Introduction
Immune checkpoint inhibitors (ICIs) are promising
drugs that can potentiate the immune system of cancer patients for
disease treatment. ICIs block endogenous factors, such as cytotoxic
T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1),
thereby enhancing the antitumor effect. ICIs, such as nivolumab
(Nivo), have reportedly extended the patient overall survival in
cases of different types of cancer and have been approved in
several countries. Meanwhile, Immune-related adverse events
(irAEs), which often occur in association with immune checkpoint
inhibitor (ICI) treatment, require early detection and appropriate
management considering their potentially fatal outcomes. Among
irAEs, diarrhea/colitis occurs particularly frequently, and serious
complications, such as intestinal perforation, may follow unless
timely and appropriate treatment is provided (1). The American Society of Clinical
Oncology guideline includes an organ system-based management
algorithm showing recommended management procedures for various
irAEs according to grade (2). In
line with this, steroid therapy with approximately 1 mg/kg/day
prednisolone (PSL) equivalent is immediately initiated for grade 3
diarrhea/colitis. However, when symptoms do not improve, infliximab
(IFX) treatment, generally at a dose of 5 mg/kg/day according to
the administration for ulcerative colitis (3-11),
has been recommended. Single-dose IFX administration has often been
selected as an irAE treatment, with additional doses administered
only when no improvements occur after the first dose. Perez-Ruiz
et al (12) have
demonstrated that the inhibition of tumor necrosis factor-α (TNF-α)
after ICI administration may prevent the occurrence of severe
colitis. IFX, approved in 1999 in the United States and in 2003 in
Japan, is an anti-TNF-α antibody drug that binds to and neutralizes
the action of TNF-α, which plays a key role in the development and
exacerbation of rheumatoid arthritis. While transient headache and
nausea can occur as short-term adverse reactions to IFX, these
symptoms are mild. However, it is imperative to pre-emptively
identify symptoms of medium- and long-term adverse reactions such
as infectious diseases, demyelinating diseases, aplastic anemia,
malignant tumors, autoimmune diseases, and heart failure, among
which infectious diseases are of particular concern. Several case
reports have described successful treatment of steroid-resistant
ICI-induced diarrhea/colitis with IFX in patients receiving
ipilimumab (IPI), an anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4)
antibody preparation (3-6),
as well as anti-programmed cell death-1 (PD-1) antibody or
anti-programmed cell death-ligand 1 (PD-L1) antibody preparations
(7-11).
However, we were unable to identify any report systematically
dealing with the required number of IFX doses or the time to switch
to after treatment.
Therefore, this study investigated the outcomes of
IFX treatment among Japanese cancer patients who developed severe
steroid-resistant irAEs induced by various ICIs and examined its
efficacy and safety for severe steroid-resistant irAEs.
Materials and methods
Patients
Among nine Japanese patients with cancer who
underwent IFX treatment for ICI-induced steroid-resistant irAEs at
the Osaka International Cancer Institute (hereinafter referred to
as the ‘Institution’) between January 2018 and June 2019, eight
whose electronic medical records contained the necessary treatment
information were included; one patient who participated in a
clinical trial was excluded.
We obtained ‘consent to the publication of a paper
related to the course of irAE treatment’ from a patient who was
alive and able to undergo follow-up examinations (case no. 2). For
the patients who could not provide consent, regardless of whether
they were deceased or owing to other reasons, we used the opt-out
submitted when we requested an approval from the Institutional
Review Board as a substitute for the consent.
Information collected
Electronic medical records were retrospectively
investigated to collect information, such as patient background,
treatment progress, examination data, and imaging data. AEs were
assessed using the Common Terminology Criteria for Adverse Events
version 4.0. The first of 7 consecutive days during which diarrhea
severity remained grade 0 was defined as the day of resolution.
Statistical analysis
In this study, we used the statistical software
Microsoft Excel 2013 to calculate only the median.
Results
Background and irAE treatment
details
Patient background and irAE treatment details are
summarized in Table I. Six male and
two female patients, with a median (range) age and body weight of
66 (58-74) years and 60.0 (38.0-85.1) kg, respectively, were
included in this study. Four patients presented with malignant
melanoma, three with lung cancer, and one with kidney cancer.
Specific ICIs used included anti-PD-1, anti-PD-L1, and anti-CTLA-4
antibody preparations in seven, two, and five patients,
respectively.
 | Table IBackground and irAE treatment
details. |
Table I
Background and irAE treatment
details.
| | Safety of IFX | |
|---|
| No. | Age, years | Sex | Body weight, kg | Name of neoplastic
disease | Past medical
history | ICI (before onset of
irAE) | irAE (for which
infliximab was used) | CMV | CD | Dosage and
administration of steroids and infliximab (days after the last dose
of ICI) | Outcome |
|---|
| 1 | 44 | M | 70 | Malignant melanoma
(stage IV); left cervical lymph node metastasis, multiple cutaneous
and subcutaneous metastases, lung metastasis, brain metastasis | Psoriasis vulgaris,
hypertension | Nivolumab 5 courses ⇒
PD ⇒ Ipilimumab 2 courses | Day 16: Diarrhea/
colitis (Grade 3) | NO | NO | Day 16: Betamethasone
(p.o.) 1 mg (brought in by patient) + Prednisolone (p.o.) 20 mg;
Prednisolone (i.v.); Day 17-23: 70 mg (1 mg/kg) ≪Day 22: Infliximab
340 mg (5 mg/kg)≫; Day 24-28: 60 mg; Day 29-34: 50 mg; Day 35-37:
40 mg; Prednisolone (p.o.); Day 38-41: 40 mg; Day 42-47: 30 mg; Day
48-54: 20 mg; Day 55-60: 15 mg; Day 61-74: 10 mg; Discharge | Effective; Day 31:
Grade 0 |
| 2 | 58 | M | 78 | Adenocarcinoma of
superior lobe (stage IIIA); Visceral pleural invasion, mediastinal
lymph node metastasis | Chronic hepatitis B,
chronic gastritis, refractory gastric ulcer, reflux
esophagitis | Durvalumab 7
courses | Day 15: Diarrhea/
colitis (Grade 3) | Day 17: NO; Day 30:
YES; Day 36: YES; Day 44: NO | NO | Prednisolone (i.v.);
Day 15-21: 80 mg (1 mg/kg); ≪Day 19: Infliximab 375 mg (5 mg/kg)≫;
Day 22-24: 70 mg; Day 25-27: 60 mg; Day 28-30: 50 mg; Day 31-36: 40
mg; ≪Day 33: Infliximab 350 mg (5 mg/kg)≫; Day 37-68: 30 mg;
Prednisolone (p.o.); Day 69-75: 30 mg; Day 76-82: 25 mg; Day 83-89:
20 mg; Day 89-95: 17.5 mg; Day 96-108: 15 mg; Day 109-122: 10 mg;
Day 123-135: 5 mg; Day 136-149: 2.5 mg | 【Ineffective
(relapse)】 Day 19: Grade 3 Day 30: Grade 2 Day 33: Grade 3 Day 44:
Grade 2 Day 51: Grade 0 |
| 3 | 70 | M | 60 | Adenocarcinoma of the
left superior lobe (stage IVA) | None | Nivolumab 35 courses
⇒ PD ⇒ Atezolizumab 3 courses | Day 11: Diarrhea/
colitis (Grade 3) | NO | Day 19: NO; Day 40:
YES; Day 96: NO | Prednisolone (i.v.);
Day 25-27: 60 mg (1 mg/kg); Prednisolone (p.o.); Day 28-35: 50 mg;
≪Day 34: Infliximab 300 mg (5 mg/kg)≫; Prednisolone (i.v.); Day
36-41: 50 mg; Day 42-48: 40 mg; Prednisolone (p.o.);Day 49-53: 35
mg; Day 54-61: 30 mg; Day 62-75: 25 mg; Day 76-89: 20 mg; Day
90-96: 15 mg; Day 97-119: 10 mg; Day 120-161: 5 mg; Day 162-189: 3
mg; Day 190-217: 2 mg; Day 218-273: 1 mg; Discharge | Ineffective
(protracted); Grade 0-3 diarrhea persisted. |
| 4 | 65 | W | 53.5 | Right lacrimal sac;
Malignant melanoma (stage IV); Lung metastasis, pleural
dissemination, meningeal dissemination | None | Nivolumab +
Ipilimumab 1 course | Day 23: hepatitis
(Grade 2); Day 27: hepatitis (Grade 3); Day 31: hepatitis (Grade
1); Day 53: Diarrhea/ colitis/enteritis (Grade 3) | Day 55: NO; Day 71:
YES; Day 80: YES; Day 85: NO | NO | Methyl prednisolone
sodium succinate (i.v.); Day 23-26: 50 mg; Day 27-41: 100 mg; Day
42-46: 80 mg; Day 47-86: 70 mg; ≪Day 62: Infliximab 250 mg (5
mg/kg)≫; Day 87-97: 60 mg; Day 98-105: 50 mg; Day 106-112: 40 mg;
Day 113-118: 30 mg; ≪Day 117: Infliximab 300 mg (5 mg/kg) ≫; Day
119-126: 20 mg; Prednisolone (i.v.); Day 127-133: 20 mg; Day
134-140: 15 mg; Day 141-146: 10 mg; Day 147-155: 5 mg
(discontinued); ≪Day159: Infliximab 300mg(5mg/kg)≫ | Ineffective
(protracted); Grade 1-3 diarrhea persisted. |
| 5 | 67 | M | 64 | Lateral border of
right foot; Malignant melanoma (stage IV) | Gastric ulcer | Nivolumab 6 courses
⇒PD ⇒Nivolumab + Ipilimumab 1 course | Day 10: Diarrhea/
colitis (Grade 3) | Day 12: NO; Day 48:
YES; Day 54: YES; Day 61: NO | NO | Methyl prednisolone
sodium succinate (i.v.); Day 10-12: 70 mg; Prednisolone (i.v.); Day
13-32: 70 mg; ≪Day 20: Infliximab 300 mg (5 mg/kg)≫; Day 33-39: 60
mg; Day 40-52: 50 mg; ≪Day 50: Infliximab 300 mg (5 mg/kg)≫; Day
53-57: 40 mg; Day 58-62: 30 mg; Day 63-70: 20 mg; ≪Day 64:
Infliximab 300 mg (5 mg/kg)≫; Day 71-80: 10 mg; Day 81-95: 5 mg;
Discharge | Effective; Day 22:
Grade 0; Thereafter Grade 0-1 (Improved); Day 48: Grade 3 (diarrhea
relapsed); Day 52: Grade 0; Thereafter Grade 0-1 (Improved) |
| 6 | 74 | M | 56 | Adenosquamous cell
lung cancer (stage IVB); Adrenal metastasis, brain metastasis | Esophageal cancer,
adrenal metastasis, brain metastasis | Pembrolizumab +
CBDCA + NabPTX 1 course | Day 5: Diarrhea/
colitis (Grade 3) | NO | NO | Hydrocortisone
sodium phosphate (i.v.); Day 5: 100 mg; Methyl prednisolone sodium
succinate (i.v.); Day 6-7: 500 mg; ≪Day 7: Infliximab 275 mg (5
mg/kg)≫ | Effectiveness
unknown; Day 8: Died |
| 7 | 62 | W | 38 | Rectal malignant
melanoma (stage IV); Multiple lymph node metastases, multiple lung
metastases, liver metastases | None | Nivolumab +
Ipilimumab 3 courses | Day 1: Diarrhea/
colitis (Grade 2); Day 18: worsened to Grade 3 | NO | NO | 20 days before the
onset of Grade 2 diarrhea/colitis to 5 days after the onset: For
exacerbation of brain metastasis and meningeal dissemination;
Betamethasone (i.v.) 4 mg; Prednisolone (i.v.); Day 6-18: 40 mg (2
mg/kg including Betamethasone (i.v.)); Day 19-22: 30 mg; ≪Day 21:
Infliximab 200 mg (5 mg/kg)≫; Day 23-26: 20 mg; Day 27-30: 40
mg | Effectiveness
unknown; Day 31: Died |
| 8 | 66 | M | 85.1 | Right kidney cancer
(stage IV) | Hypertension,
dyslipidemia, gastric ulcer, chronic hepatitis C, myocarditis | Nivolumab +
Ipilimumab 4 courses ⇒ Nivolumab 8 courses | Day 34: DIC
exacerbation, myocarditis, skin problems | Day 34: NO; Day 50:
NO; Day 58: YES; Day 63: YES; Day 69: YES; Day 75: YES; Day 82:
NO | Not tested | Prednisolone
(i.v.); Day 34-36: 80 mg; Methyl prednisolone sodium succinate
(i.v.); Day 37-39: 1000 mg; Prednisolone (i.v.); Day 40-44: 80 mg;
≪Day 40: Infliximab 425 mg (5 mg/kg)≫; Day 45-47: 60 mg; Day 48-53:
50 mg; Day 54-60: 40 mg; ≪Day 54: Infliximab 400 mg (5 mg/kg)≫;
Prednisolone (p.o.); Day 61-64: 30 mg; Day 65-68: 25 mg; Day 69-74:
20 mg; Day 75-81: 15 mg; Day 82-88: 10 mg; Day 89-95: 5 mg;
Discharge | Effective; Day 49:
DIC improved; Day 69: myocarditis improved |
irAE treatment was switched from steroids to IFX
since systemic steroid treatment was ineffective for grade ≥3
diarrhea/colitis (case nos. 1-7) or for disseminated intravascular
coagulation and myocarditis resulting from activated autoimmunity
(case no. 8). Grade 3 diarrhea/colitis occurred at a median (range)
of 119 (5-1,011) days after the first ICI dose or at a median
(range) of 15.5 (5-75) days after the last ICI dose. Grade 3
hepatitis preceded diarrhea/colitis in one case (case no. 4).
Three patients who responded to IFX satisfied the
defined resolution (case nos. 1, 5 and 8). Accordingly, responses
to IFX occurred after one dose in case no. 1 and after two doses in
case nos. 5 and 8 (in case no. 5, an accessory third dose was
administered, but the defined resolution was satisfied after two
IFX doses). Although the treatment was transiently effective in one
case (case no. 2), irAEs relapsed. Two patients did not respond to
IFX (case nos. 3 and 4), while two patients (case nos. 6 and 7)
underwent rapid deterioration of their general condition after IFX
treatment initiation and died before the IFX effect was noted. The
cause of death was irAE diarrhea and multi-organ failure in case
no. 6 and respiratory failure due to multiple lung metastases and
pulmonary congestion in case no. 7. No causal relationships were
observed between IFX treatment and deaths. The median (range)
number of days between systemic steroid treatment and IFX
initiation was 9 (2-39) days. In three IFX responders, resolution
to grade 0 required a median (range) of 18 (9-32) days after IFX
treatment initiation. No AEs were attributable to IFX. No patients
developed cytomegalovirus (CMV) or Clostridium difficile
(CD) infection when grade 3 irAEs occurred.
Among four patients who experienced the relapse of
grade 3 diarrhea/colitis after IFX administration or were
refractory to IFX, three (case nos. 2, 4 and 5) developed CMV
infection and one (case no. 3) developed CD infection after IFX
initiation. Detailed treatment courses are described in separate
sections for case no. 1, in whom IFX was effective, and case no. 2,
in whom irAE relapsed repeatedly.
Since different hormone level between women in
menstruation period and women without menstruation period would
impact the resistance to steroid, case reports did not list
premenopausal women.
Case reports Case no. 1
The case was a 44-year-old man with a body weight of
69.8 kg.
Diseases: Malignant melanoma of the head, with left
cervical lymph node, multiple cutaneous/subcutaneous, lung, and
multiple brain metastases (stage IV)
Past medical history: Psoriasis vulgaris and
hypertension
History of the present illness: The patient
underwent extended resection and dissection of the left cervical
lymph node metastases in June 2017. Multiple cutaneous metastases
were noted in November 2017, for which extended resection, flap
surgery, and resection of a subcutaneous mass in his left back were
performed. Although five courses of nivolumab (Nivo) monotherapy
had been administered since December 2017, the disease still
progressed. Therefore, the treatment was switched to IPI
monotherapy as the second-line treatment in February 2018.
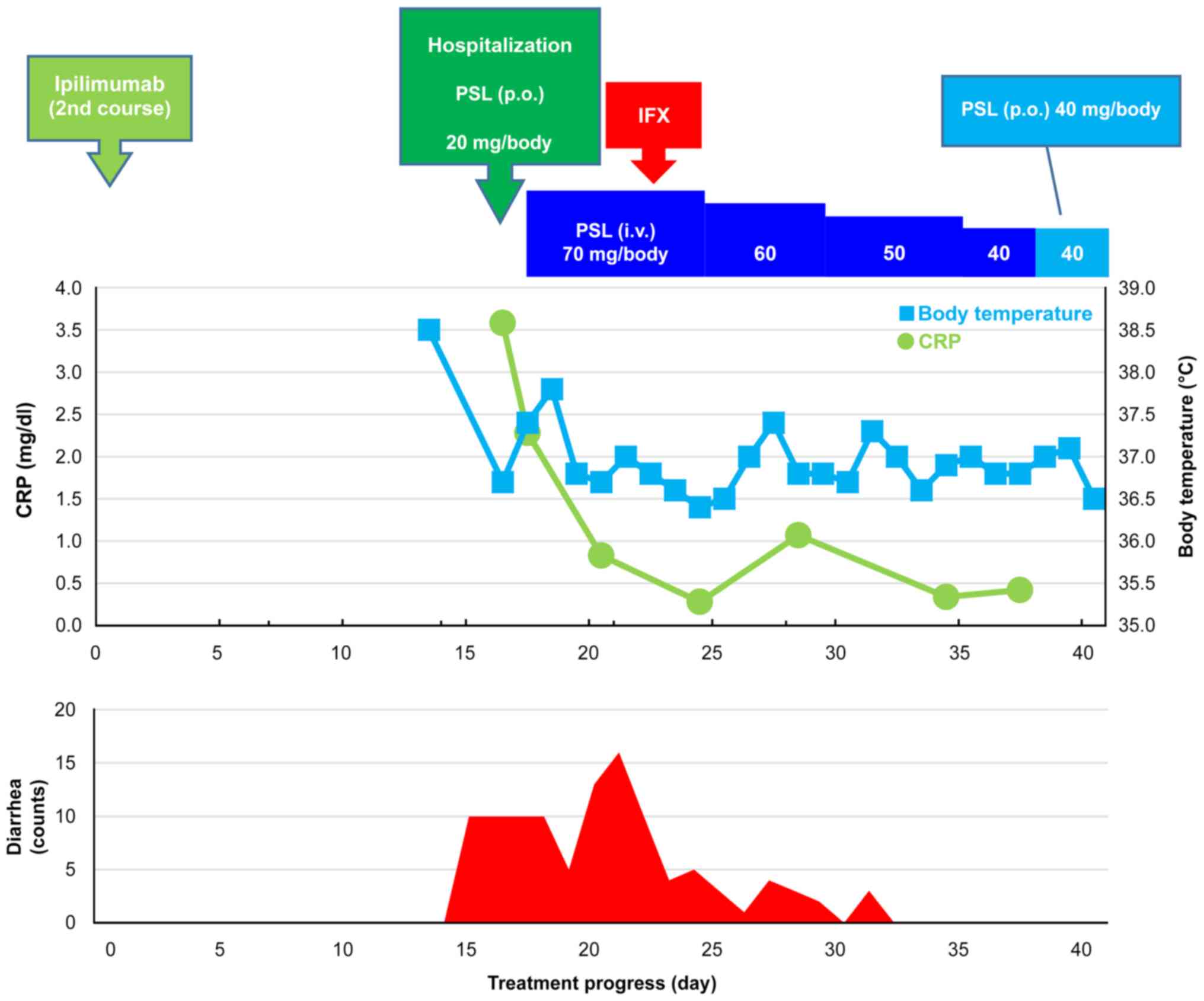
Treatment course: The clinical course after two
courses of IPI is presented in Fig.
1. The day on which the second IPI course was introduced was
set as day 0. The patient was febrile (38.5˚C) on day 13, and grade
3 colitis/diarrhea occurred on day 15. The patient was subsequently
hospitalized on day 16 due to diarrhea occurring 10 times/day
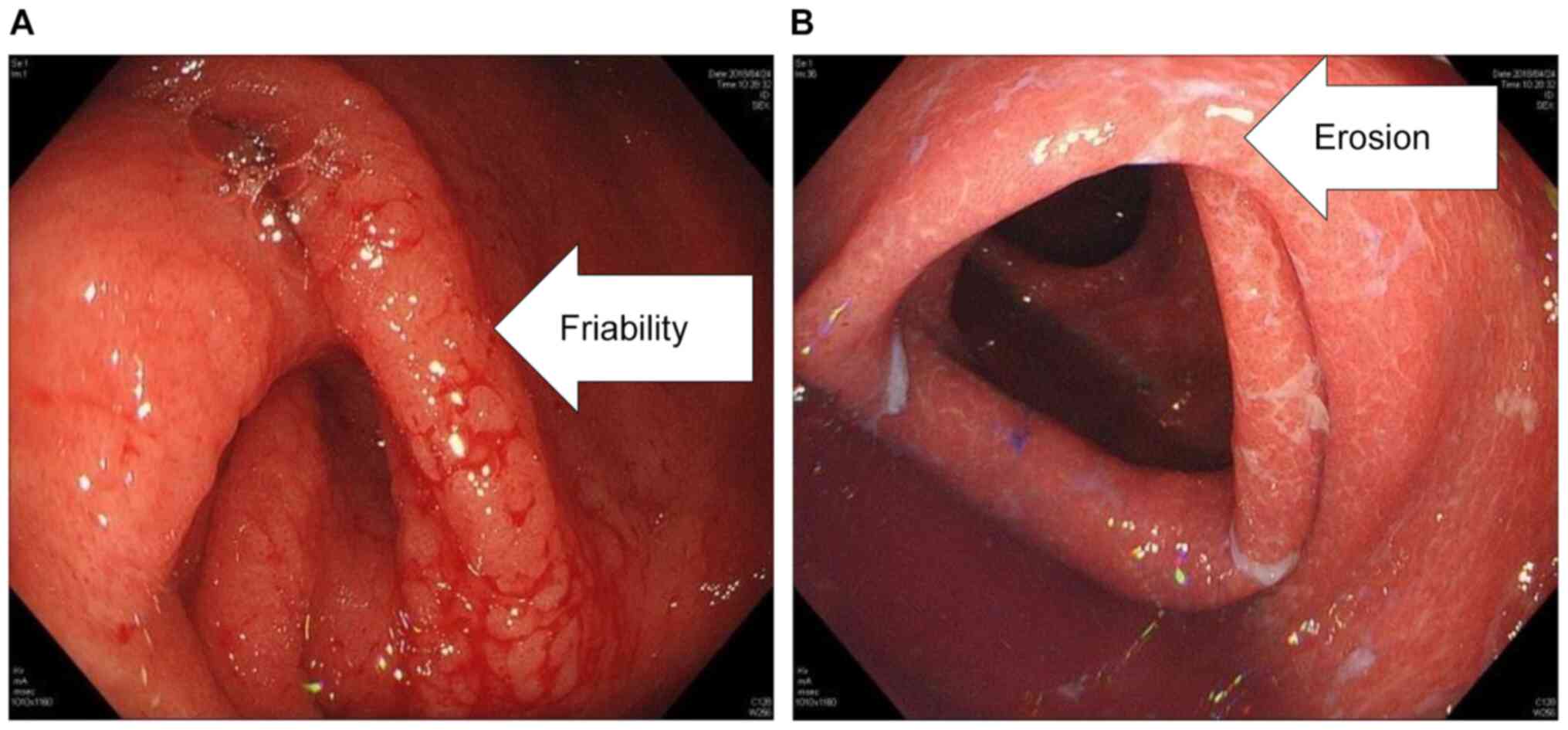
(grade 3). Endoscopy findings revealed mucosal friability and
erosion in the lower gastrointestinal tract (Fig. 2). Laboratory findings on admission
are summarized in Table II. The
patient was instructed to fast, and replacement fluid and PSL 20
mg/body per os were initiated. Although the PSL dose was increased
to 1 mg/kg/day (70 mg/body/day) on day 17, no improvements were
noted even after several days; thus, a diagnosis of
steroid-resistant colitis/diarrhea was established. Accordingly,
IFX 5 mg/kg/day (340 mg/body/day) was administered intravenously on
the seventh hospital day (day 22). The number of bowel movements
started decreasing a day after IFX administration (day 23), and
stool passage normalized 9 days after IFX administration (day 31).
The patient was subsequently discharged on day 45.
 | Table IICase no. 1: Laboratory findings on
admission. |
Table II
Case no. 1: Laboratory findings on
admission.
| Variable | Value |
|---|
| Hematology | |
|
WBC |
6.56x103/µl |
|
Neutro | 65.9% |
|
Lympho | 15.8% |
|
Mono | 10.6% |
|
Eosino | 3.1% |
|
Baso | 1.0% |
|
RBC |
4.96x104/µl |
|
Hb | 14.1 g/dl |
|
Ht | 42.5% |
|
PLT |
28.3x104/µl |
|
CMV
antigen | (-) |
|
Fecal
culture | (-) |
|
Fecal CD
toxin | (-) |
| Biochemistry | |
|
Alb | 3.4 g/dl |
|
AST | 41 U/l |
|
ALT | 30 U/l |
|
LDH | 3,119 U/l |
|
ALP | 219 U/l |
|
γ-GTP | 60 U/l |
|
CK | 82 U/l |
|
Cr | 1.11 mg/dl |
|
BUN | 13 mg/dl |
|
CRP | 3.58 mg/dl |
|
Na | 135 mmol/l |
|
K | 4.2 mmol/l |
|
Cl | 99 mmol/l |
|
TSH | 0.15 µU/ml |
|
FT4 | 0.9 ng/dl |
Case no. 2
The case was a 58-year-old man with a body weight of
78.3 kg.
Diseases: Adenocarcinoma of the right superior lobe,
with visceral pleural invasion and mediastinal lymph node
metastasis (stage IIIA: T2aN2M0).
Past medical history: Chronic hepatitis B, chronic
gastritis, refractory gastric ulcer, and reflux esophagitis.
History of the present illness: As part of
chemoradiation therapy (CRT), the patient received two courses of
cisplatin/vinorelbine combination therapy in November 2018. A total
dose of 64 Gy/40 Fr was used for radical irradiation of the chest
and mediastinum. Response assessment showed a stable disease.
Durvalumab monotherapy was then initiated on day 44 after RT in
January 2019 as a post-CRT maintenance therapy.
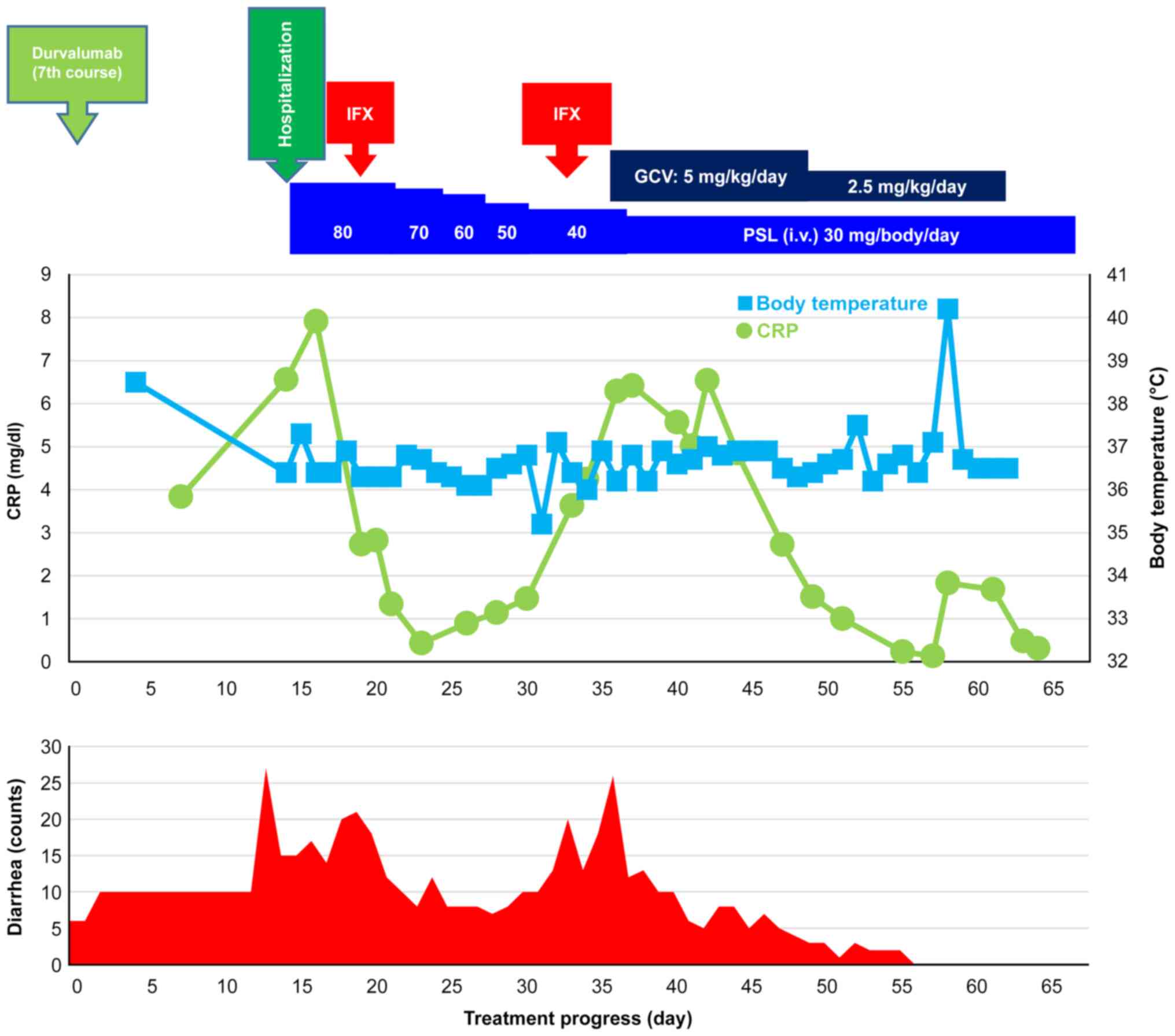
Treatment course: The clinical course after seven
courses of durvalumab is shown in Fig.
3. The day on which the seventh course of durvalumab was
introduced was set as day 0. The patient was hospitalized for
thorough examination and treatment on day 15 due to diarrhea
(bloody stool) occurring 27 times/day (grade 3). Laboratory
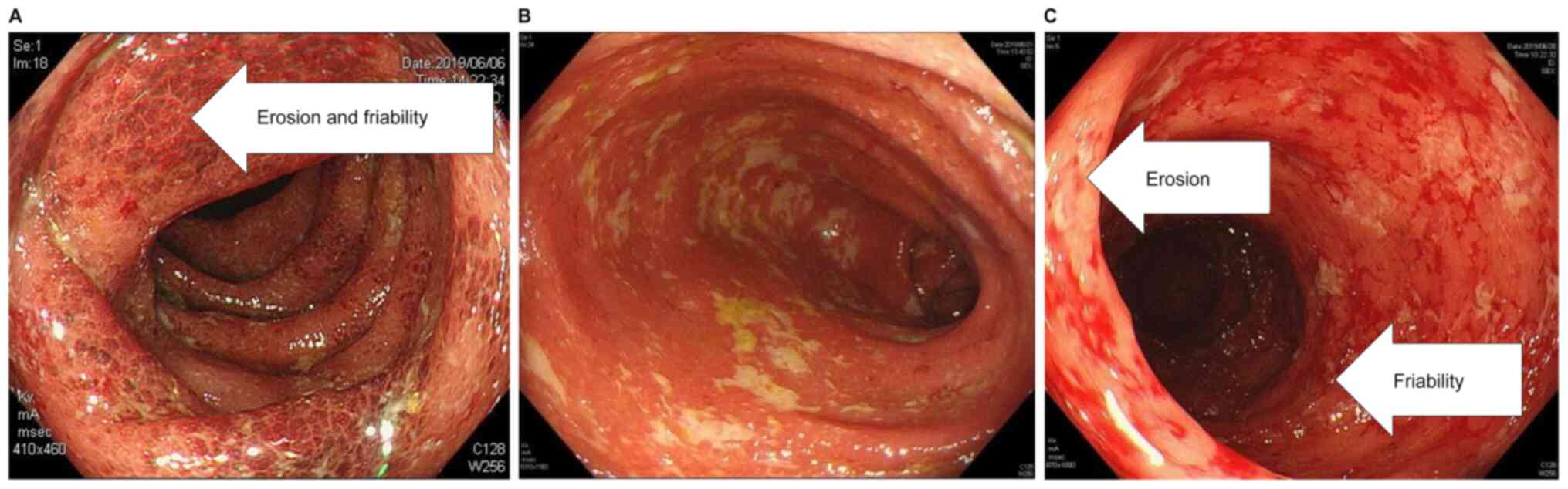
findings on admission are summarized in Table III. Colonoscopy revealed redness,
erosion, edema, and grade 3 (Mayo classification) lesions
throughout the colon (Fig. 4), and
computed tomography revealed wall thickening of the rectum, sigmoid
colon, and cecum. Based on these findings, the patient was
diagnosed with grade 3 colitis as an irAE. Treatment with
high-calorie infusion, antibacterial cefmetazole infusion, and oral
sulfamethoxazole/trimethoprim in conjunction with daily
administration of PSL 1 mg/kg/day (80 mg/body/day) was initiated in
a fasted state. On day 19, diarrhea occurred 21 times/day (grade
3), and the stool remained muddy. Thus, steroid treatment was
concluded to be ineffective. Thereafter, IFX 5 mg/kg/day (375
mg/body/day) was administered with the steroid dose being gradually
reduced, which improved his diarrhea to grade 1/2. On day 30,
diarrhea remained persistent at 10 times/day (grade 2), and
colonoscopy revealed improved albeit persistent mucosal erosion and
bleeding. Thus, IFX re-administration was planned. On day 33,
diarrhea worsened to 23 times/day (grade 3), during which he
received the second dose of IFX 5 mg/kg/day (350 mg/body/day). On
day 36, no improvement in diarrhea frequency was observed (17
times/day; grade 3). While the CMV cell test result indicated CMV
infection, the CD test result was negative. The patient was thus
diagnosed with concomitant CMV enteritis and irAE (colitis) and was
administered with ganciclovir 5 mg/kg infusion twice daily. On day
44, the CMV cell test result was negative, and diarrhea frequency
improved to 9 times/day (grade 2). Ganciclovir dose was
subsequently reduced to 2.5 mg/kg/dose x2 doses/day on day 50. On
day 51, diarrhea was resolved (once/day; grade 0), and on day 69,
treatment was switched to oral PSL 30 mg/body/day. PSL treatment
was continued thereafter with gradually tapering of doses, during
which no diarrhea occurred.
 | Table IIICase no. 2: Laboratory findings on
admission. |
Table III
Case no. 2: Laboratory findings on
admission.
| Variable | Value |
|---|
| Hematology | |
|
WBC |
8.69x103/µl |
|
Neutro | 75.3% |
|
Lympho | 12.5% |
|
Mono | 5.7% |
|
Eosino | 3.9% |
|
Baso | 0.3% |
|
RBC |
4.58x104/µl |
|
Hb | 12.5 g/dl |
|
Ht | 40.2% |
|
PLT |
33.4x104/µl |
|
CMV
antigen | (-) |
|
Fecal
culture | (-) |
|
Fecal CD
toxin | (-) |
| Biochemistry | |
|
TP | 6.8 g/dl |
|
Alb | 3.4 g/dl |
|
AST | 14 U/l |
|
ALT | 13 U/l |
|
LDH | 199 U/l |
|
γ-GTP | 23 U/l |
|
Cr | 0.85 mg/dl |
|
CRP | 6.56 mg/dl |
|
Na | 142 mmol/l |
|
K | 4.0 mmol/l |
|
Cl | 104 mmol/l |
Discussion
Various irAEs have been reported for Nivo and other
ICIs considering their utility for the treatment of various
cancers, including non-small cell lung cancer. In Japan, ICIs were
first approved for malignant melanoma. Given the expected increase
in opportunities for the use of ICIs, the incidence of grade ≥3
diarrhea/colitis as a serious irAE has been predicted to increase
accordingly. In particular, anti-CTLA-4 antibody preparations have
been associated with a high incidence of severe diarrhea/colitis
and possible poor outcomes, such as long-term hospitalization and
death (13-15). In contrast, anti-PD-1 and anti-PD-L1 antibody
preparations have been associated with relatively lower incidences
of severe diarrhea/colitis (16-19).
Berman et al (20) reported that IPI-induced
diarrhea/colitis can be attributable to the dysregulation of
gastrointestinal mucosal immunity. Although the aforementioned
study did not describe anti-PD-1 and anti-PD-L1 antibody
preparations, these classes of ICIs presumably alter intestinal
immunity in a manner similar to anti-CTLA-4 antibodies.
One study showed that tumor necrosis factor-α
(TNF-α) was elevated in PD-1-knockout irAE model mice (21). While the anti-TNF-α antibody
preparation IFX, a standard therapeutic agent for common ulcerative
colitis, has been suggested as an effective treatment for irAEs, no
specific IFX doses and dosing intervals have been recommended for
this. Moreover, many reports have used IFX for enteritis in
accordance with the treatment of ulcerative colitis (3-11).
Pagès et al (6) proposed
early administration of IFX 5-10 mg/kg/day when systemic steroid
treatment failed to produce any appreciable symptomatic improvement
2/3 days after the onset of IPI-induced irAEs. In the present
study, seven patients with grade 3 diarrhea/colitis received a
single or multiple doses of IFX 5.0 mg/kg/day according to the
dosage and administration for ulcerative colitis. The median
interval between systemic steroid administration and the first IFX
dose was 9 (range, 2-39) days. However, we cannot exclude the
possibility that such a long interval before initiating IFX
administration affected treatment outcomes. Patients included in
this study, who received multiple IFX doses, presented differing
IFX dosing intervals given that the second and subsequent doses
were provided according to relapse severity or prolonged symptoms.
In the three responders to IFX, the effect appeared at a median of
18 (range, 9-32) days after IFX administration, which maintained
diarrhea/colitis at grade 0 for at least 7 consecutive days. This
finding suggests that IFX requires certain duration to produce its
efficacy. Case nos. 6 and 7 experienced rapid deterioration of
their general condition after IFX administration and ultimately
died before IFX could show any beneficial effects. However, whether
IFX administration had any causal relationship with the deaths
remains unclear. Careful consideration may be warranted before IFX
administration, particularly among patients showing rapid
deterioration of the general condition.
Johnson et al (11) reported that severe diarrhea/colitis
as an irAE improved in 72% (26/36) patients after a single dose of
IFX, in 22% (8/36) after two doses, and in 6% (2/36) after three
doses. Furthermore, Soularue et al (1) pointed out that severe diarrhea/colitis
as an irAE exhibits many clinical similarities with ulcerative
colitis but rarely develops into chronic autoimmune diseases and
that the former is likely to involve transient immune activation.
Case nos. 4 and 5 in the present study received three or more doses
of IFX and required long treatment periods for severe
diarrhea/colitis (107 days and 86 days, respectively).
Other causes of grade ≥2 diarrhea/colitis, including
bacterial and viral enteritis, such as CMV and CD infections, must
be ruled out (22). Accordingly,
none of the eight patients developed any infection concurrent with
irAEs. In case no. 2, however, neither systemic steroid treatment
nor IFX was effective, with the patient ultimately being diagnosed
with concomitant CMV enteritis based on reexamination results for
infectious diseases. After immediate initiation of ganciclovir
infusion, diarrhea improved to grade 0 after 14 days. Franklin
et al (23) reported that
12.2% (5/41) patients with ICI-induced severe diarrhea/colitis were
refractory to immunomodulatory treatment with steroids and IFX,
showed more severe inflammation during colon biopsy, and tested
positive for CMV. Kuo et al (24) detected CD in a case of severe
diarrhea/colitis occurring after IPI administration in which
symptoms improved transiently with steroids and IFX but relapsed
after 1 month. While the mentioned reports do not discuss the
mechanisms underlying CMV or CD infection, administration of the
strong immunosuppressant IFX together with long-term systemic
administration of high-dose steroids might have compromised the
intestinal immunity, rendering the patient susceptible to
opportunistic infections. In this study, patients in whom IFX
exhibited its effects relatively earlier may be less susceptible to
infection owing to the shorter treatment duration. The single dose
of IFX used herein induced a full response only in case no. 1. In
the remaining cases, CMV and CD infections were detected despite
appreciable IFX effects and anti-CMV and antibacterial treatment
for infections was accordingly administered in parallel. These data
suggest that periodic assessment for CMV and CD infections is
necessary when IFX is administered.
In addition, the immunosuppressant cyclosporine
(CyA) and vedolizumab, a humanized α4β7 integrin monoclonal
antibody, are worth considering as third-line treatments for severe
diarrhea/colitis as irAE. Accordingly, Iyoda et al (25) reported that oral CyA 50 mg/body/day
as a third-line treatment for Nivo-induced grade 3 enteritis,
following the unsuccessful first- and second-line treatments with
oral PSL 30-60 mg for 50 days and two doses of IFX 5 mg/kg,
respectively, decreased the frequency of diarrhea after 3 days and
resolved diarrhea after 2 weeks. Moreover, Bergqvist et al
(26) reported that vedolizumab
promoted remission in 6 of the 7 patients with malignant melanoma
or lung cancer who developed IPI- or Nivo-induced enteritis
refractory to steroids and IFX at a median of 56 days after
administration with no related AEs.
According to Postow et al (27) it is possible that immunosuppression
with IFX, steroids, and other agents reduce the antitumor efficacy
of ICIs. They compared the antitumor efficacy of ICIs in patients
who received immunosuppressants for the treatment of irAE with that
in those who did not, and they found no significant reduction
between the two groups; however, they did not eliminate the
possibility of reduction because no prospective studies have been
conducted. Nevertheless, using immunosuppressants for irAE
treatment has been reported to increase the likelihood of
contracting opportunistic infections, as was the case in this
study.
The use of IFX for irAE treatment is not covered by
national health insurance, the guideline on optimal usage issued by
the Ministry of Health, Labour and Welfare of Japan states that
‘when corticosteroids do not improve adverse reactions, the
addition of immunosuppressants other than corticosteroids should be
considered,’ thereby officially recommending the use of
immunosuppressants as needed. Accordingly, attending physicians
administered the immunosuppressants recommended for patient's irAE
according to the ASCO or other guidelines. We retrospectively
included and analyzed cases in which IFX was administered at
discretion of individual attending physicians, with no new
interventions. Therefore, we categorized our study as a
retrospective observational study.
Limitations of this study were its retrospective
design based on electronic medical records and a small number of
patients as it was a single-center study, which predisposes it to
various biases due to insufficient statistical power. Therefore, a
multi-center study involving a larger number of patients is
necessary for more accurate assessments.
In conclusion, early initiation of IFX treatment in
conjunction with systemic steroid therapy should be considered for
severe diarrhea/colitis and other irAEs. However, reevaluation for
possible infections and prompt revision of the treatment strategy,
such as switching to oral CyA or vedolizumab, may be necessary when
irAEs do not respond to steroids/IFX.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Through research conferences, MT, TY, KF, TIn, SO,
YN, SY, TO, RI, TIs, TK, KN and FI established comprehensive
research goals and aims, and developed and designed methodologies.
TIn, SO, YN, SY, TO, RI, TIs, TK and KN provided patient samples.
YK, MT, TY, KF, TIn, SO, YN, SY, TO, RI, TIs, TK, KN and FI
performed experiments, collected data and conducted administrative
activities to maintain the survey data. YK and AT conducted data
analysis. YK drafted the manuscript with help from AT. TIn, SO, YN,
SY, TO and KN assessed the authenticity of all the raw data to
ensure its legitimacy. MT, TY, KF, TIn, SO, YN, TO, SY, RI, TIs,
TK, KN and FI conducted critical reviews, visualizations and edits.
AT, KF and FI were responsible for oversight and leadership in
planning and carrying out research activities. All authors agreed
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted with approval from
the Ethical Review Board of the Osaka International Cancer
Institute (Osaka, Japan) and in accordance with ethical guidelines
on clinical research (approval no. 19086). Due consideration was
given toward protecting personal information, with data being
handled after anonymization. Information on the present study is
available at the institution's website. The patients could withdraw
their consent for participation at any period throughout the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soularue E, Lepage P, Colombel JF, Coutzac
C, Faleck D, Marthey L, Collins M, Chaput N, Robert C and Carbonnel
F: Enterocolitis due to immune checkpoint inhibitors: A systematic
review. Gut. 67:2056–2067. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Minor DR, Chin K and Kashani-Sabet M:
Infliximab in the treatment of anti-CTLA4 antibody (Ipilimumab)
induced immune-related colitis. Cancer Biother Radiopharm.
24:321–325. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Beck KE, Blansfield JA, Tran KQ, Feldman
AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM,
Kleiner D, et al: Enterocolitis in patients with cancer after
antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J
Clin Oncol. 24:2283–2289. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hillock NT, Heard S, Kichenadasse G, Hill
CL and Andrews J: Infliximab for ipilimumab-induced colitis: A
series of 13 patients. Asia Pac J Clin Oncol. 13:e284–e290.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pagès C, Gornet JM, Monsel G, Allez M,
Bertheau P, Bagot M, Lebbé C and Viguier M: Ipilimumab-Induced
acute severe colitis treated by infliximab. Melanoma Res.
23:227–230. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Geier M, Gouva S and Geier B:
Enterocolitis due to nivolumab treated by infliximab. ASGIS.
2:14–16. 2019.
|
|
8
|
Yanai S, Nakamura S and Matsumoto T:
Nivolumab-induced colitis treated by infliximab. Clin Gastroenterol
Hepatol. 15:e80–e81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nassri AB, Muenyi V, Al-Khasawneh A, De
Souza Ribeiro B, Scolapio JS, Malespin M and de Melo SW Jr:
Ipilimumab and nivolumab induced steroid-refractory colitis treated
with infliximab: A case report. World J Gastroint Pharmacol Ther.
10:29–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Delasos L, Desai A, Lia NL, Kethireddy N
and Ray C: A case of immunotherapy-induced colitis complicated by
perforation and treated with infliximab postoperatively. Case Rep
Oncol Med. 2019(9069354)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Johnson DH, Zobniw CM, Trinh VA, Ma J,
Bassett RL Jr, Abdel-Wahab N, Anderson J, Davis JE, Joseph J,
Uemura M, et al: Infliximab associated with faster symptom
resolution compared with corticosteroids alone for the management
of immune-related enterocolitis. J Immunother Cancer.
6(103)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Perez-Ruiz E, Minute L, Otano I, Alvarez
M, Ochoa MC, Belsue V, de Andrea C, Rodriguez-Ruiz ME, Perez-Gracia
JL, Marquez-Rodas I, et al: Prophylactic TNF blockade uncouples
efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy.
Nature. 569:428–432. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grobb JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gupta A, De Felice KM, Loftus EV Jr and
Khanna S: Systematic review: Colitis associated with anti-CTLA-4
therapy. Aliment Pharmacol Ther. 42:406–417. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-Programmed-Death-Receptor-1 treatment with pembrolizumab
in Ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Berman D, Parker SM, Siegel J, Chasalow
SD, Weber J, Galbraith S, Targan SR and Wang HL: Blockade of
cytotoxic T-lymphocyte antigen-4 by ipilimumab results in
dysregulation of gastrointestinal immunity in patients with
advanced melanoma. Cancer Immun. 24:10–11. 2010.PubMed/NCBI
|
|
21
|
Liu J, Blake SJ, Harjunpää H, Fairfax KA,
Yong MC, Allen S, Kohrt HE, Takeda K, Smyth MJ and Teng MW:
Assessing immune-related adverse events of efficacious combination
immunotherapies in preclinical models of cancer. Cancer Res.
76:5288–5301. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Weber JS, Kähler KC and Hauschild A:
Management of immune-related adverse events and kinetics of
response with ipilimumab. J Clin Oncol. 30:2691–2697.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Franklin C, Rooms I, Fiedler M, Reis H,
Milsch L, Herz S, Livingstone E, Zimmer L, Schmid KW, Dittmer U, et
al: Cytomegalovirus reactivation in patients with refractory
checkpoint inhibitor-induced colitis. Eur J Cancer. 86:248–256.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kuo JR, Davis AD, Rodriguez EA, Vela MF,
Heigh RI, Salomao MA and Gurudu SR: Severe diarrhea in the setting
of immune checkpoint inhibitors. Case Rep Gastroenterol.
12:704–708. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Iyoda T, Kurita N, Takada A, Watanabe H
and Ando M: Resolution of infliximab-refractory nivolumab-induced
acute severe enterocolitis after cyclosporine treatment in a
patient with non-small cell lung cancer. Am J Case Rep. 27:360–364.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bergqvist V, Hertervig E, Gedeon P,
Kopljar M, Griph H, Kinhult S, Carneiro A and Marsal J: Vedolizumab
treatment for immune checkpoint inhibitor-induced enterocolitis.
Cancer Immunol Immunother. 66:581–592. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Postow MA, Sidlow R and Hellmann MD:
Immune-Related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|