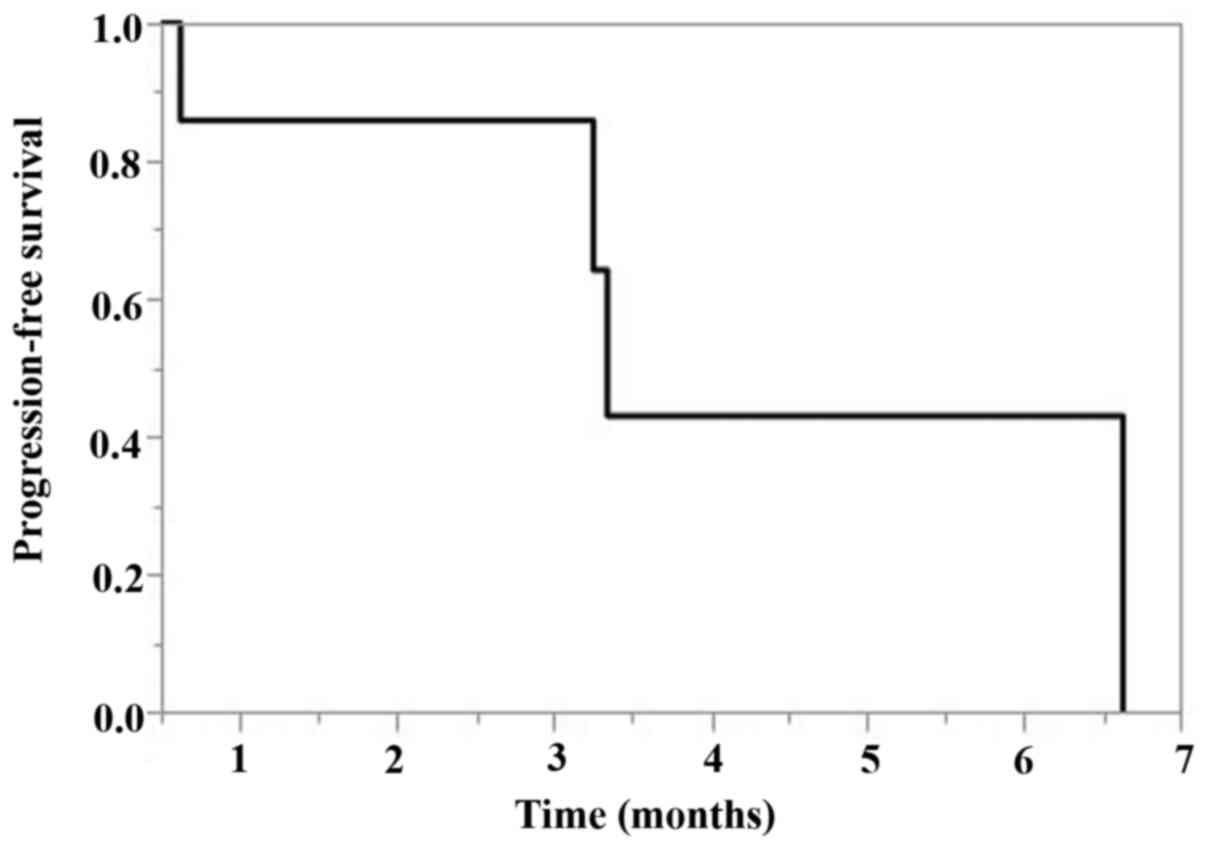
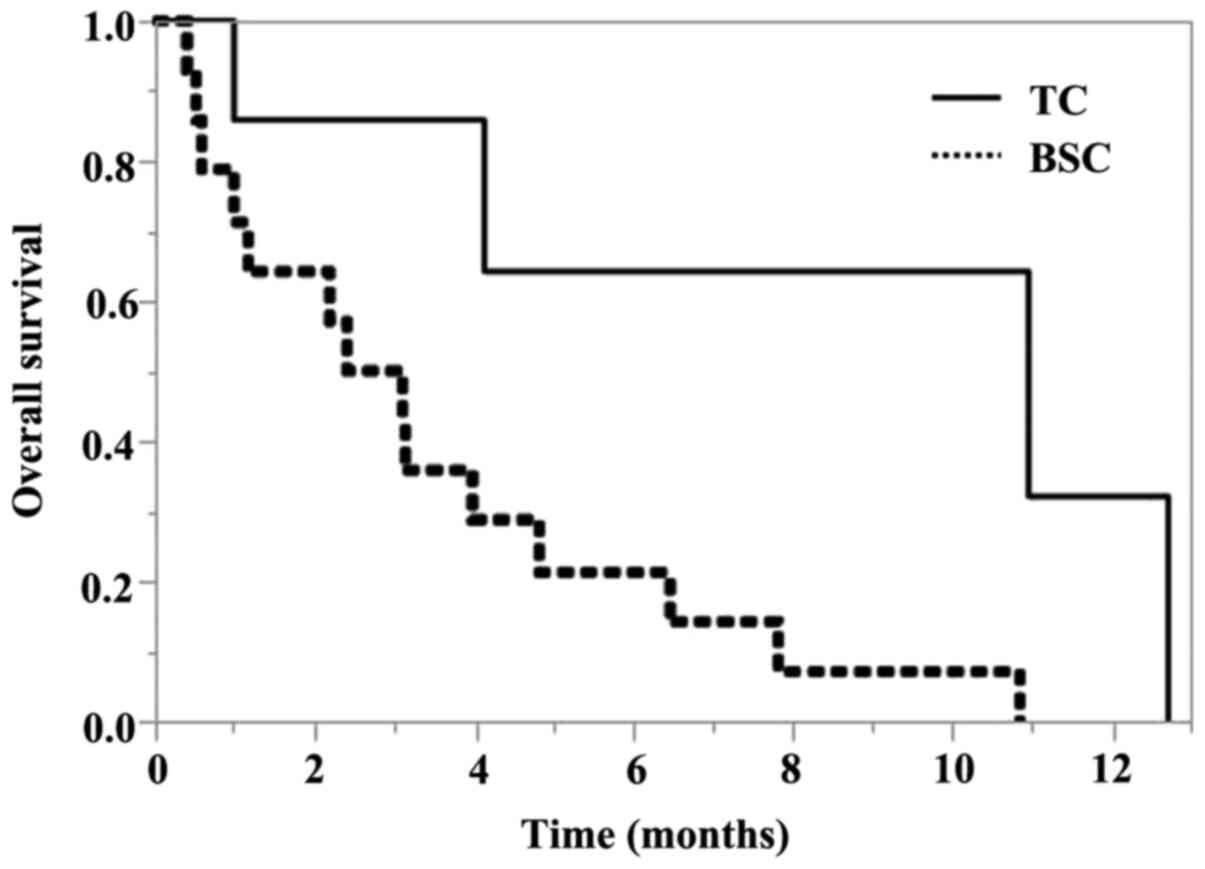
|
1
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A,
Karyakin O, et al: Phase III trial of vinflunine plus best
supportive care compared with best supportive care alone after a
platinum-containing regimen in patients with advanced transitional
cell carcinoma of the urothelial tract. J Clin Oncol. 27:4454–4461.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Loehrer PJ Sr, Einhorn LH, Elson PJ,
Crawford ED, Kuebler P, Tannock I, Raghavan D, Stuart-Harris R,
Sarosdy MF, Lowe BA, et al: A randomized comparison of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 10:1066–1073.
1992.PubMed/NCBI View Article : Google Scholar
|
|
3
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pronzato P, Vigani A, Pensa F, Vanoli M,
Tani F and Vaira F: Second line chemotherapy with ifosfamide as
outpatient treatment for advanced bladder cancer. Am J Clin Oncol.
20:519–521. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vaughn DJ, Broome CM, Hussain M, Gutheil
JC and Markowitz AB: Phase II trial of weekly paclitaxel in
patients with previously treated advanced urothelial cancer. J Clin
Oncol. 20:937–940. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yuasa T, Urakami S and Yonese J: Recent
advances in medical therapy for metastatic urothelial cancer. Int J
Clin Oncol. 23:599–607. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
Last Updated September 21, 2020.
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL,
Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi
A, et al: Randomized phase III KEYNOTE-045 trial of pembrolizumab
versus paclitaxel, docetaxel, or vinflunine in recurrent advanced
urothelial cancer: Results of >2 years of follow-up. Ann Oncol.
30:970–976. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Furubayashi N, Negishi T, Yamashita T,
Kusano S, Taguchi K, Shimokawa M and Nakamura M: The combination of
paclitaxel and carboplatin as second-line chemotherapy can be a
preferred regimen for patients with urothelial carcinoma after the
failure of gemcitabine and cisplatin chemotherapy. Mol Clin Oncol.
7:1112–1118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antonia SJ, Mirza N, Fricke I, Chiappori
A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, et
al: Combination of p53 cancer vaccine with chemotherapy in patients
with extensive stage small cell lung cancer. Clin. Cancer Res.
12:878–887. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Radfar S, Wang Y and Khong HT: Activated
CD4+ T cells dramatically enhance chemotherapeutic tumor
responses in vitro and in vivo. J Immunol. 183:6800–6807.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chiappori AA, Soliman H, Janssen WE,
Antonia SJ and Gabrilovich DI: INGN-225: A dendritic cell-based p53
vaccine (Ad.p53-DC) in small cell lung cancer: Observed association
between immune response and enhanced chemotherapy effect. Expert
Opin Biol Ther. 10:983–991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ramakrishnan R, Antonia S and Gabrilovich
DI: Combined modality immunotherapy and chemotherapy: A new
perspective. Cancer Immunol Immunother. 57:1523–1529.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gribben JG, Ryan DP, Boyajian R, Urban RG,
Hedley ML, Beach K, Nealon P, Matulonis U, Campos S, Gilligan TD,
et al: Unexpected association between induction of immunity to the
universal tumor antigen CYP1B1 and response to next therapy. Clin
Cancer Res. 11:4430–4436. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wheeler CJ, Das A, Liu G, Yu JS and Black
KL: Clinical responsiveness of glioblastoma multiforme to
chemotherapy after vaccination. Clin Cancer Res. 10:5316–5326.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schvartsman G, Peng SA, Bis G, Lee JJ,
Benveniste MFK, Zhang J, Roarty EB, Lacerda L, Swisher S, Heymach
JV, et al: Response rates to single-agent chemotherapy after
exposure to immune checkpoint inhibitors in advanced non-small cell
lung cancer. Lung Cancer. 112:90–95. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Szabados B, van Dijk N, Tang YZ, van der
Heijden MS, Wimalasingham A, Gomez de Liano A, Chowdhury S, Hughes
S, Rudman S, Linch M and Powles T: Response rate to chemotherapy
after immune checkpoint inhibition in metastatic urothelial cancer.
Eur Urol. 73:149–152. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sonpavde G, Sternberg CN, Rosenberg JE,
Hahn NM, Galsky MD and Vogelzang NJ: Second-line systemic therapy
and emerging drugs for metastatic transitional-cell carcinoma of
the urothelium. Lancet Oncol. 11:861–870. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Osa A, Uenami T, Naito Y, Hirata H, Koyama
S, Takimoto T, Shiroyama T, Futami S, Nakatsubo S, Sawa N, et al:
Monitoring antibody binding to T cells in a pembrolizumab-treated
patient with lung adenocarcinoma on hemodialysis. Thorac Cancer.
10:2183–2187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
National Comprehensive Cancer Network:
Guidelines on bladder cancer. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
Accessed Jun 1, 2020.
|
|
26
|
Loriot Y, Necchi A, Park SH, Garcia-Donas
J, Huddart R, Burgess E, Fleming M, Rezazadeh A, Mellado B,
Varlamov S, et al: Erdafitinib in locally advanced or metastatic
urothelial carcinoma. N Engl J Med. 381:338–348. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rosenberg JE, O'Donnell PH, Balar AV,
McGregor BA, Heath EI, Yu EY, Galsky MD, Hahn NM, Gartner EM,
Pinelli JM, et al: Pivotal trial of enfortumab vedotin in
urothelial carcinoma after platinum and anti-programmed death
1/programmed death ligand 1 therapy. J Clin Oncol. 37:2592–2600.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roth BJ, Dreicer R, Einhorn LH, Neuberg D,
Johnson DH, Smith JL, Hudes GR, Schultz SM and Loehrer PJ:
Significant activity of paclitaxel in advanced transitional-cell
carcinoma of the urothelium: A phase II trial of the Eastern
cooperative oncology group. J Clin Oncol. 12:2264–2270.
1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burch PA, Richardson RL, Cha SS, Sargent
DJ, Pitot HC IV, Kaur JS and Camoriano JK: Phase II study of
paclitaxel and cisplatin for advanced urothelial cancer. J Urol.
164:1538–1542. 2000.PubMed/NCBI
|
|
30
|
Vaishampayan UN, Faulkner JR, Small EJ,
Redman BG, Keiser WL, Petrylak DP and Crawford ED: Phase II trial
of carboplatin and paclitaxel in cisplatin-pretreated advanced
transitional cell carcinoma: A Southwest oncology group study.
Cancer. 104:1627–1632. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Soga N, Onishi T, Arima K and Sugimura Y:
Paclitaxel carboplatin chemotherapy as a second-line chemotherapy
for advanced platinum resistant urothelial cancer in Japanese
cases. Int J Urol. 14:828–832. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Esteban-Fernández D, Verdaguer JM,
Ramírez-Camacho R, Palacios MA and Gómez-Gómez MM: Accumulation,
fractionation, and analysis of platinum in toxicologically affected
tissues after cisplatin, oxaliplatin, and carboplatin
administration. J Anal Toxicol. 32:140–146. 2008.PubMed/NCBI View Article : Google Scholar
|