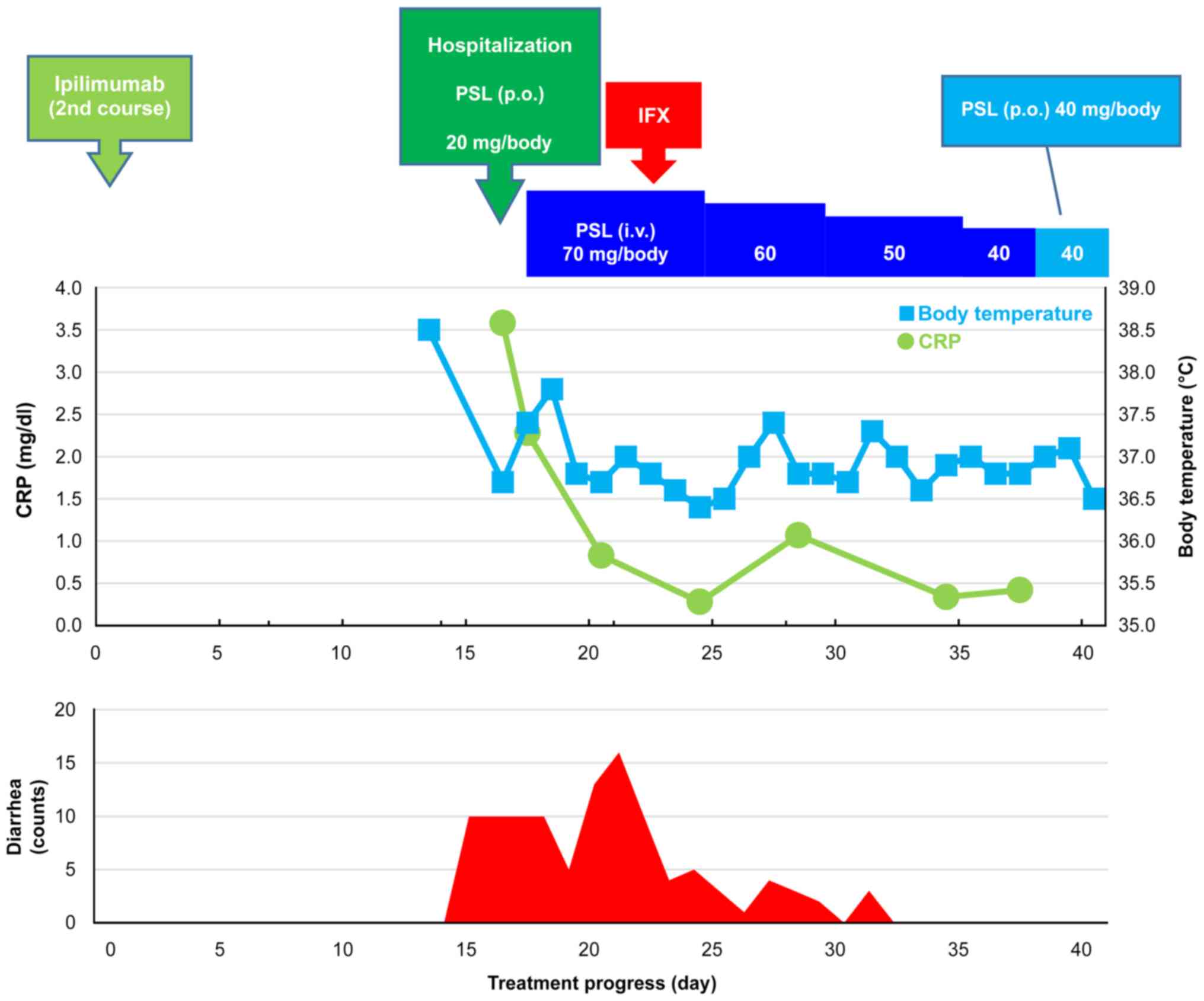
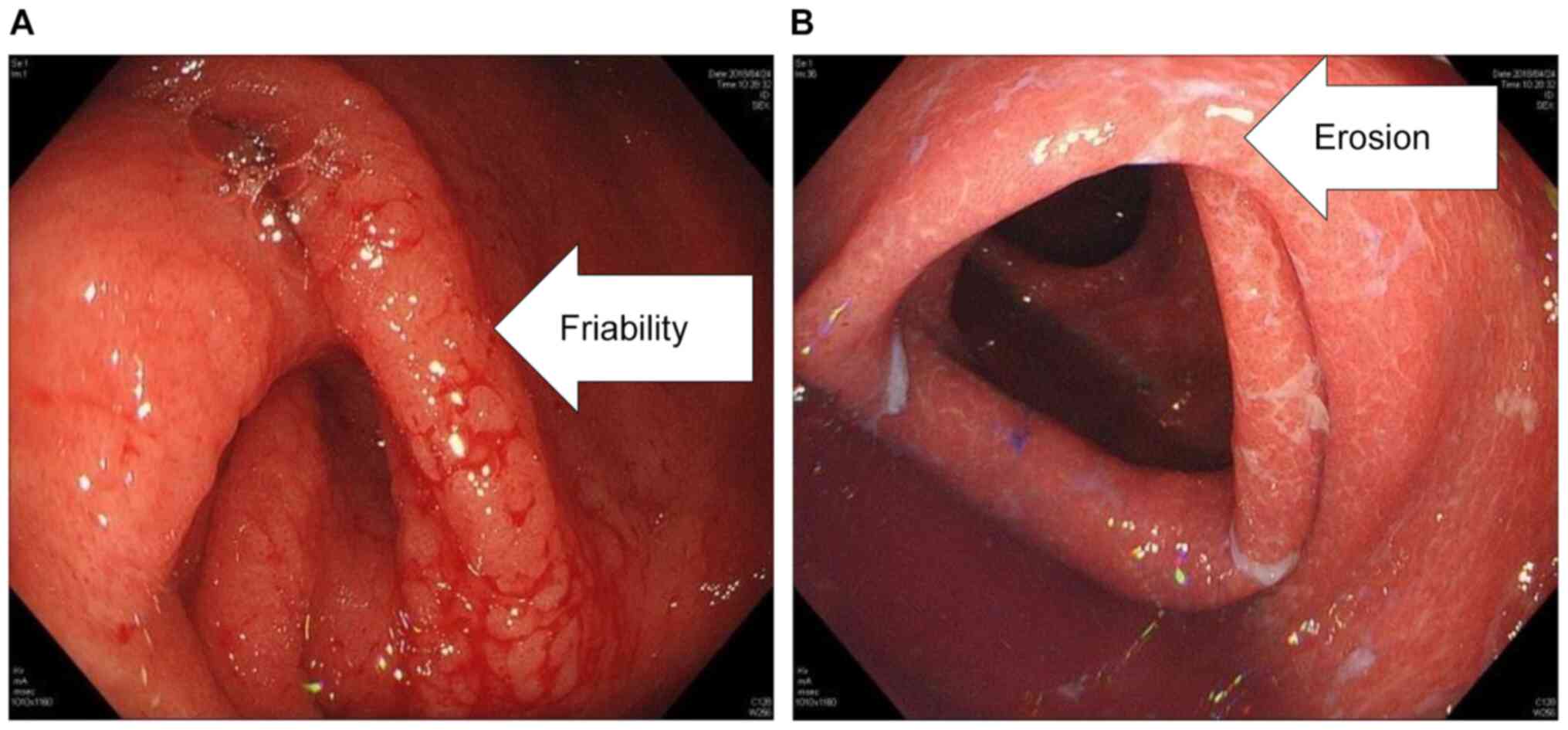
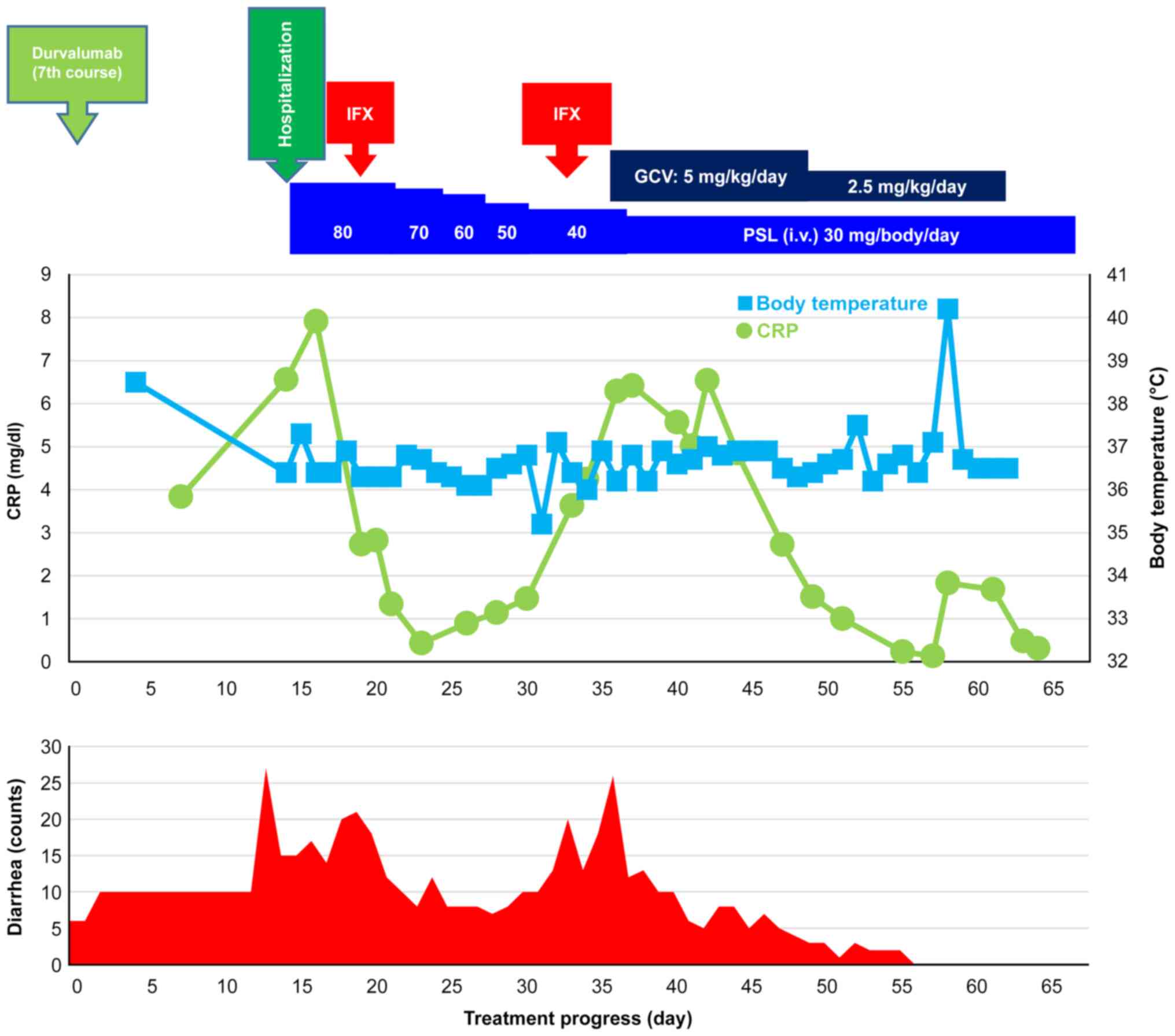
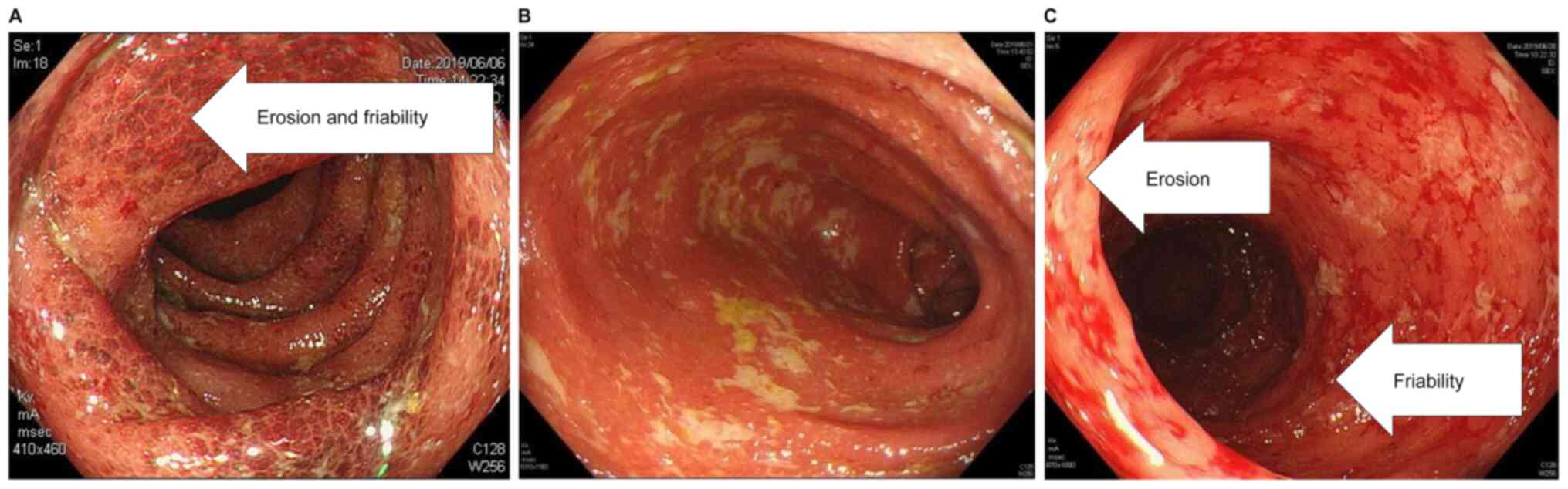
|
1
|
Soularue E, Lepage P, Colombel JF, Coutzac
C, Faleck D, Marthey L, Collins M, Chaput N, Robert C and Carbonnel
F: Enterocolitis due to immune checkpoint inhibitors: A systematic
review. Gut. 67:2056–2067. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Minor DR, Chin K and Kashani-Sabet M:
Infliximab in the treatment of anti-CTLA4 antibody (Ipilimumab)
induced immune-related colitis. Cancer Biother Radiopharm.
24:321–325. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Beck KE, Blansfield JA, Tran KQ, Feldman
AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM,
Kleiner D, et al: Enterocolitis in patients with cancer after
antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J
Clin Oncol. 24:2283–2289. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hillock NT, Heard S, Kichenadasse G, Hill
CL and Andrews J: Infliximab for ipilimumab-induced colitis: A
series of 13 patients. Asia Pac J Clin Oncol. 13:e284–e290.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pagès C, Gornet JM, Monsel G, Allez M,
Bertheau P, Bagot M, Lebbé C and Viguier M: Ipilimumab-Induced
acute severe colitis treated by infliximab. Melanoma Res.
23:227–230. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Geier M, Gouva S and Geier B:
Enterocolitis due to nivolumab treated by infliximab. ASGIS.
2:14–16. 2019.
|
|
8
|
Yanai S, Nakamura S and Matsumoto T:
Nivolumab-induced colitis treated by infliximab. Clin Gastroenterol
Hepatol. 15:e80–e81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nassri AB, Muenyi V, Al-Khasawneh A, De
Souza Ribeiro B, Scolapio JS, Malespin M and de Melo SW Jr:
Ipilimumab and nivolumab induced steroid-refractory colitis treated
with infliximab: A case report. World J Gastroint Pharmacol Ther.
10:29–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Delasos L, Desai A, Lia NL, Kethireddy N
and Ray C: A case of immunotherapy-induced colitis complicated by
perforation and treated with infliximab postoperatively. Case Rep
Oncol Med. 2019(9069354)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Johnson DH, Zobniw CM, Trinh VA, Ma J,
Bassett RL Jr, Abdel-Wahab N, Anderson J, Davis JE, Joseph J,
Uemura M, et al: Infliximab associated with faster symptom
resolution compared with corticosteroids alone for the management
of immune-related enterocolitis. J Immunother Cancer.
6(103)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Perez-Ruiz E, Minute L, Otano I, Alvarez
M, Ochoa MC, Belsue V, de Andrea C, Rodriguez-Ruiz ME, Perez-Gracia
JL, Marquez-Rodas I, et al: Prophylactic TNF blockade uncouples
efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy.
Nature. 569:428–432. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grobb JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gupta A, De Felice KM, Loftus EV Jr and
Khanna S: Systematic review: Colitis associated with anti-CTLA-4
therapy. Aliment Pharmacol Ther. 42:406–417. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-Programmed-Death-Receptor-1 treatment with pembrolizumab
in Ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Berman D, Parker SM, Siegel J, Chasalow
SD, Weber J, Galbraith S, Targan SR and Wang HL: Blockade of
cytotoxic T-lymphocyte antigen-4 by ipilimumab results in
dysregulation of gastrointestinal immunity in patients with
advanced melanoma. Cancer Immun. 24:10–11. 2010.PubMed/NCBI
|
|
21
|
Liu J, Blake SJ, Harjunpää H, Fairfax KA,
Yong MC, Allen S, Kohrt HE, Takeda K, Smyth MJ and Teng MW:
Assessing immune-related adverse events of efficacious combination
immunotherapies in preclinical models of cancer. Cancer Res.
76:5288–5301. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Weber JS, Kähler KC and Hauschild A:
Management of immune-related adverse events and kinetics of
response with ipilimumab. J Clin Oncol. 30:2691–2697.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Franklin C, Rooms I, Fiedler M, Reis H,
Milsch L, Herz S, Livingstone E, Zimmer L, Schmid KW, Dittmer U, et
al: Cytomegalovirus reactivation in patients with refractory
checkpoint inhibitor-induced colitis. Eur J Cancer. 86:248–256.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kuo JR, Davis AD, Rodriguez EA, Vela MF,
Heigh RI, Salomao MA and Gurudu SR: Severe diarrhea in the setting
of immune checkpoint inhibitors. Case Rep Gastroenterol.
12:704–708. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Iyoda T, Kurita N, Takada A, Watanabe H
and Ando M: Resolution of infliximab-refractory nivolumab-induced
acute severe enterocolitis after cyclosporine treatment in a
patient with non-small cell lung cancer. Am J Case Rep. 27:360–364.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bergqvist V, Hertervig E, Gedeon P,
Kopljar M, Griph H, Kinhult S, Carneiro A and Marsal J: Vedolizumab
treatment for immune checkpoint inhibitor-induced enterocolitis.
Cancer Immunol Immunother. 66:581–592. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Postow MA, Sidlow R and Hellmann MD:
Immune-Related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|