Introduction
Locally advanced or metastatic urothelial carcinoma
(UC) is an incurable disease with a poor long-term survival
(1). Until recently, cytotoxic
chemotherapy was the only treatment available for advanced UC, and
platinum-based chemotherapy had been the gold-standard treatment
(2,3). However, platinum resistance develops
rapidly, and nearly 80% of cases will relapse. The prognosis is
extremely poor after failure of platinum-based chemotherapy.
Second-line chemotherapy regimens with various single agents and
combinations of agents have been reported, but the prognosis is
extremely poor (1,4,5).
Pembrolizumab, a humanized monoclonal antibody
targeting programmed death receptor-1 (PD-1), was a second-line
treatment for platinum-refractory patients with significantly
longer overall survival (approximately 3 months) and a lower
incidence of treatment-related adverse events in comparison to
chemotherapy in the KEYNOTE-045 phase III trial (6). This open-label, international, phase 3
trial also showed that the objective response rate was
significantly higher in the pembrolizumab group (21.1%) than in the
chemotherapy group (11.4%) (P=0.001). Since December 2017,
pembrolizumab has been approved in Japan as a second-line treatment
for radically unresectable UC, that has worsened after chemotherapy
(7). However, the objective
response rate in the pembrolizumab group was not still satisfactory
at 21.1%, so most patients develop progressive disease after
pembrolizumab and choose best support care (BSC) or chemotherapy if
they desire aggressive treatment, as there is no standard care
after platinum-based chemotherapy and pembrolizumab.
However, to the best of our knowledge, there are no
published studies describing the results of paclitaxel and
carboplatin (TC) chemotherapy alone in metastatic UC when
administered to patients with progression during treatment with
platinum-based chemotherapy and antibodies targeting PD-1
(pembrolizumab). We retrospectively compared the outcomes and
toxicities of patients with metastatic UC after failure of
platinum-based chemotherapy and pembrolizumab who selected TC
chemotherapy with those in patients who received BSC.
Patients and methods
Patients and methods
Thirty-six patients received pembrolizumab for
metastatic UC at four institutions from January 2018 to August
2019. Of the 21 patients who progressed after pembrolizumab, 7
received TC chemotherapy (TC group), and 14 selected the BSC (BSC
group). All patients with UC had histopathologically diagnosed and
radiologically confirmed disease progression after platinum-based
chemotherapy and pembrolizumab (8).
The TC regimen, paclitaxel (175 mg/m2) and carboplatin
(area under the curve: 5), was administered intravenously on day 1
every 21 days and was repeated until disease progression or
unacceptable adverse events occurred. Tumors were generally
measured by computed tomography before and after every 2-3 cycles
of TC chemotherapy. Decisions on adverse events were made in
accordance with the Common Terminology Criteria for Adverse Events,
version 5.0(9). The tumor response
was assessed as the best response according to the Response
Evaluation Criteria in Solid Tumors, version 1.1(10).
All of the patients provided their written informed
consent to participate in this study, and the study protocol was
approved by the Ethics Committee of the Kyushu Cancer Center
(Fukuoka, Japan).
Statistical analyses
The statistical analyses were carried out using the
JMP® Pro, version 14.2.0 software package (SAS
Institute, Inc., Cary, NC, USA). The objective response rate is
defined as the proportion of patients who achieve a partial or
complete response to TC chemotherapy. The progression-free survival
(PFS) in the TC group was calculated from the day on which
chemotherapy was started until the date when patients who were
alive and without disease progression or who were lost to follow-up
had their data censored at the time of the final tumor assessment.
In the BSC group, in general no computed tomography findings were
examined after patients selected BSC. Therefore, the PFS was not
calculated in the BSC group. The overall survival (OS) was
calculated from the day that BSC was selected or the day on which
chemotherapy was started until the date of the last follow-up
examination or death from any cause. The Mann-Whitney U test was
used to assess the differences between the BSC and TC groups. The
Kaplan-Meier method was used to evaluate progression-free survival
(PFS) and OS, and the differences between the BSC and TC groups was
determined by the log-rank test. P-values of <0.05 were
considered to indicate statistical significance.
Results
Patient characteristics
The clinical characteristics are listed in Table I. Twenty-one patients were enrolled
in this study (male, n=17, 91%; median age, 70 years; range, 57-85
years). The patients selected BSC or TC chemotherapy after the
failure of platinum-based chemotherapy and pembrolizumab for UC.
Eight patients had bladder UC, seven had upper urinary tract UC,
and six had both types of UC. All patients except one had visceral
metastasis. With regard to the treatment after pembrolizumab, 14
patients (66.7%) selected BSC, and 7 (33.3%) received TC
chemotherapy. The median time from the fist-line chemotherapy
treatment was 13.9 months (95% CI, 12.0-27.2 months). There were no
statistically significant differences in any characteristics
between the BSC and TC chemotherapy groups. Regarding the regimens
administered prior to TC or BSC, 13 patients received gemcitabine +
cisplatin (GC) and pembrolizumab, 2 patients received GC,
methotrexate + vinblastine + doxorubicin + cisplatin (MVAC) and
pembrolizumab, 2 patients received GC, TC, MVAC and pembrolizumab,
2 patients received GC, avelumab and pembrolizumab, 1 patient
received gemcitabine+carboplatin (GCBDCA), GC, gemcitabine +
paclitaxel and pembrolizumab, and 1 patient received GC, GCBDCA, G
and pembrolizumab.
 | Table IClinicopathological characteristics of
patients with urothelial carcinoma (n=21). |
Table I
Clinicopathological characteristics of
patients with urothelial carcinoma (n=21).
| Characteristics | Paclitaxel and
carboplatin chemotherapy (n=7) | Best supportive care
(n=14) | P-value |
|---|
| Sex, male, n (%) | 4(57) | 13(93) | 0.054 |
| Median age (range),
years | 62 (57-79) | 70 (58-85) | 0.108 |
| ECOG PS, n (%) | | | 0.546 |
|
0 | 3(43) | 3(21) | |
|
1 | 1(14) | 4(29) | |
|
≥2 | 3(43) | 7(50) | |
| Primary tumor site, n
(%) | | | 0.874 |
|
Bladder | 2(28) | 6(43) | |
|
Upper
urinary tract | 2(28) | 5(36) | |
|
Bladder +
upper urinary tract | 3(43) | 3(21) | |
| No. of chemotherapy
before pembrolizumab | | | 0.931 |
|
1 | 4(57) | 9(64) | |
|
2 | 2(28) | 2(15) | |
|
3 | 1(14) | 3(21) | |
| Median time from
first-line chemotherapy, months (95% CI) | 11.8 (4.7-31.1) | 15.8 (10.4-31.7) | 0.371 |
| Metastasis sites, n
(%) | | | 0.129 |
|
Lymph nodes
only | 1(14) | 0 (0) | |
|
Visceral
disease | 6(86) | 14(100) | |
The PFS of TC chemotherapy and OS
according to treatments after pembrolizumab
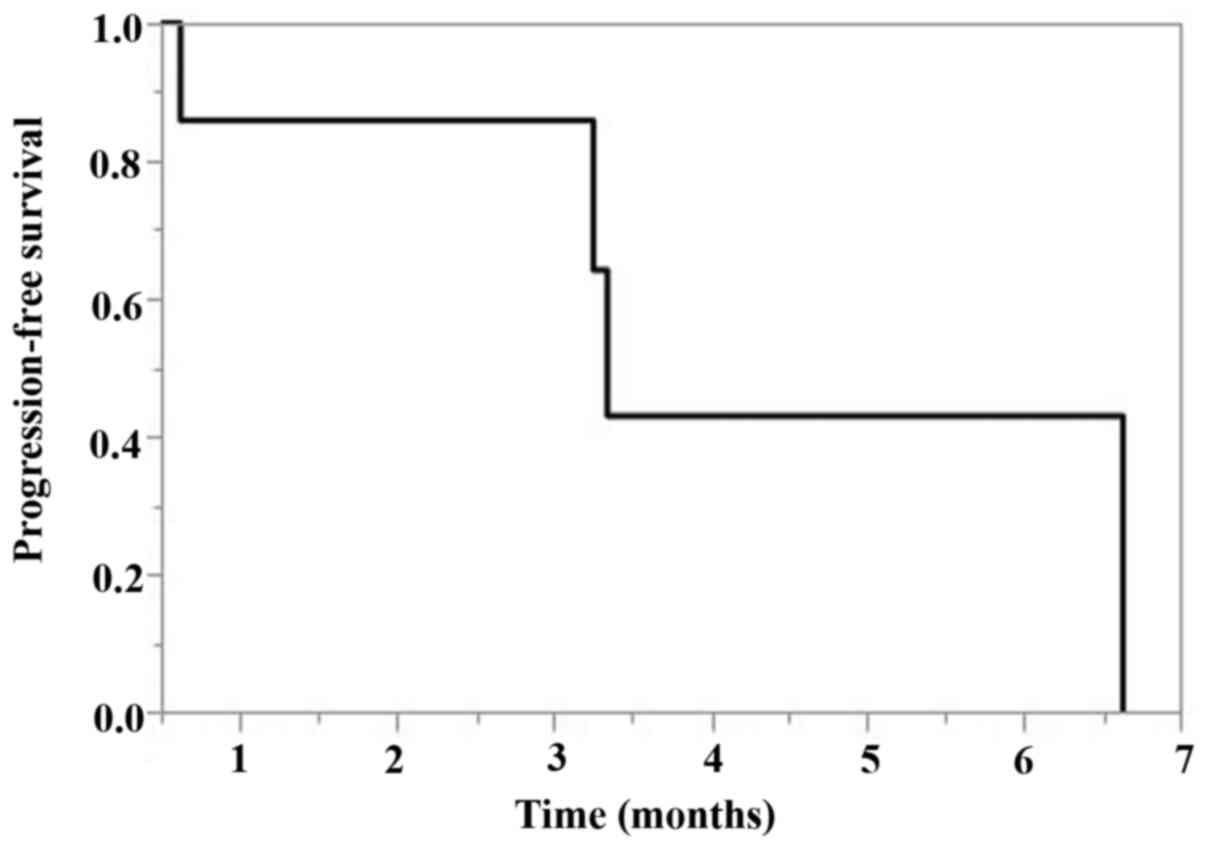
The PFS of TC chemotherapy after pembrolizumab was
3.4 months [95% confidence interval (CI), 0.6-6.6 months] (Fig. 1). The OS according to treatment
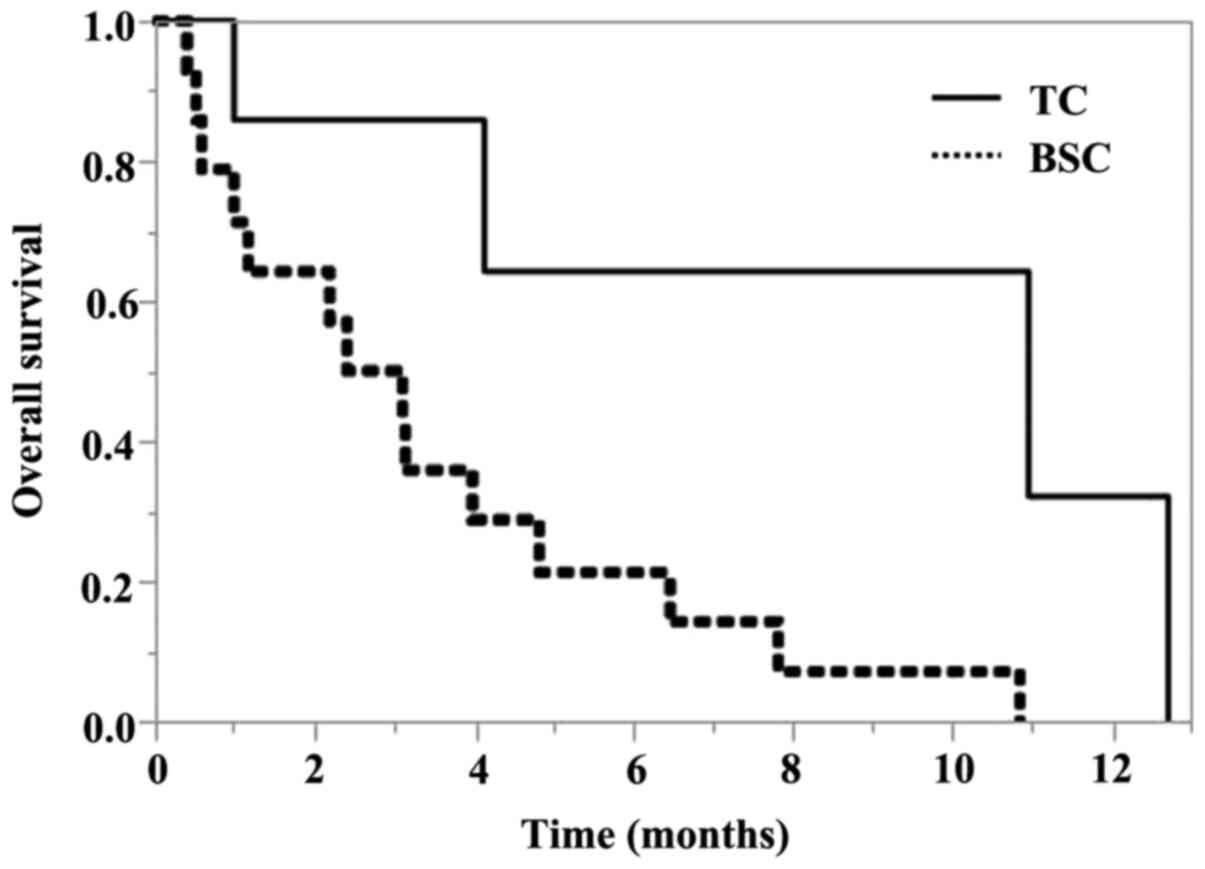
after pembrolizumab is shown in Fig.
2. The median OS for BSC was 2.7 months (95% CI, 0.6-4.8
months), and the median OS for TC was 10.9 months (95% CI, 0.9-12.7
months). The estimated OS rate was 75% at 6 months and 30% at 12
months in the TC chemotherapy group.
A log-rank test revealed a statistically significant
difference in OS between BSC and TC (P=0.0156).
The response analysis and toxicities
in patients who received TC chemotherapy after pembrolizumab
The objective tumor responses are shown in Table II. Among the 7 patients who
received TC chemotherapy after pembrolizumab, a complete response
(CR) was not confirmed in any patients, while 2 patients (28.6%)
showed a partial response (PR), with an objective response rate of
28.6%. The disease control rate (defined by the achievement of CR,
PR or SD) was 85.7%, which was defined as a tumor response of CR,
PR or stable disease [SD].
 | Table IIAnalysis of the responses of patients
who received paclitaxel and carboplatin chemotherapy (n=7). |
Table II
Analysis of the responses of patients
who received paclitaxel and carboplatin chemotherapy (n=7).
| Response | No. of patients | Response rate,
% |
|---|
| CR | 0 | 0 |
| PR | 2 | 28.6 |
| SD | 4 | 57.1 |
| PD | 1 | 14.3 |
| Overall response
rate (CR + PR) | 2 | 28.6 |
| Disease control
rate (CR + PR + SD) | 6 | 85.7 |
Table III shows
the adverse events associated with TC chemotherapy after
pembrolizumab. Myelosuppression was the most common toxicity. Grade
≥3 thrombocytopenia and neutropenia occurred in 3 patients (42.9%),
while febrile neutropenia was observed in 2 patients (28.6%); no
patients showed severe infection. Grade 3 anemia occurred in 1
patient (14.3%). With regard to non-hematological toxicities, grade
3 fatigue developed in 1 patient. All other toxicities were less
than grade 3 in severity, and no immune-related adverse events
occurred. No treatment-related deaths occurred among the seven
patients.
 | Table IIIToxicities in patients treated with
paclitaxel and carboplatin chemotherapy (n=7). |
Table III
Toxicities in patients treated with
paclitaxel and carboplatin chemotherapy (n=7).
| Adverse events | Grade 1, n | Grade 2, n | Grade 3, n | Grade 4, n | Grade ≥3, % |
|---|
|
Thrombocytopenia | 0 | 1 | 3 | 0 | 42.9 |
| Neutropenia | 0 | 0 | 1 | 2 | 42.9 |
| Febrile
neutropenia | NA | NA | 2 | 0 | 28.6 |
| Anemia | 1 | 1 | 1 | 0 | 14.3 |
| Fatigue | 0 | 1 | 1 | 0 | 14.3 |
| Neuropathy | 1 | 1 | 0 | 0 | 0.0 |
| Rash
maculo-papular | 0 | 1 | 0 | NA | 0.0 |
| Alopecia | 0 | 3 | NA | NA | NA |
Discussion
This study represents the first specific report of
outcomes focusing on TC chemotherapy after the failure of
platinum-based chemotherapy and pembrolizumab for advanced UC
patients. In patients with metastatic UC who had previously been
treated with both platinum-based chemotherapy and pembrolizumab, TC
chemotherapy led to a 28.6% objective response rate, and the OS was
10.9 months. This systematic review of the efficacy of chemotherapy
in the setting after platinum-based chemotherapy and immune
checkpoint inhibitor treatment suggests that anti-PD1 may have a
delayed synergistic effect on subsequent cytotoxic therapy.
There is currently no data on chemotherapy for
advanced UC after pembrolizumab. There is also no data on what is
being done in the post-treatment of pembrolizumab arm in
KEYNOTE-045 study (11). Therefore,
the chemotherapy regimen that has been used in the second-line
setting before pembrolizumab is expected to be able to be
administered to patients who desire aggressive treatment after
pembrolizumab failure. Our previous study reviewed the tolerability
and efficacy of TC therapy as second-line therapy for UC that is
resistant to gemcitabine and cisplatin (GC) as a primary
chemotherapy regimen (12). We
reported that the median OS was 12.7 months (95% CI, 3.1-25.4
months), the objective response rate (CR 6.2%, PR 12.5%) was 18.7%,
and the disease control rate was 56.2% in patients who received TC
chemotherapy as a second-line regimen. In the present study, we
reviewed the tolerability and efficacy of TC chemotherapy after the
failure of platinum-based chemotherapy and pembrolizumab for
advanced UC patients. It revealed that the median OS was 10.9
months (95% CI, 0.9-12.7 months), the objective response rate was
28.6%, and the disease control rate was 85.7% in patients who
received TC chemotherapy after the failure of platinum-based
chemotherapy and pembrolizumab. These data may suggest a lack of
cross-resistance between chemotherapy and immune checkpoint
inhibitors in metastatic UC. In addition, chemotherapy may be more
effective after immune checkpoint inhibitors, even as a third-line
treatment. The safety was virtually the same as in our previous
report, and no new safety signals were recognized. This result also
shows that TC chemotherapy can be safely administered even after
immune checkpoint inhibitors.
Exposure to vaccine-based immunotherapy followed by
chemotherapy has been previously reported to be associated with an
improved response to treatment in other types of cancer (13-18).
Similarly, other reports of patients with advanced tumors have
shown improved response rates and improved survival in patients who
received chemotherapy administered after various vaccine-based
treatment strategies (17,18). Although chemotherapy has
historically been considered immunosuppressive, several mechanisms
have been proposed for the enhancement of tumor immunity with
certain agents, such as increasing neoantigen presentation and cell
recognition, abrogating myeloid-derived suppressor cell and
T-regulatory cell activity and enhancing cross-priming and
promoting anti-tumor CD4+ T-cell phenotype (19). A recent phase 2 randomized trial
report on non-small-cell lung cancer supports there is a positive
interaction between chemotherapy and checkpoint inhibitors. This
trial included 123 patients who were randomized to receive
front-line carboplatin and pemetrexed with or without
pembrolizumab; the combination group showed a favorable objective
response rate (55% vs. 29%) and PFS (13.0 vs. 8.9 months, P=0.01)
(20). In addition, it was also
reported that the confirmed objective response rate to single-agent
chemotherapy after immunotherapy exposure was higher than that
described in historical data from the pre-anti-PD1 era and was
similar to the objective response rate to first-line platinum-based
chemotherapy in non-small-cell lung cancer patients (21). A previous study compared the
response rates of patients with metastatic urothelial carcinoma who
received third-line chemotherapy treatment (after chemotherapy and
immune checkpoint inhibitors) and second-line chemotherapy
treatment (after only immune checkpoint inhibitors) (22). Patients who receive chemotherapy for
the first time after immune checkpoint inhibitors showed a high
response rate to the chemotherapy (64%), which suggests that there
is no cross-resistance between the two classes of agents. The same
appears to apply to patients who have previously received both
chemotherapy and immune checkpoint inhibitors. The chemotherapy
response rate of 21% is in line with the expected results in
patients who have previously failed chemotherapy without a history
of immune checkpoint inhibitor exposure (23). Taken together these results suggest
that the chemotherapy responses are maintained, regardless of the
history of exposure to immune checkpoint inhibitors in cases of
metastatic urothelial carcinoma.
In the present study, the efficacy of TC
chemotherapy after the failure of platinum-based chemotherapy and
pembrolizumab for advanced UC was better than the previously
reported efficacy of TC chemotherapy as a second-line regimen for
advanced UC showing resistance to GC as a first-line chemotherapy
regimen (objective response rate 28.6% vs. 18.7%) (12). These results suggest that synergy
exists between immune checkpoint inhibitors and chemotherapy. In a
previous study, the duration of pembrolizumab, a PD-1-blocking
antibody, in T cells of non-small-cell lung cancer patients was
systematically assessed, and complete binding to T cells was
reportedly lost after approximately 20 to 25 weeks (24). Anti-PD1 also may provide a delayed
synergistic effect on subsequent cytotoxic therapy and may
contribute to improved therapeutic efficacy through the overlap of
circulating anti-PD1 and cytotoxic agents. No standard
subsequent-line therapy after platinum-based chemotherapy and
pembrolizumab has been established in Japan. Erdafitinib (a
tyrosine kinase inhibitor of fibroblast growth factor receptor 1-4)
and enfortumab vedotin (Nectin-4-directed antibody-drug conjugate)
are recommended as the preferred subsequent-line systemic therapy
options according to the National Comprehensive Cancer Network
guidelines (25-27),
but these drugs are not yet approved in Japan. Therefore, we
administer TC chemotherapy as salvage chemotherapy for such
patients. Paclitaxel is an antimitotic spindle drug that promotes
microtubular aggregation and interferes with certain cell
functions, including cell mitosis, transport and motility.
Single-agent paclitaxel was shown to have an overall response rate
of 42% in previously untreated patients with UC (28) and 70% when administered in
combination with cisplatin (29).
Platinum-based agents have also been frequently included in salvage
chemotherapy, which is provided even after the failure of a
platinum-based regimen; the efficacy of this agent against
platinum-resistant disease has been reported (30,31).
However, patients with UC often have an impaired renal function due
to an advanced age, history of platinum-containing chemotherapy,
history of nephrectomy and/or disease-related hydronephrosis.
Carboplatin is a less nephrotoxic and emetogenic platinum compound
than cisplatin (32); thus,
carboplatin is considered a favorable agent for second-line
regimens. Therefore, salvage chemotherapy (TC chemotherapy was
selected in the present study) after progression on platinum-based
chemotherapy and immune checkpoint inhibitors may also be options
for treatment in patients with metastatic UC. We previously
reported the utility of TC chemotherapy as second-line treatment
after the failure of GC chemotherapy (12), and the efficacy of TC chemotherapy
was compared with that of BSC. The present study reported the
efficacy of TC chemotherapy after the failure of GC chemotherapy
and pembrolizumab and compared its efficacy with that of BSC.
Although the present study was a retrospective study, we believe
that comparing the results of TC chemotherapy under similar
conditions will show that TC therapy can be an effective treatment
method even after the failure of GC chemotherapy and
pembrolizumab.
Several limitations associated with the present
study warrant mention. First, we evaluated the clinical practice
data related to the efficacy and tolerability of TC chemotherapy
after the failure of platinum-based chemotherapy and pembrolizumab
for metastatic UC in a retrospective, non-randomized, trial.
Second, the median observation period for the present study was
short at 4.1 months. When comparing OS and BSC, a longer
observation period might be necessary. However, the overall
survival (natural history) after the failure of platinum-based
chemotherapy and pembrolizumab in patients who selected BSC has
rarely been reported. The present study showed that the
progression-free survival was not very long, even if patients
selected TC chemotherapy. Therefore, extending the observation
period is expected to be difficult. Third, the population of the
current study was relatively small population; thus, further
studies are needed to confirm our data in a larger study
population. In the present analysis, in a small study population,
TC chemotherapy achieved a 28.6% objective response rate and the
toxicity profile was tolerable as third-line or beyond treatment in
patients with advanced or metastatic UC who had previously received
platinum-based chemotherapy and pembrolizumab. TC chemotherapy is a
suitable option for patients who desire aggressive treatment after
failure of platinum-based chemotherapy and pembrolizumab in
advanced or metastatic UC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NF, YH and MN conceived and designed the analysis.
All authors acquired the data. FM, TN, TI and MK confirmed the
authenticity of the data and analyzed the data. NF and YH drafted
the manuscript. TT, KK, NT and MN acquired the data, assisted with
statistical analysis, supervised the study and critically revised
the manuscript for important intellectual content. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the National Hospital Organization Kyushu Cancer
Center (approval no. 2014-99). Written informed consent was
provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A,
Karyakin O, et al: Phase III trial of vinflunine plus best
supportive care compared with best supportive care alone after a
platinum-containing regimen in patients with advanced transitional
cell carcinoma of the urothelial tract. J Clin Oncol. 27:4454–4461.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Loehrer PJ Sr, Einhorn LH, Elson PJ,
Crawford ED, Kuebler P, Tannock I, Raghavan D, Stuart-Harris R,
Sarosdy MF, Lowe BA, et al: A randomized comparison of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 10:1066–1073.
1992.PubMed/NCBI View Article : Google Scholar
|
|
3
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pronzato P, Vigani A, Pensa F, Vanoli M,
Tani F and Vaira F: Second line chemotherapy with ifosfamide as
outpatient treatment for advanced bladder cancer. Am J Clin Oncol.
20:519–521. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vaughn DJ, Broome CM, Hussain M, Gutheil
JC and Markowitz AB: Phase II trial of weekly paclitaxel in
patients with previously treated advanced urothelial cancer. J Clin
Oncol. 20:937–940. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yuasa T, Urakami S and Yonese J: Recent
advances in medical therapy for metastatic urothelial cancer. Int J
Clin Oncol. 23:599–607. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
Last Updated September 21, 2020.
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL,
Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi
A, et al: Randomized phase III KEYNOTE-045 trial of pembrolizumab
versus paclitaxel, docetaxel, or vinflunine in recurrent advanced
urothelial cancer: Results of >2 years of follow-up. Ann Oncol.
30:970–976. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Furubayashi N, Negishi T, Yamashita T,
Kusano S, Taguchi K, Shimokawa M and Nakamura M: The combination of
paclitaxel and carboplatin as second-line chemotherapy can be a
preferred regimen for patients with urothelial carcinoma after the
failure of gemcitabine and cisplatin chemotherapy. Mol Clin Oncol.
7:1112–1118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antonia SJ, Mirza N, Fricke I, Chiappori
A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, et
al: Combination of p53 cancer vaccine with chemotherapy in patients
with extensive stage small cell lung cancer. Clin. Cancer Res.
12:878–887. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Radfar S, Wang Y and Khong HT: Activated
CD4+ T cells dramatically enhance chemotherapeutic tumor
responses in vitro and in vivo. J Immunol. 183:6800–6807.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chiappori AA, Soliman H, Janssen WE,
Antonia SJ and Gabrilovich DI: INGN-225: A dendritic cell-based p53
vaccine (Ad.p53-DC) in small cell lung cancer: Observed association
between immune response and enhanced chemotherapy effect. Expert
Opin Biol Ther. 10:983–991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ramakrishnan R, Antonia S and Gabrilovich
DI: Combined modality immunotherapy and chemotherapy: A new
perspective. Cancer Immunol Immunother. 57:1523–1529.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gribben JG, Ryan DP, Boyajian R, Urban RG,
Hedley ML, Beach K, Nealon P, Matulonis U, Campos S, Gilligan TD,
et al: Unexpected association between induction of immunity to the
universal tumor antigen CYP1B1 and response to next therapy. Clin
Cancer Res. 11:4430–4436. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wheeler CJ, Das A, Liu G, Yu JS and Black
KL: Clinical responsiveness of glioblastoma multiforme to
chemotherapy after vaccination. Clin Cancer Res. 10:5316–5326.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schvartsman G, Peng SA, Bis G, Lee JJ,
Benveniste MFK, Zhang J, Roarty EB, Lacerda L, Swisher S, Heymach
JV, et al: Response rates to single-agent chemotherapy after
exposure to immune checkpoint inhibitors in advanced non-small cell
lung cancer. Lung Cancer. 112:90–95. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Szabados B, van Dijk N, Tang YZ, van der
Heijden MS, Wimalasingham A, Gomez de Liano A, Chowdhury S, Hughes
S, Rudman S, Linch M and Powles T: Response rate to chemotherapy
after immune checkpoint inhibition in metastatic urothelial cancer.
Eur Urol. 73:149–152. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sonpavde G, Sternberg CN, Rosenberg JE,
Hahn NM, Galsky MD and Vogelzang NJ: Second-line systemic therapy
and emerging drugs for metastatic transitional-cell carcinoma of
the urothelium. Lancet Oncol. 11:861–870. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Osa A, Uenami T, Naito Y, Hirata H, Koyama
S, Takimoto T, Shiroyama T, Futami S, Nakatsubo S, Sawa N, et al:
Monitoring antibody binding to T cells in a pembrolizumab-treated
patient with lung adenocarcinoma on hemodialysis. Thorac Cancer.
10:2183–2187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
National Comprehensive Cancer Network:
Guidelines on bladder cancer. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
Accessed Jun 1, 2020.
|
|
26
|
Loriot Y, Necchi A, Park SH, Garcia-Donas
J, Huddart R, Burgess E, Fleming M, Rezazadeh A, Mellado B,
Varlamov S, et al: Erdafitinib in locally advanced or metastatic
urothelial carcinoma. N Engl J Med. 381:338–348. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rosenberg JE, O'Donnell PH, Balar AV,
McGregor BA, Heath EI, Yu EY, Galsky MD, Hahn NM, Gartner EM,
Pinelli JM, et al: Pivotal trial of enfortumab vedotin in
urothelial carcinoma after platinum and anti-programmed death
1/programmed death ligand 1 therapy. J Clin Oncol. 37:2592–2600.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roth BJ, Dreicer R, Einhorn LH, Neuberg D,
Johnson DH, Smith JL, Hudes GR, Schultz SM and Loehrer PJ:
Significant activity of paclitaxel in advanced transitional-cell
carcinoma of the urothelium: A phase II trial of the Eastern
cooperative oncology group. J Clin Oncol. 12:2264–2270.
1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burch PA, Richardson RL, Cha SS, Sargent
DJ, Pitot HC IV, Kaur JS and Camoriano JK: Phase II study of
paclitaxel and cisplatin for advanced urothelial cancer. J Urol.
164:1538–1542. 2000.PubMed/NCBI
|
|
30
|
Vaishampayan UN, Faulkner JR, Small EJ,
Redman BG, Keiser WL, Petrylak DP and Crawford ED: Phase II trial
of carboplatin and paclitaxel in cisplatin-pretreated advanced
transitional cell carcinoma: A Southwest oncology group study.
Cancer. 104:1627–1632. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Soga N, Onishi T, Arima K and Sugimura Y:
Paclitaxel carboplatin chemotherapy as a second-line chemotherapy
for advanced platinum resistant urothelial cancer in Japanese
cases. Int J Urol. 14:828–832. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Esteban-Fernández D, Verdaguer JM,
Ramírez-Camacho R, Palacios MA and Gómez-Gómez MM: Accumulation,
fractionation, and analysis of platinum in toxicologically affected
tissues after cisplatin, oxaliplatin, and carboplatin
administration. J Anal Toxicol. 32:140–146. 2008.PubMed/NCBI View Article : Google Scholar
|