. http://gco.iarc.fr/today/data/factsheets/populations/991-who-africa-region-afro-fact-sheets.pdf.
Accessed April 20, 2020.
|
|
20
|
Boudreaux AA, Smith LL, Cosby CD, Bason
MM, Tappero JW and Berger TG: Intralesional vinblastine for
cutaneous Kaposi's sarcoma associated with acquired
immunodeficiency syndrome. A clinical trial to evaluate efficacy
and discomfort associated with infection. J Am Acad Dermatol.
28:61–65. 1993.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brambilla L, Bellinvia M, Tourlaki A,
Scoppio B, Gaiani F and Boneschi V: Intralesional vincristine as
first-line therapy for nodular lesions in classic Kaposi sarcoma: A
prospective study in 151 patients. Br J Dermatol. 162:854–859.
2010.PubMed/NCBI View Article : Google Scholar
|