Introduction
Oral mucositis affects 40-80% of patients undergoing
chemotherapy and almost all patients undergoing head and neck
radiotherapy (1-3).
Oral mucositis causes dysphagia, dysarthria, and odynophagia and is
a possible gateway for opportunistic infections; therefore, it
leads to a decreased quality of life and is considered an important
non-hematological complication of antitumor treatment (4-8).
Typical macroscopic and microscopic characteristics
of oral mucositis reflect a natural history following radiation
therapy or the chemotherapy cycle, in which the initial wound of an
erythematous plaque evolves into isolated ulcerations that converge
and result in more extensive and deeper wounds, with symptoms
ranging from burning to severe pain (4). The erythematous wound
histopathologically represents epithelial hypoplasia associated
with inflammatory reaction on the underlying lamina propria caused
by an antitumor drug that secondarily inhibits normal epithelial
renewal; the healing phase occurs with the end of the
antineoplastic cycle, showing spontaneous resolution of the ulcers
(7,9-11).
The in situ cytokine production by oral
inflammatory cells influences the establishment and remission of
ulcers. Parkin and Cohen (12)
suggested that the cytokine profile of the lamina propria cells
affects the mitotic and apoptotic activities of oral epithelial
tissue. Notable among these cytokines, are tumor necrosis
factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), and
interferon-gamma (IFN-γ). TNF-α is a pro-inflammatory cytokine that
is primarily produced by activated macrophages. TGF-β enhances the
proliferation of several cells of mesenchymal origin and increases
extracellular matrix synthesis by T-lymphocytes and platelets.
IFN-γ is produced by Th1 lymphocytes and natural killer cells; it
regulates the proliferation and differentiation of various cell
types and has the ability to modulate the immune system (13-15).
Besides cytokines, nitric oxide is one of the ten
smallest molecules existing in nature and is a highly soluble
reactive free radical produced by the NO synthase (NOS) enzyme from
L-arginine. This molecule remains in the tissue for only a few
seconds, but its presence can be indirectly detected through NOS
based on its tissue expression namely eNOS (endothelial), nNOS
(neuronal), and iNOS (inducible) (16-18).
Experimental and clinical studies suggest that both overproduction
and inhibition of NO are associated with greater potential for
tissue damage or maintenance of a chronic response, respectively
(17).
Nitric oxide also has a destructive effect on
invading microorganisms and is therefore released into the inflamed
site by neutrophils and macrophages (19).
Some immunomodulatory drugs have been employed to
control the exacerbation of oral mucositis wounds (20-22).
Among these, pentoxifylline (PTX) is an anti-bleeding and
anti-thrombogenic agent with the ability to inhibit the TNF-α
expression by inhibiting its genetic transcription (23,24).
Atorvastatin has an anti-inflammatory and anti-thrombogenic action
that is capable of reducing C-reactive protein levels and TNF-α
expression in situ (20,25).
Studies conducted over the past decade have also
demonstrated the effectiveness of the anti-inflammatory action of
trans-caryophyllene, a molecule isolated from the copaiba oil-resin
of the Brazilian medicinal plant Copaifera langsdorffii
(25-28).
Although the current control of oral wounds is
non-specific and palliative-preventing their establishment is
critical to cancer prognosis, since severe wounds limit and disrupt
treatment (4,6,29,30).
In our previous study, utilizing the same
experimental design as the current study, we determined the
influence of these drugs on white blood cell counts of animals
undergoing chemotherapy with 5-fluorouracil (5-FU). The results
demonstrated that atorvastatin significantly prevented leukocyte
reduction in comparison to other experimental groups, thus
demonstrating excellent potential for the prevention of leukopenia
(25).
Considering the increase in the number of patients
submitted to chemotherapy and/or radiotherapy treatments for cancer
control and, consequently, sequelae caused by those treatments,
this article discusses the need to control these manifestations
with the objective of improving the quality of life of the
aforementioned patients. The large number of cancer cases makes the
use of such therapies to increase even further, as they are
considered the only way to control the development of the disease.
As a consequence of the use of these therapies, oral mucositis
might appear, which leads to poor nutrition of the patient,
difficulties to swallow and, generally, causes the abandonment of
treatment due to lack of control. Given the fact that there is
little knowledge on the control and prevention of such oral ulcers,
new studies are necessary to unveil information that might be
useful in such situations.
The aim of the present study was to investigate the
preventive potential of pentoxifylline, atorvastatin and
trans-caryophyllene based on histopathological analysis of wounds
in the oral mucosa induced in 5-FU-treated Wistar rats, and to
evaluate their immunomodulatory effect on serum nitrite production,
in situ IFN-γ and TNF-α, and TGF-β and TNF-α in tissues.
Materials and methods
Approval and separation of
animals
The entire experimental protocol was approved by the
Animal Research Committee of the Federal University of Juiz de Fora
(Notion No. 062/2011).
Male Wistar rats (Rattus norvegicus) (N=32)
from the Animal Facility of the Reproductive Biology Center (CBR)
of the Federal University of Juiz de Fora were used for this study,
with an average age of 9 weeks and average body weight of 250 g.
The rats were allowed ad libitum access to food and water.
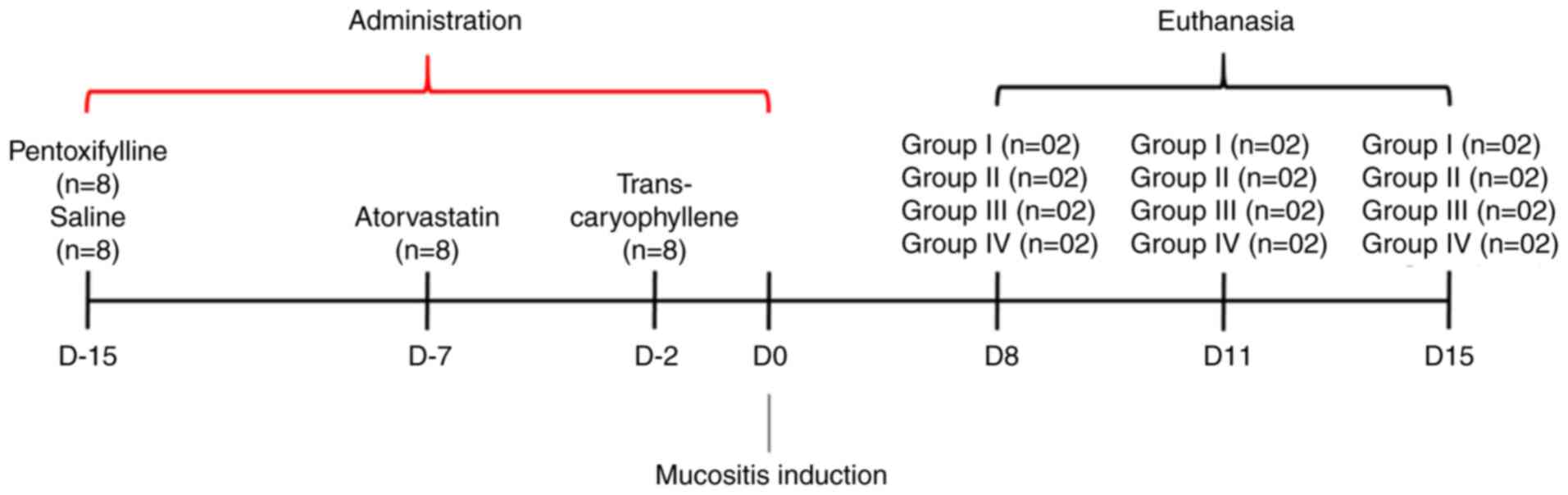
Fifteen days before induction of oral mucositis, the
animals were divided into four groups: Group I-saline-treated
animals with chemotherapy-induced mucositis (n=8), Group
II-trans-caryophyllene-treated animals with chemotherapy-induced
mucositis (n=8), Group III-pentoxifylline-treated animals with
chemotherapy-induced mucositis (n=8), and Group
IV-atorvastatin-treated animals with chemotherapy-induced mucositis
(n=8).
Drug delivery protocol
Trans-caryophyllene gavage administration began two
days before 5-FU treatment and was continued for 2 days after
medication (totalling 9 days of treatment) with a dose of 50 mg/kg
(0.102 ml) (31-33).
Pentoxifylline dissolved in sterile saline of 0.9%
at a concentration of 100 mg/kg/day that was administered
intraperitoneally for 15 consecutive days (34).
Atorvastatin was administered intraperitoneally at
10 mg/kg/day for 1 week before 5-FU treatment (20).
The drug delivery schedule is outlined in Fig. 1.
Induction of oral mucositis
Oral mucositis was induced by the administration of
the chemotherapeutic agent 5-FU (Eurofarma, São Paulo, Brazil) via
intraperitoneal injection on Day 0 (100 mg/kg) and on Day 2 (60
mg/kg) of the experiment (35).
On Days 3 and 4, the mouth's mucosa of 8 pre-treated
animals from each group, after being anesthetized with Ketamine
(100 mg/kg) and Xylazine (10 mg/kg), was bilaterally scarified
twice by the same operator using the tip of a sterile needle
(35,36).
Determination of when the animals
should be sacrificed
The animals were separated into groups to be
euthanized at 8, 11 and 15 days after induction of oral mucositis.
However, clinical evaluation standards were used that could cause
the anticipation of deaths: Marked weight loss, ocular or nasal
hemorrhage, decreased consumption of water and feed, loss of
movement in the cage, demonstration of pain.
Euthanasia method
The method used was deep anestesia with the
combination of Xylazine 10 mg/kg + 100 mg/kg Ketamine, mixed in the
same syringe, intraperitoneally.
The death of the animals was confirmed through
cardiac and respiratory arrest, absence of reflexes (seen through
the hind legs), drop in body temperature.
After the drug is applied, exsanguination is
performed, performed by means of cardiac puncture or large blood
vessels, when rodent serum is obtained.
Sample collection and analysis
After deep anesthesia with Ketamine (100 mg/kg) and
Xylazine (10 mg/kg), via intraperitoneal injection, followed by
euthanasia, the mouth's mucosa was excised.
The schedule of euthanasia, as well as the drug
delivery, is outlined in Fig.
1.
The excised mouth's mucosa was dissected to obtain
the mucositis-affected areas. The samples were immediately fixed in
10% buffered formalin and submitted to routine histological
processing for H&E staining.
The sections were analyzed with a x400 magnification
Zeiss microscope (Hallbergmoos) in three fields of each sample by
an Axion Cam ICC 5 (Zeiss) computer system digital capture
performed by means of a digital camera attached to an optical
microscope. The captured images were processed in an automatic
morphometry Zen 2012 (Blue Edition) program, in which
semi-automatic microscopic field morphometry of leukocytes and
vascular sections was performed on the lamina propria underlying
the epithelial wound.
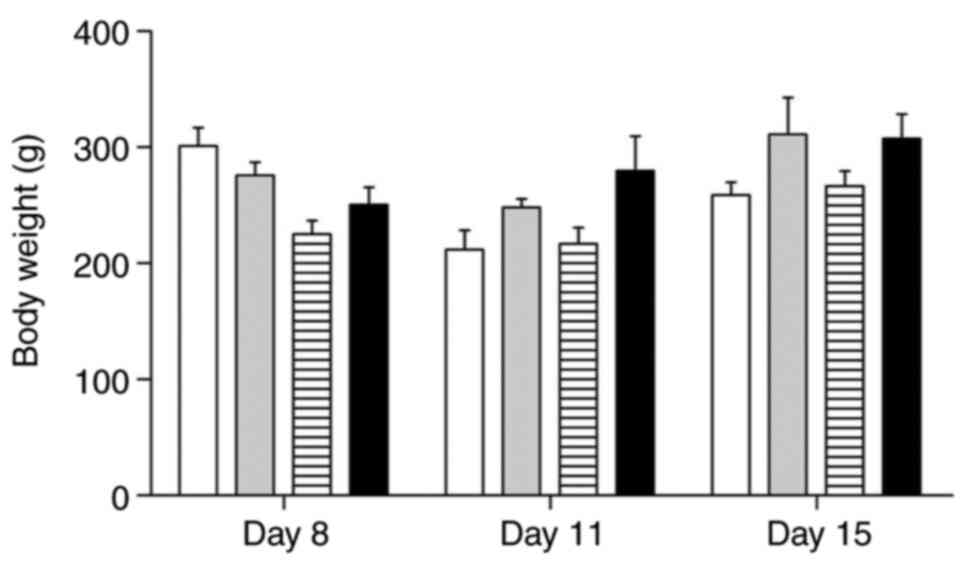
Body weight
Animals should not lose more than 30% of their
initial body weight. For this, both body weight and feed intake
should were evaluated daily. As the animals were controlled daily,
when there was excessive weight loss, they were euthanized.
In situ detection of TNF-α and TGF-β
expression
The Starr Trek Universal HRP Detection System was
used to analyze the expression of TGF-β and TNF-α in the lamina
propria cells that were considered positive by intracytoplasmic
brown pigmentation. Three field counts were averaged, in addition
to total counts for each group.
TNF-α and IFN-γ serum levels
TNF-α and IFN-γ (both from PeproTech Inc.) levels
were examined by the ELISA (Enzyme Linked ImmunoSorbent Assay)
method, which is based on antigen-antibody interactions detected
through enzymatic reactions. In this method, an antigen is bound to
a solid base-the ELISA plate- and is subsequently tested. In the
case of a positive result, an antigen-antibody binding occurs,
which is detected by adding a secondary antibody that targets the
immunoglobulins of the target species (37).
Serum nitrite levels
Serum nitrite was quantified using the Griess
method, by generating a standard curve covering concentrations
between 3.12 and 100 µM. The results were expressed in moles/ml.
The Griess method or test is a chemical reaction that detects the
presence of organic nitrites through its reaction with
sulfanilamide in an acidic medium. To measure nitrite production,
100 µl aliquots of samples were incubated with 100 µl of the Griess
reagent (50 µl 1% sulfanilamide solution and 50 µl 0.1% N-naphthyl
ethylenediamine dihydrochloride solution in 2.5%
H2PO4) at room temperature for 10 min. This
reaction produces a bright red staining compound that can be
estimated from 10 min to 2 h after mixing (38).
Statistical analysis
The data are presented as mean ± standard deviation
of the mean (SEM). For comparison between groups, we used a
Kruskal-Wallis test with Dunn's Multiple Comparison post-hoc test.
In all instances, the significance level was set at 5% (P<0.05).
The analyses were performed with GraphPad Prism version 5.0 for
Windows (GraphPad Software).
Results
Body weight
Although all animals showed weight loss, there was a
significant variation between different groups. The results were
expressed as the mean body weight per group on different days of
the experiment (Fig. 2).
Group I animals showed marked weight loss of around
50% on the 11th day and slight recovery at the end of the
experiment; Group II animals showed slight initial weight loss and
recovery greater than 50% by the end of it; Group III showed weight
loss leading to cachexia on the 11th day and slight recovery until
the 15th day; Group IV animals showed a weight gain greater than
50% on all days of the experiment.
Histopathological and
histomorphometric analysis
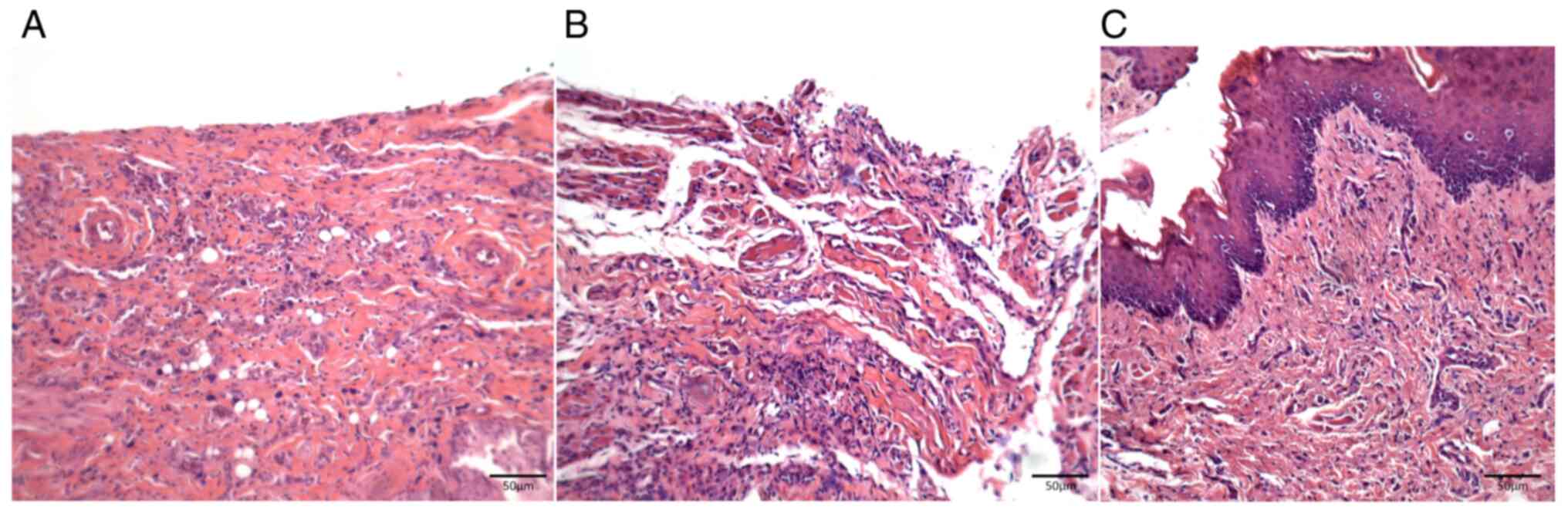
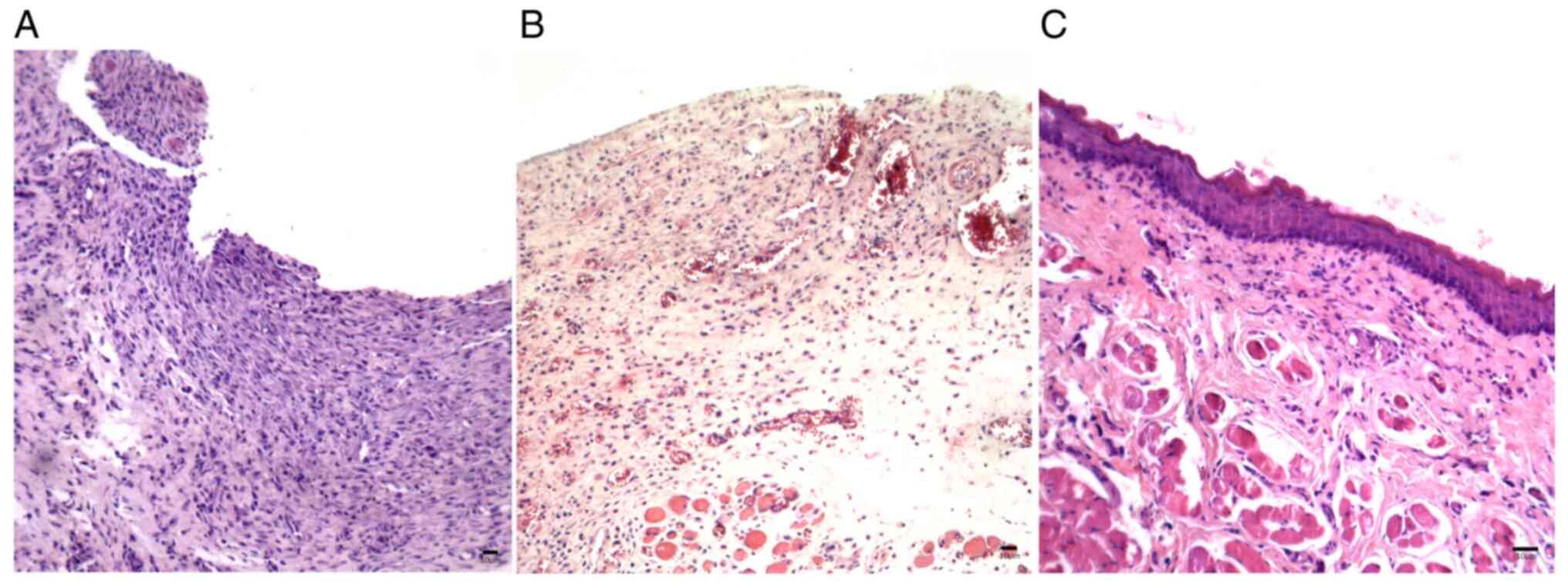
At 8 days after delivery of the chemotherapeutic
drug, samples from the saline-treated control group of animals
(Group I) showed loss of epithelium in the oral mucosa with mild
inflammatory mononuclear infiltrates. At 11 days after chemotherapy
in these animals, the oral wounds progressed to ulcers with severe
diffuse inflammatory infiltrates and at 15 days after chemotherapy,
the wounds showed mild re-epithelialization associated with an
underlying inflammatory infiltrate still present (Fig. 3A-C).
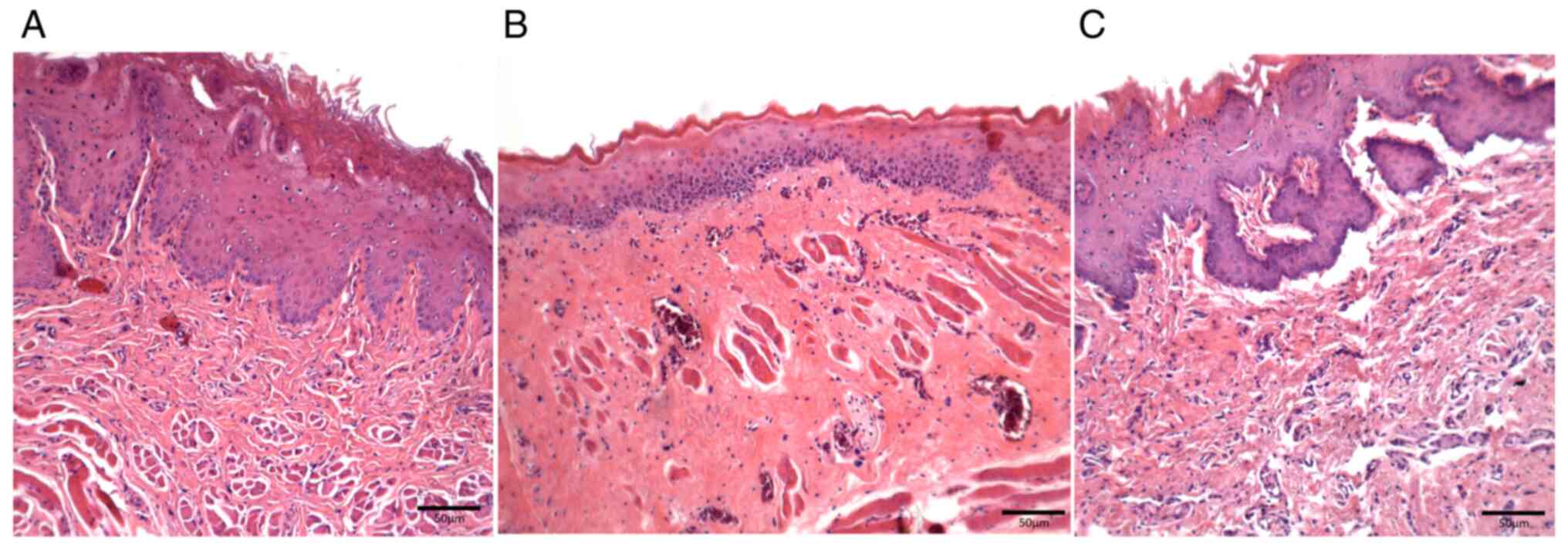
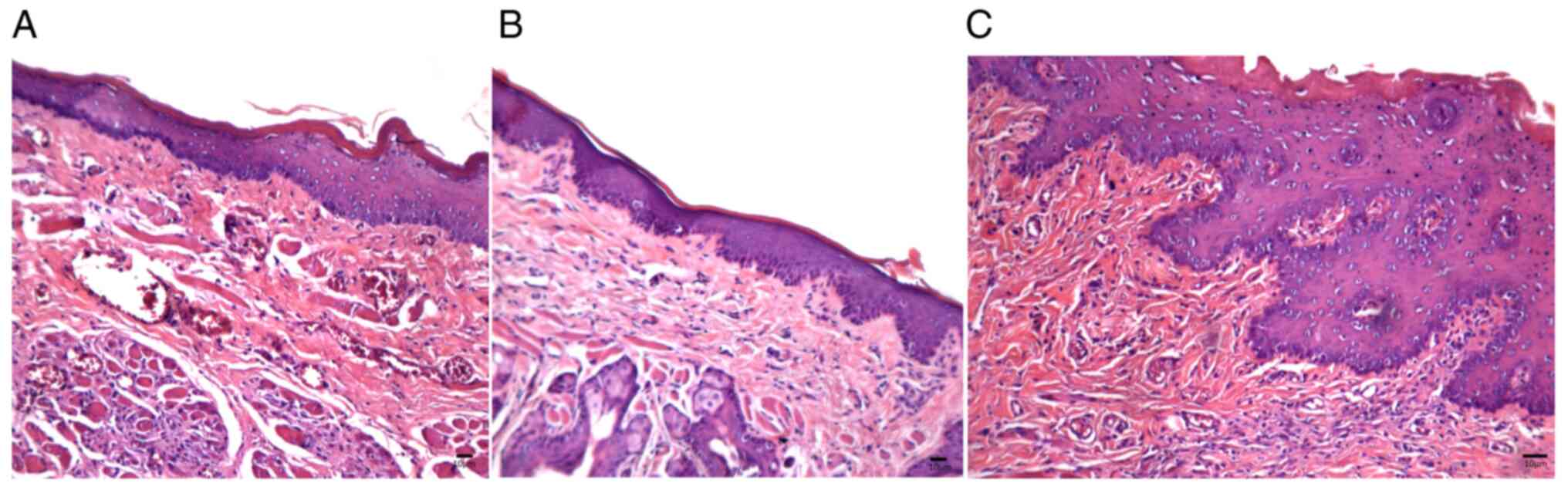
Mucosal samples in trans-caryophyllene-treated
animals (Group II) showed a preserved thin epithelial lining
associated with focal perivascular inflammatory infiltrates on Day
8 and areas with no leukocyte infiltration were occasionally
identified; no inflammation was observed in the samples on Day 11,
and on Day 15 keratinized epithelial restoration was observed.
Moreover, the amount of vascular sections decreased on Day 11 and
showed recovery on Day 15 (Fig.
4A-C).
From Day 8 onwards, samples from the
pentoxifylline-treated animals (Group III) showed early and
complete epithelial loss in oral wounds with severe inflammatory
infiltrates and occasional necrotic areas; on Day 15 slight
re-epithelialization associated with a mild and diffuse
inflammatory infiltrate was observed. The number of blood vessels
decreased in animals from this group on Day 11 before it increased
again on Day 15 (Fig. 5A-C).
On Day 8, samples from atorvastatin-treated animals
(Group IV) displayed no epithelial dissolution, with preserved thin
lining and mild diffuse inflammatory infiltrates; on Days 11 and 15
there was a progressive increase in inflammatory infiltrates, but
with epithelial preservation. Similar to other groups, recovery of
the number of vascular sections was observed on Day 15, after a
decrease on Day 11 (Fig. 6A-C).
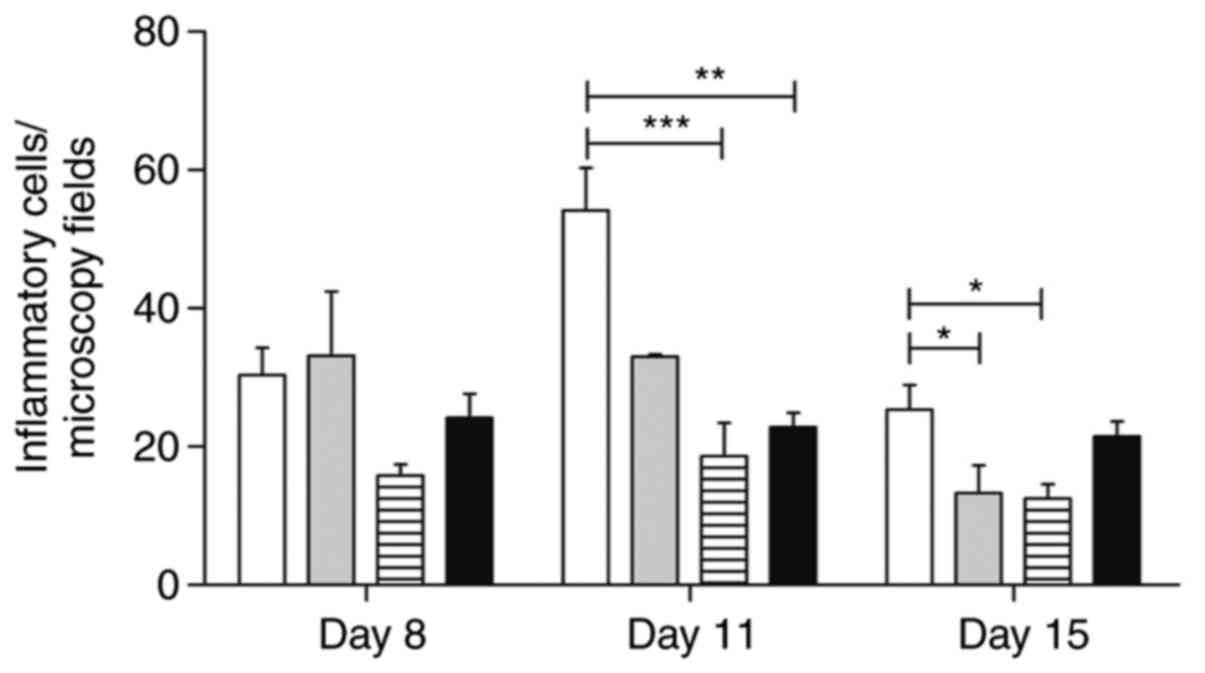
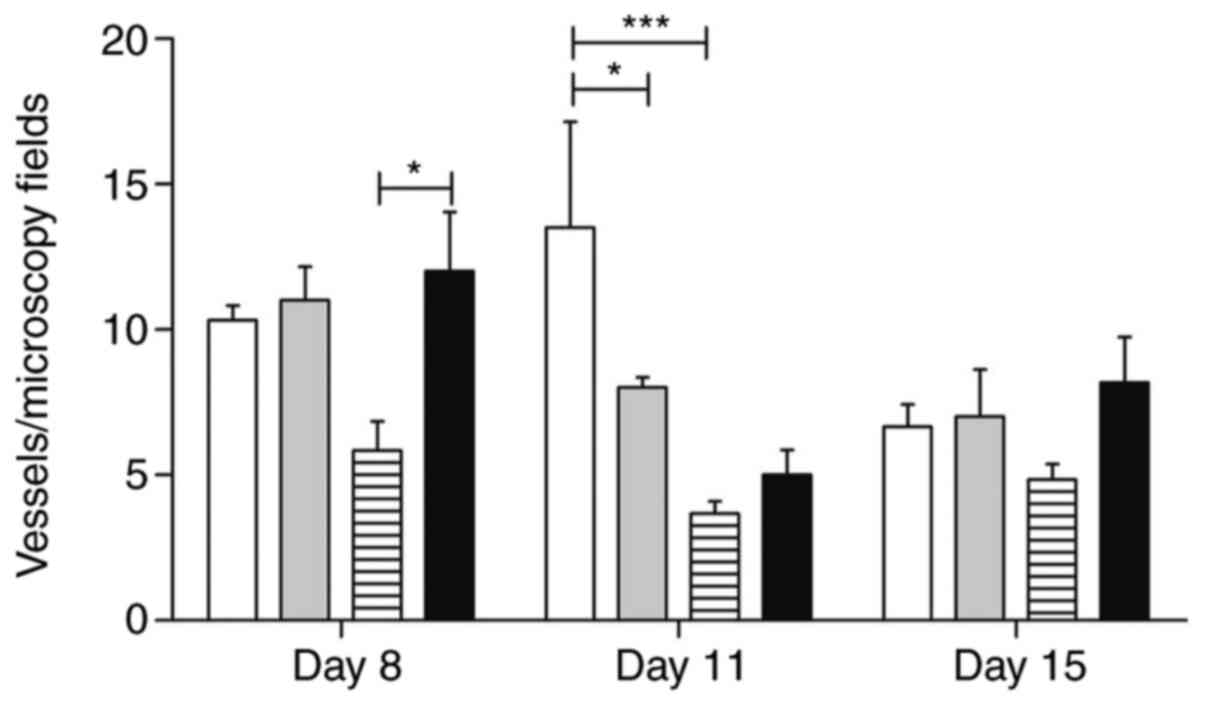
The morphometric analysis of the inflammatory
infiltrates and the wound area vascularization is shown in Figs. 7 and 8.
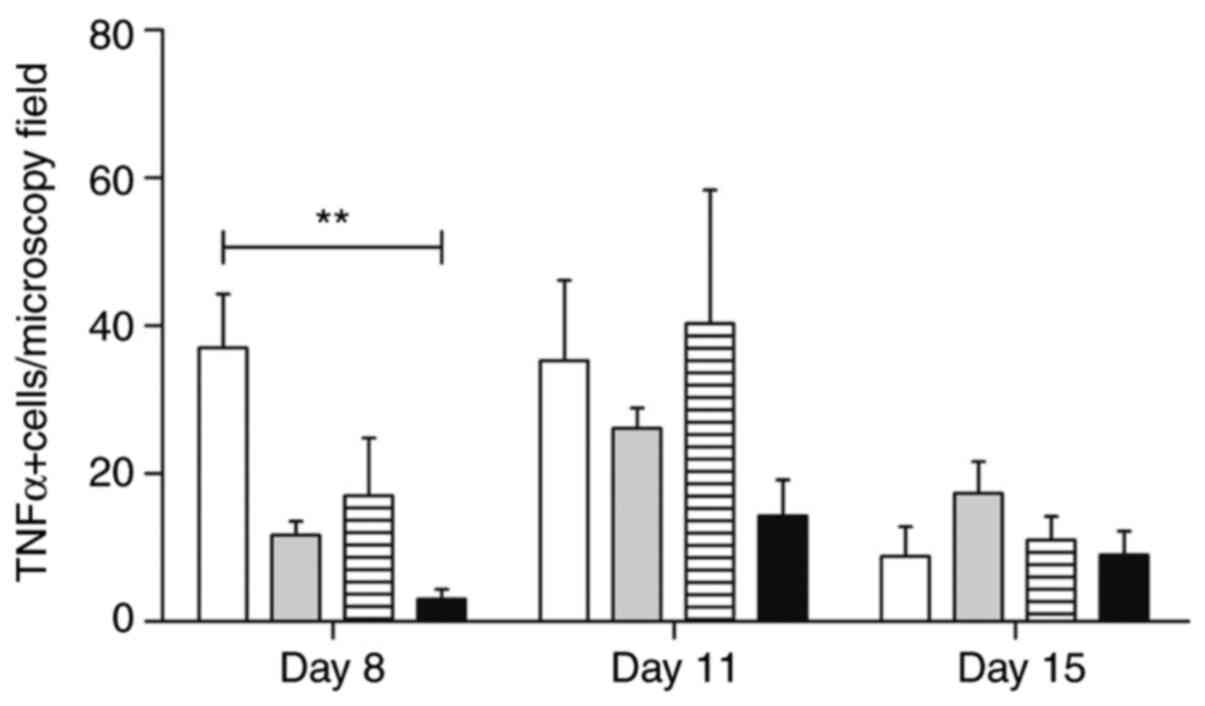
In situ analysis of TNF-α and TGF-β
expression
The analysis of TNF-α expression revealed many
positive mononuclear cells, mainly in samples from
trans-caryophyllene-treated animals (Fig. 9). Atorvastatin negatively modulated
the levels of this cytokine during mucositis development at all
stages (P<0.01). Pentoxifylline-treated animals maintained a
number of positive cells similar to the control group (Fig. 10).
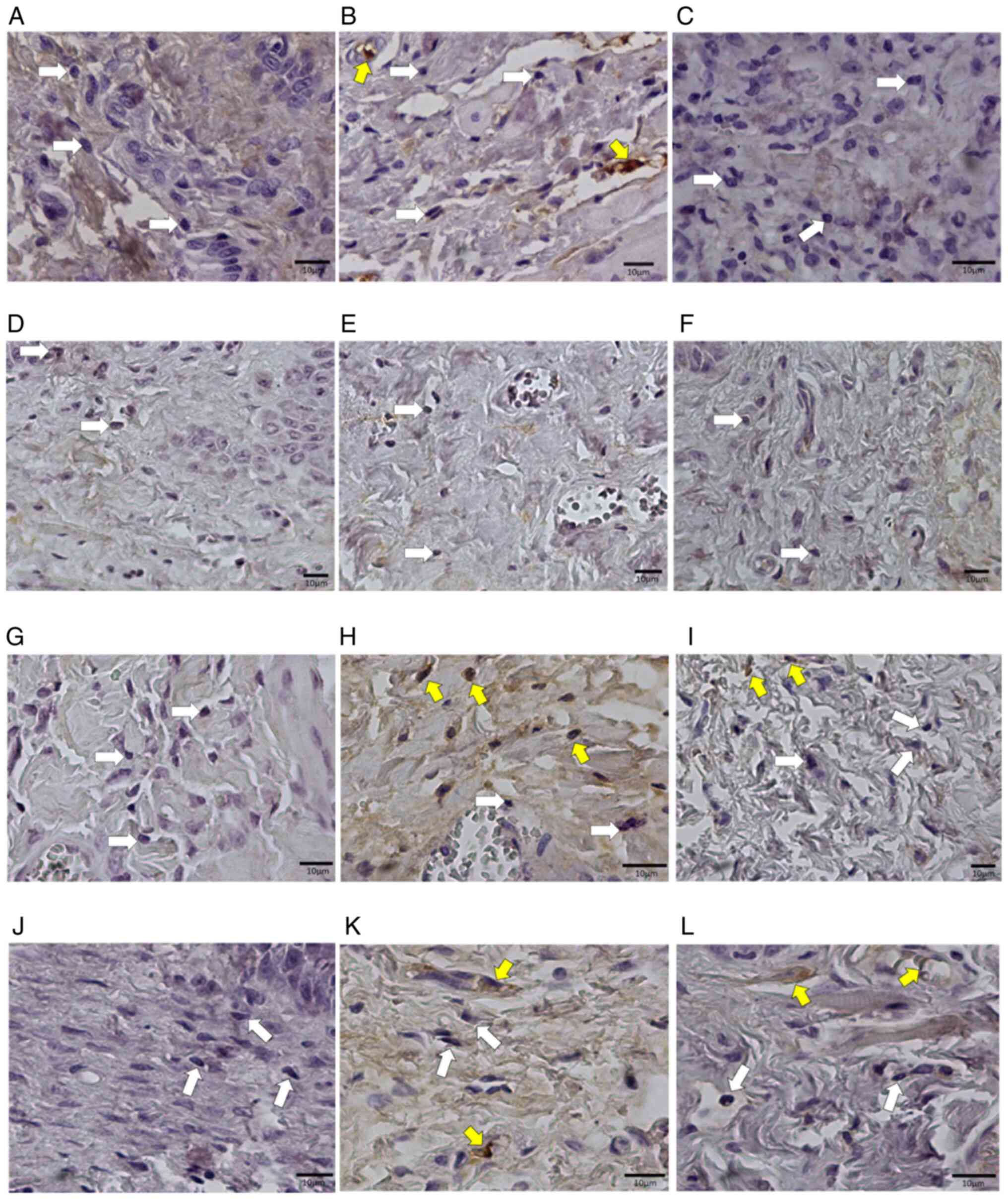 | Figure 9Photomicrographs of histological
sections of oral mucosa samples from Wistar rats subjected to
chemotherapy. (A) Animal in the control group, without medication,
euthanized on day 8. (B) Animal in the control group, without
medication, euthanized on day 11. (C) Animal in the control group,
without medication, euthanized on the 15th day. (D) Animal that
received trans-karyophylene, euthanized on day 8. (E) Animal that
received euthanized trans-karyophylene on day 11. (F) Animal that
received trans-karyophylene, euthanized on the 15th day. (G) Animal
that received pentoxifylline, euthanized on day 8. (H) Animal that
received pentoxifylline, euthanized on day 11. (I) Animal that
received pentoxifylline, euthanized on day 15. (J) Animal that
received atorvastatin, euthanized on the 8th day. (K) Animal that
received atorvastatin, euthanized on the 11th day. (L) Animal that
received atorvastatin, euthanized on day 15. Magnification, x400.
White arrows show negative cells and yellow arrows indicate
positive cells. Anti-TNF-α immunohistochemical reaction. |
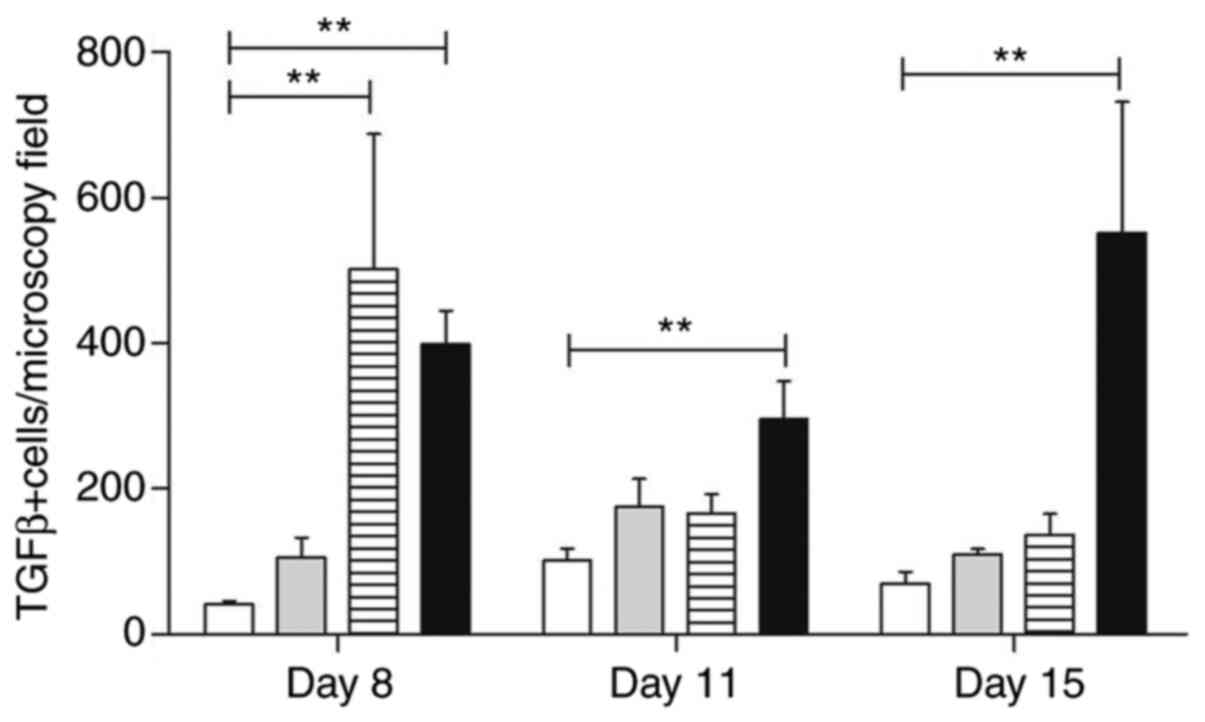
Similarly, the analysis of TGF-β expression in the
lamina propria infiltrating inflammatory cells contained in oral
mucosa samples revealed several positive mononuclear cells,
observed mainly in samples from atorvastatin-treated animals
(Fig. 11). The lowest number of
cells was observed in the group treated with trans-caryophyllene
(P<0.01) (Fig. 12).
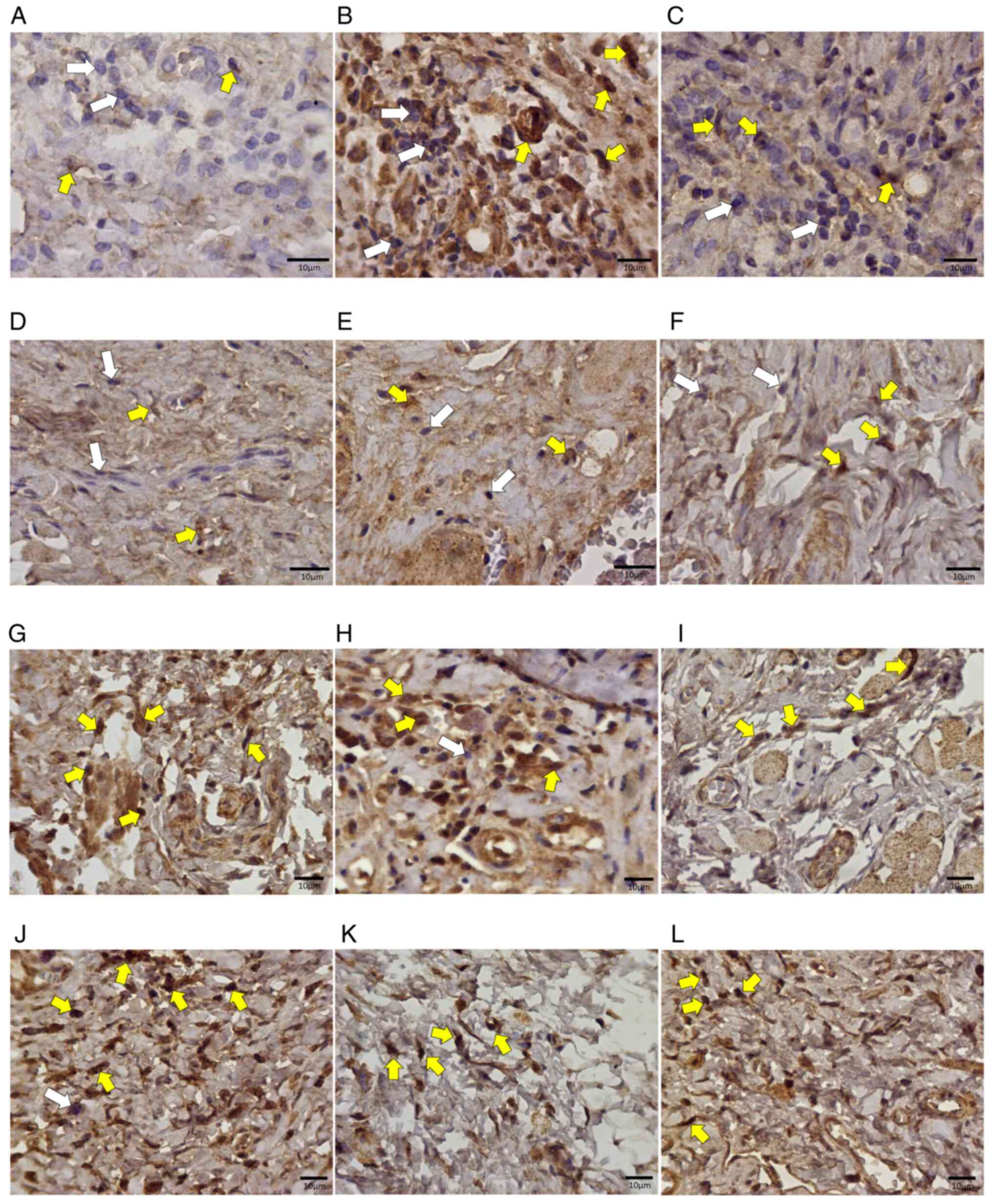 | Figure 11Photomicrographs of histological
sections of oral mucosa samples from Wistar rats subjected to
chemotherapy. (A) Animal in the control group, without medication,
euthanized on day 8. (B) Animal in the control group, without
medication, euthanized on day 11. (C) Animal in the control group,
without medication, euthanized on the 15th day. (D) Animal that
received trans-karyophylene, euthanized on day 8. (E) Animal that
received euthanized trans-karyophylene on day 11. (F) Animal that
received trans-karyophylene, euthanized on the 15th day. (G) Animal
that received pentoxifylline, euthanized on day 8. (H) Animal that
received pentoxifylline, euthanized on day 11. (I) Animal that
received pentoxifylline, euthanized on day 15. (J) Animal that
received atorvastatin, euthanized on the 8th day. (K) Animal that
received atorvastatin, euthanized on the 11th day. (L) Animal that
received atorvastatin, euthanized on day 15. Magnification, x400.
White arrows indicate negative cells and yellow arrows indicate
positive cells for the anti-TGF-β immunohistochemical reaction. |
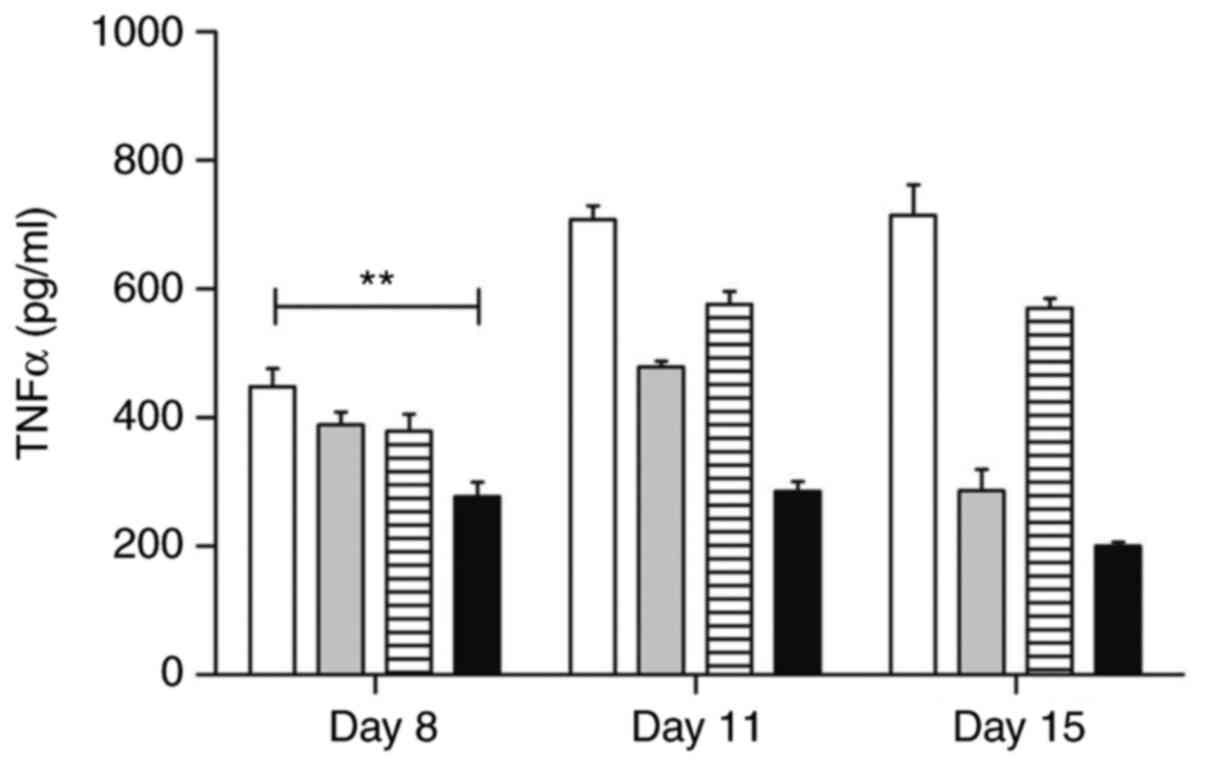
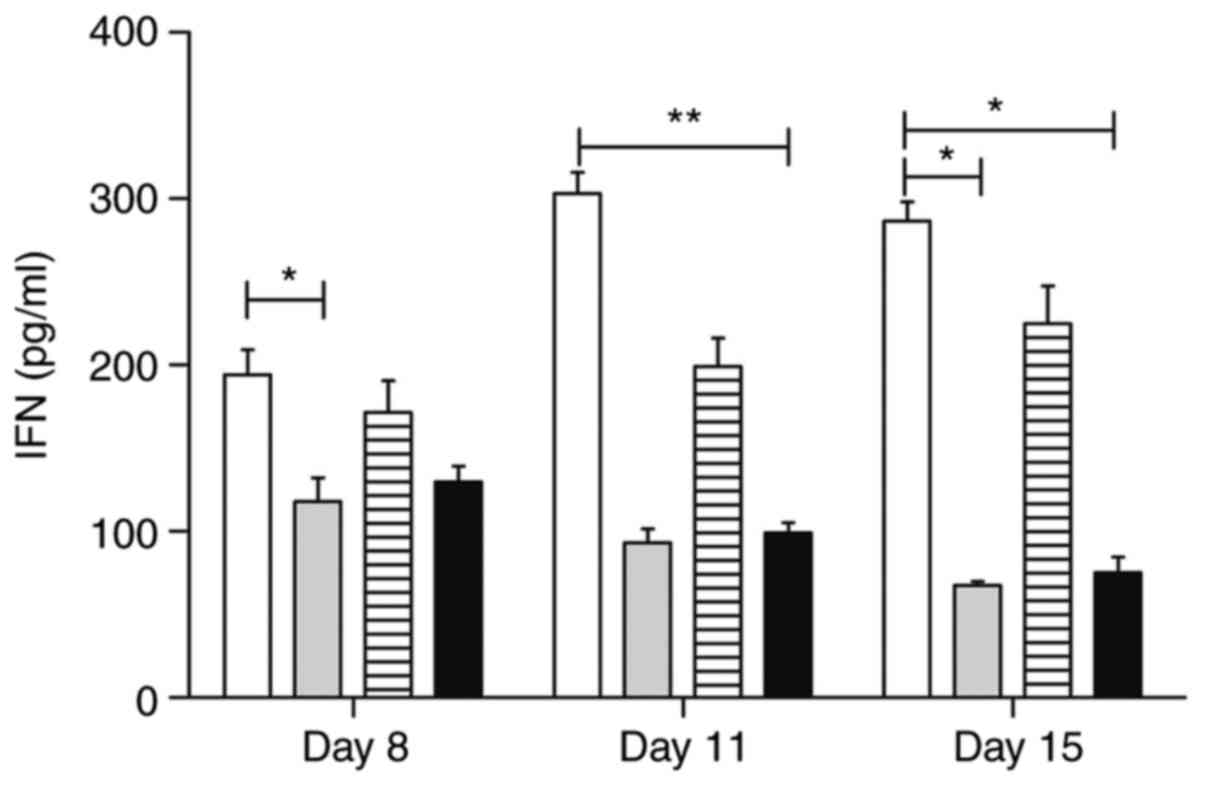
Serological evaluation of TNF-α and
INF-γ
Preventive treatment with atorvastatin has been
shown to modulate TNF-α levels in serum samples during all stages
evaluated in the experiment (P<0.01) (277.3±45.12 on Day 8;
285.3±30.63 on Day 11; 200.3±12.20 on Day 15) in relation to the
samples from the control group (194.0±30.19 on Day 8; 303.0±25.60
on Day 11; and 286.50±23.10 on Day 15) (Fig. 13).
On the other hand, the trans-caryophyllene treatment
negatively modulated the serum levels of IFN-γ on the first day of
analysis (P<0.05) (117.8±28.91), compared to the control group
(194.0±30.19), whereas atorvastatin delivery was effective in
modulating this cytokine on Day 11 (99.00±12.25). On Day 15, the
treatments with atorvastatin and trans-caryophyllene maintained
significantly lower IFN-γ levels than those of the control group
(75.00±19.27, and 67.50±4.65, respectively). Pentoxifylline
treatment did not influence the IFN-γ serum levels in the animals
at any stage evaluated in the study. Details of the IFN-γ serum
levels are shown in Fig. 14.
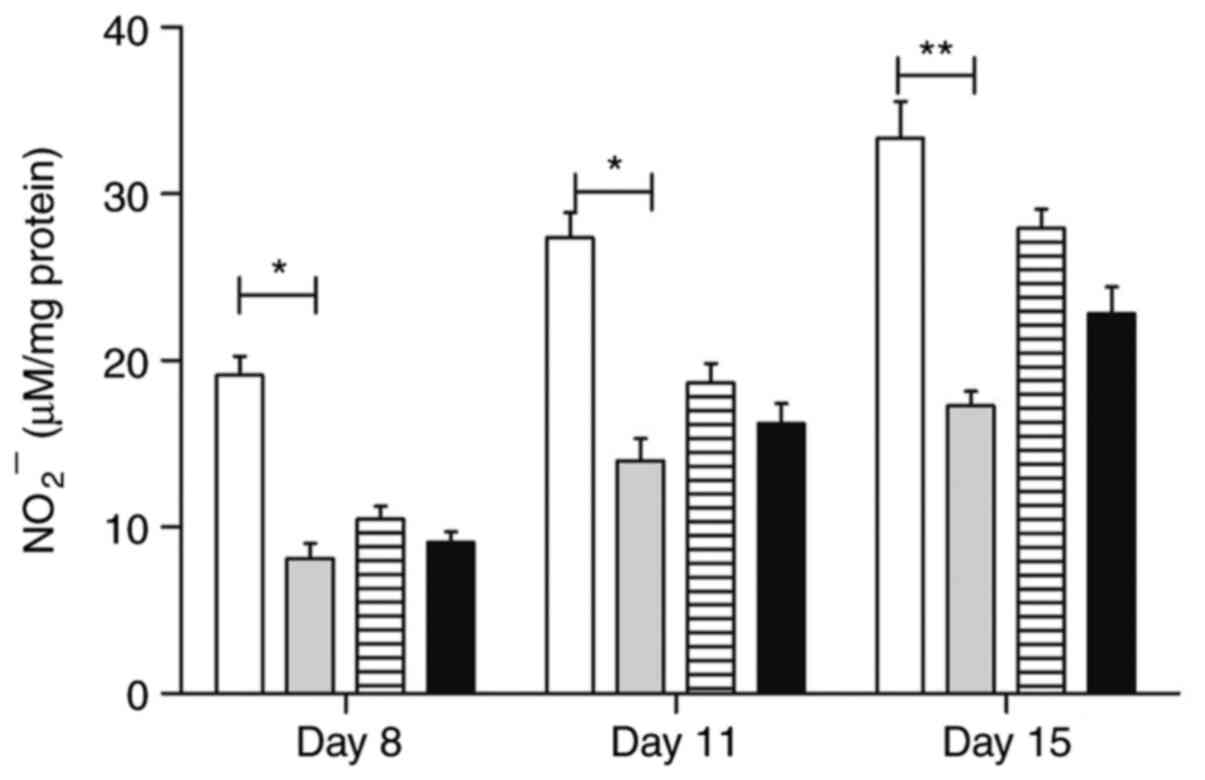
Serological nitrite evaluation
The serum nitrite levels were significantly
modulated by trans-caryophyllene on all evaluated days (P<0.05)
(8.12±1.8; 13.97±2.69; 17.28±1.75); atorvastatin, in turn, was
effective only in the last period (22.8±3.25, Day 15), compared to
the control group on the same days. Pentoxifylline treatment did
not influence the serum nitrite levels in the different evaluation
periods. Fig. 15 shows the serum
nitrite levels in different groups of animals throughout the
study.
Discussion
Oral mucositis has been the focus of several
experimental and clinical studies because its control allows more
effective anti-cancer radiation therapy and/or chemotherapy.
However, despite its importance, no control or preventive treatment
for oral mucositis has been established or filed so far (6,7,9,39-44).
To this end, several experimental models have been developed to
investigate the mechanisms associated with the oral mucositis
development, and to assess the effects of different therapeutic
agents on the evolution of oral ulcers (1,8,9,20,22,30,35,45).
In this study, histopathological and
histomorphometric analyses of oral mucosa samples demonstrated that
treatment with atorvastatin and pentoxifylline proved effective in
modulating the cellularity of the inflammatory infiltrate on Day 11
of the experiment, corresponding to the exacerbation of oral
mucositis. Trans-caryophyllene, as well as pentoxifylline, showed
significant effects on Day 15, in the remission stage of oral
ulcers. The delivery of pentoxifylline and trans-caryophyllene
favored angiogenesis on the lamina propria of Day 11 samples. These
data suggest that there was no correlation between the severity of
the inflammatory infiltrate and the amount of vascular sections
analyzed per microscopic field. However, the local inflammatory
infiltrate influenced the loss of epithelial continuity. Lima et
al (46) showed the development
of local ulcers and abscesses associated with leukopenia and a
change in body mass in a hamster model. Jain et al (47) used the oral mucositis model in rats
to prove that complex biological events mediated by a number of
inflammatory cytokines and their direct effect on the basal layer
of the epithelium result in the destruction of the anatomical
barrier of the oral mucosa.
Results from studies by Ward and Clissold (34) and Allen et al (48) suggested that controlling the TNF-α
expression by inhibiting its gene transcription allows it to
modulate leukopenia and inflammatory infiltrates in the mucosa
affected by oral mucositis (23).
This study showed that the TNF-α serum levels were significantly
lower in samples that were treated preventively with atorvastatin
compared to the control group and other experimental groups. The
in situ expression of this cytokine was lower in the
trans-caryophyllene-treated group and was histopathologically
associated with lower inflammation of the oral ulcers and their
satisfactory re-epithelialization. These results suggest that
preventive therapy with atorvastatin is capable of controlling
ulceration of the oral mucosa by inhibiting TNF-α production.
Preventive treatment with trans-caryophyllene has
been significantly effective in preventing oral mucositis and is
relevant in retaining epithelial integrity and mild inflammation of
the lamina propria underlying the slightly eroded areas.
Systemically, trans-caryophyllene modulated IFN-γ levels at the
establishment and remission stages of oral mucositis (Days 8 and 15
of the experiment, respectively), suggesting that this cytokine
does not influence exacerbation (Day 11) of the ulcers directly.
Other studies were performed using trans-caryophyllene, which
focused on the immunomodulatory effects of the drug (49-51).
Wright et al (52) performed jejunal integrity studies in
rats using the herbal extract-Iberogast- and concluded that this
plant partially improved the histopathological features of 5-FU
induced mucositis, but conferred no significant protection.
In 2014, Cheah et al (53) investigated the effects of grape seed
extract in colon cancer and it demonstrated reduced severity of
intestinal mucositis in patients undergoing 5-FU chemotherapy.
In addition, preventive treatment with atorvastatin
displayed absence of epithelial dissolution with preserved
integrity and mildly diffused inflammatory infiltrates.
Systemically, atorvastatin negatively modulated the levels of TNF-α
and INF-γ during the development of mucositis at all stages (Days
8, 11 and 15).
Serological analysis showed that trans-caryophyllene
significantly reduces IFN-γ levels, whereas atorvastatin reduces
TNF-α levels. In addition to these results, there was a significant
inhibition of leukopenia, as per previously published data
(25).
Histologically, oral wounds showed that prevention
with trans-caryophyllene was effective and developed no ulcers,
whereas atorvastatin was associated with better surface recovery of
the ulcerative wounds. Together, these results suggest that
trans-caryophyllene prevents oral mucositis and reduces the
systemic production of pro-inflammatory mediators.
Finally, we highlight the importance of exploring
drugs to establish preventive protocols for oral mucositis that are
secondary to chemotherapy and radiotherapy, since only symptomatic
treatment is currently recommended, and only includes painkillers
associated with nutritional support (46).
Acknowledgements
The authors would like to thank Professor Akinori
Cardozo Nagato (Department of Physiology, Federal University of
Juiz de Fora, Brazil) for statistical analysis.
Funding
This work received support of Rede Mineira de Bioterismo/FAPEMIG
(Minas Gerais State Research Support Foundation) number 31/11 and
TOXIFAR (Minas Gerais Network of Toxicological and Pharmacological
Tests of Therapeutic Products) number 26/11; Post Graduation
Program at the Federal University of Juiz de Fora.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors participated in the design,
interpretation of the experiments, analysis of the data, and review
of the manuscript. MIDCC, CNC, BJVA and FMA designed the
experiments. MIDCC, BJVA and FMA performed pathologic anatomy.
MIDCC and JOAC performed the immunohistochemistry experiments.
MIDCC and JOAC were involved in ELISA. MIDCC, CNC, BJVA and FMA
wrote the text. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental research was carried out on rodents
(Rattus novergicus) having been approved by the Animal
Research Ethics Committee of the Federal University of Juiz de Fora
under number 062/2011. All stages of the research were explained in
the proper form and, after being verified by three evaluators, it
was approved without changes or considerations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kostler WJ, Hejna M, Wenzel C and
Zielinski CC: Oral mucositis complicating chemotherapy and/or
radiotherapy: Options for prevention and treatment. CA Cancer J
Clin. 51:290–315. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sonis ST: Mucositis as a biological
process: A new hypothesis for the development of
chemotherapy-induced stomatotoxicity. Oral Oncol. 34:39–43.
1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sonis ST: New thoughts on the initiation
of mucositis. Oral Dis. 16:597–600. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sonis ST: The pathobiology of mucositis.
Nat Rev Cancer. 4:277–284. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Sonis ST and Costello KA: A database for
mucositis induced by cancer chemotherapy. Eur J Cancer B Oral
Oncol. 31B:258–260. 1995.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stone R, Fliedner MC and Smiet AC:
Management of oral mucositis in patients with cancer. Eur J Oncol
Nurs. 9 (Suppl 1):S24–S32. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Trotti A, Bellm LA, Epstein JB, Frame D,
Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L and Zilberberg MD:
Mucositis incidence, severity and associated outcomes in patients
with head and neck cancer receiving radiotherapy with or without
chemotherapy: A systematic literature review. Radiother Oncol.
66:253–262. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trucci VM, Veeck EB and Morosolli ARC:
Current strategies for the management of oral mucositis induced by
radiotherapy or chemotherapy. Rev Odonto Cienc. 24:309–314.
2009.
|
|
9
|
Lalla RV, Sonis ST and Peterson DE:
Management of oral mucositis in patients who have cancer. Dent Clin
North Am. 52:61–77. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peterson DE: New strategies for management
of oral mucositis in cancer patients. J Support Oncol. 4 (Suppl
1):S9–S13. 2006.PubMed/NCBI
|
|
11
|
Yeoh AS, Gibson RJ, Yeoh EE, Bowen JM,
Stringer AM, Giam KA and Keefe DM: A novel animal model to
investigate fractionated radiotherapy-induced alimentary mucositis:
The role of apoptosis, p53, nuclear factor-kappaB, COX-1, and
COX-2. Mol Cancer Ther. 6:2319–2327. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Parkin J and Cohen B: An overview of the
immune system. Lancet. 357:1777–1789. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goldman L and Ausiello D (eds): Principles
of cancer treatment. In: Internal Medicine Treaty. Campus Elsevier,
Rio de Janeiro, 2005.
|
|
14
|
Kolios G, Petoumenos C and Nakos A:
Mediators of inflammation: Production and implication in
inflammatory bowel disease. Hepatogastroenterology. 45:1601–1609.
1998.PubMed/NCBI
|
|
15
|
Mitchell R, Kumar V, Abbas A and Fausto N:
Robbins and Cotran Pathologic Basis of Diseases. 7th edition.
Elsevier, Rio de Janeiro, 2006.
|
|
16
|
Aarestrup B: In situ evaluation of
mediators of the inflammatory response and apoptosis process in
chronic periodontitis in patients with AIDS. Niteroi, Universidade
Federal Fluminense, 2006.
|
|
17
|
Baliga RS, Chaves AA, Jing L, Ayers LW and
Bauer JA: AIDS-related vasculopathy: Evidence for oxidative and
inflammatory pathways in murine and human AIDS. Am J Physiol Heart
Circ Physiol. 289:H1373–H1380. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Duda A, Stange A, Lüftenegger D, Stanke N,
Westphal D, Pietschmann T, Eastman SW, Linial ML, Rethwilm A and
Lindemann D: Prototype foamy virus envelope glycoprotein leader
peptide processing is mediated by a Furin-like cellular protease,
but cleavage is not essential for viral infectivity. J Virol.
78:13865–13870. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Consolaro A: Cellular stress: Precedes and
is present in inflammation. In: Inflammation and Repair. Dental
Press, Maringá, 2009.
|
|
20
|
Azevedo IM, Kumakura HS, Alloufa SL,
Mourão TS, Souza PM, Carvalho MDF, Medeiros VB, Araújo-Filho I,
Rêgo ACM and Medeiros AC: Effect of simvastatin in attenuation of
mucositis induced by methotrexate in rats. J Surg Clin Res.
1:22–32. 2010.
|
|
21
|
Flores D and Lisart R: Effectiveness of
palifermin in the prevention of oral mucositis in patients with
haematological cancers. Farm Hosp. 34:163–169. 2010.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
22
|
Spielberger R, Stiff P, Bensinger W,
Gentile T, Weisdorf D, Kewalramani T, Shea T, Yanovich S, Hansen K,
Noga S, et al: Palifermin for oral mucositis after intensive
therapy for hematologic cancers. N Engl J Med. 351:2590–2598.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schmidt-Choudhury A, Furuta GT, Lavigne
JA, Galli SJ and Wershil BK: The regulation of tumor necrosis
factor-alpha production in murine mast cells: Pentoxifylline or
dexamethasone inhibits IgE-dependent production of TNF-alpha by
distinct mechanisms. Cell Immunol. 171:140–1446. 1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Raber-Durlacher JE, von Bültzingslöwen I,
Logan RM, Bowen J, Al-Azri AR, Everaus H, Gerber E, Gomez JG,
Pettersson BG, Soga Y, et al: Systematic review of cytokines and
growth factors for the management of oral mucositis in cancer
patients. Support Care Cancer. 21:343–355. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Campos MI, Vieira WD, Campos CN, Aarestrup
FM and Aarestrup BJ: Atorvastatin and trans-caryophyllene for the
prevention of leukopenia in an experimental chemotherapy model in
Wistar rats. Mol Clin Oncol. 3:825–828. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Basile AC, Sertie JA, Freitas PC and
Zanini AC: Anti-inflammatory activity of oleoresin from Brazilian
Copaifera. J Ethnopharmacol. 22:101–109. 1988.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pereira FJ, Martins FT, Corrêa RS, Moreira
ME, Costa A, Santos MH, Polo M and Barbosa LC: Isolation, chemical
composition and anti-inflammatory activity of Copaifera
langsdorffii Desf. fruit peels essential oil according to
successive hydrodistillations. Acta Farm Bonaer. 27:369–374.
2008.
|
|
28
|
Vilanova CM, Ribeiro SM, Machado RC,
Vieira SM, Lima SG, Nunes PH and Martins MC: Evaluation of
oil-resin activity of Copaifera sp. On gastric emptying in
Rattus novergicus. Emir J Food Agricult. 25:394–397.
2013.
|
|
29
|
Siddiqui MA and Wellington K: Palifermin:
In myelotoxic therapy-induced oral mucositis. Drugs. 65:2139–2149.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sonis ST: Efficacy of palifermin
(keratinocyte growth factor-1) in the amelioration of oral
mucositis. Core Evid. 4:199–205. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Fernandes ES, Passos GF, Medeiros R, da
Cunha FM, Ferreira J, Campos MM, Pianowski LF and Calixto JB:
Anti-inflammatory effects of compounds alpha-humulene and
(-)-trans-caryophyllene isolated from the essential oil of Cordia
verbenacea. Eur J Pharmacol. 569:228–236. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Leandro LM, Vargas Fde S, Barbosa PC,
Neves JK, da Silva JA and da Veiga-Junior VF: Chemistry and
biological activities of terpenoids from copaiba (Copaifera
spp.) oleoresins. Molecules. 17:3866–3889. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Paiva LA, de Alencar Cunha KM, Santos FA,
Gramosa NV, Silveira ER and Rao VS: Investigation on the wound
healing activity of oleo-resin from Copaifera langsdorffi in
rats. Phytother Res. 16:737–739. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Ward A and Clissold SP: Pentoxifylline. A
review of its pharmacodynamic and pharmacokinetic properties, and
its therapeutic efficacy. Drugs. 34:50–97. 1987.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sonis ST, Tracey C, Shklar G, Jenson J and
Florine D: An animal model for mucositis induced by cancer
chemotherapy. Oral Surg Oral Med Oral Pathol. 69:437–443.
1990.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Scully C, Epstein J and Sonis S: Oral
mucositis: A challenging complication of radiotherapy,
chemotherapy, and radiochemotherapy: Part 1, pathogenesis and
prophylaxis of mucositis. Head Neck. 25:1057–1070. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cai Y, Wang Z, Li J, Li N, Wei F and Liu
Q: Evaluation of an indirect ELISA using recombinant granule
antigen Gra7 for serodiagnosis of Toxoplasma gondii infection in
cats. J Parasitol. 101:37–40. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Grisham MB, Jourd'Heuil D and Wink DA:
Nitric oxide. I. Physiological chemistry of nitric oxide and its
metabolites: Implications in inflammation. Am J Physiol.
276:G315–G321. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fekrazad R and Chiniforush N: Oral
mucositis prevention and management by therapeutic laser in head
and neck cancers. J Lasers Med Sci. 5:1–7. 2014.PubMed/NCBI
|
|
40
|
Herrstedt J: Prevention and management of
mucositis in patients with cancer. Int J Antimicrob Agents.
16:161–163. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lara RN, da Guerra EN and de Melo NS:
Macroscopic and microscopic effects of GaAIAs diode laser and
dexamethasone therapies on oral mucositis induced by fluorouracil
in rats. Oral Health Prev Dent. 5:63–71. 2007.PubMed/NCBI
|
|
42
|
Pico JL, Avila-Garavito A and Naccache P:
Mucositis: Its occurrence, consequences, and treatment in the
oncology setting. Oncologist. 3:446–451. 1998.PubMed/NCBI
|
|
43
|
Silverman S Jr: Diagnosis and management
of oral mucositis. J Support Oncol. 5:13–21. 2007.PubMed/NCBI
|
|
44
|
Wardill HR, Bowen JM and Gibson RJ: New
pharmacotherapy options for chemotherapy-induced alimentary
mucositis. Expert Opin Biol Ther. 14:347–354. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Vanderhoof JA, Park JH, Mohammadpour H and
Blackwood D: Effects of dietary lipids on recovery from mucosal
injury. Gastroenterology. 98:1226–1231. 1990.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lima V, Vidal FD, Rocha FA, Brito GA and
Ribeiro RA: Effects of tumor necrosis factor-alpha inhibitors
pentoxifylline and thalidomide on alveolar bone loss in short-term
experimental periodontal disease in rats. J Periodontol.
75:162–168. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jain P, Keservani R and Dahima R: In-vivo
characterization of hydrogel for treatment of chemo-radiotherapy
induced oral mucositis. Pharmacol Online. 1:1016–1025. 2010.
|
|
48
|
Allen R, Rapecki S and Higgs G: The role
of IL-10 in the inhibition of LPS-mediated TNF release from human
PBMCs by phosphodiesterase 4 (PDE4) inhibitors. Inflamm Res.
46(218)1997.
|
|
49
|
Dias DS, Fontes LB, Crotti AE, Aarestrup
BJ, Aarestrup FM, da Silva Filho AA and Corrêa JO: Copaiba oil
suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice
induced with experimental autoimmune encephalomyelitis (EAE).
Molecules. 19:12814–12826. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Guo K, Mou X, Huang J, Xiong N and Li H:
Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory
responses by inhibiting NF-κB activation in microglia. J Mol
Neurosci. 54:41–48. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Veiga Junior VF, Rosas EC, Carvalho MV,
Henriques MG and Pinto AC: Chemical composition and
anti-inflammatory activity of copaiba oils from Copaifera
cearensis Huber ex Ducke, Copaifera reticulata Ducke and
Copaifera multijuga Hayne-a comparative study. J
Ethnopharmacol. 112:248–254. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wright TH, Yazbeck R, Lymn KA, Whitford
EJ, Cheah KY, Butler RN, Feinle-Bisset C, Pilichiewicz AN, Mashtoub
S and Howarth GS: The herbal extract, Iberogast, improves jejunal
integrity in rats with 5-fluorouracil (5-FU)-induced mucositis.
Cancer Biol Ther. 8:923–929. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cheah KY, Howarth GS and Bastian SE: Grape
seed extract dose-responsively decreases disease severity in a rat
model of mucositis; concomitantly enhancing chemotherapeutic
effectiveness in colon cancer cells. PLoS One.
9(e85184)2014.PubMed/NCBI View Article : Google Scholar
|