|
1
|
Gourley C and Bookman MA: Evolving
concepts in the management of newly diagnosed epithelial ovarian
cancer. J Clin Oncol. 37:2386–2398. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Meyer LA, Cronin AM, Sun CC, Bixel K,
Bookman MA, Cristea MC, Griggs JJ, Levenback CF, Burger RA,
Mantia-Smaldone G, et al: Use and effectiveness of neoadjuvant
chemotherapy for treatment of ovarian cancer. J Clin Oncol.
34:3854–3863. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ,
Panici PB, et al: Neoadjuvant chemotherapy or primary surgery in
Stage IIIC or IV ovarian cancer. N Engl J Med. 363:943–953.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vergote I, Van Nieuwenhuysen E and
Vanderstichele A: How to select neoadjuvant chemotherapy or primary
debulking surgery in patients with Stage IIIC or IV ovarian
carcinoma. J Clin Oncol. 34:3827–3828. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gore ME, Fryatt I and Wiltshaw E:
Treatment of relapsed carcinoma of the ovary with cisplatin or
carboplatin following initial treatment with these compounds.
Gynecol Oncol. 36:207–211. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393.
1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ozols RF: Treatment of recurrent ovarian
cancer: Increasing options-‘recurrent’ results. J Clin Oncol.
15:2177–2180. 1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gore M, Mainwaring P, A'Hern R, MacFarlane
V, Slevin M, Harper P, Osborne R, Mansi J, Blake P, Wiltshaw E and
Shepherd J: Randomized trial of dose-intensity with single-agent
carboplatin in patients with epithelial ovarian cancer. London
Gynaecological Oncology Group. J Clin Oncol. 16:2426–2434.
1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jodrell DI, Egorin MJ, Canetta RM,
Langenberg P, Goldbloom EP, Burroughs JN, Goodlow JL, Tan S and
Wiltshaw E: Relationships between carboplatin exposure and tumor
response and toxicity in patients with ovarian cancer. J Clin
Oncol. 10:520–528. 1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McGuire WP, Hoskins WJ, Brady MF, Homesley
HD, Creasman WT, Berman ML, Ball H, Berek JS and Woodward J:
Assessment of dose-intensive therapy in suboptimally debulked
ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol.
13:1589–1599. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Markman M, Liu PY, Moon J, Monk BJ,
Copeland L, Wilczynski S and Alberts D: Impact on survival of 12
versus 3 monthly cycles of paclitaxel (175 mg/m²) administered to
patients with advanced ovarian cancer who attained a complete
response to primary platinum-paclitaxel: Follow-up of a Southwest
Oncology Group and Gynecologic Oncology Group phase 3 trial.
Gynecol Oncol. 114:195–198. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Omura GA, Brady MF, Look KY, Averette HE,
Delmore JE, Long HJ, Wadler S, Spiegel G and Arbuck SG: Phase III
trial of paclitaxel at two dose levels, the higher dose accompanied
by filgrastim at two dose levels in platinum-pretreated epithelial
ovarian cancer: An intergroup study. J Clin Oncol. 21:2843–2848.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thigpen JT: Dose-intensity in ovarian
carcinoma: Hold, enough? J Clin Oncol. 15:1291–1293.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Grénman S, Wiklund T, Jalkanen J, Kuoppala
T, Mäenpää J, Kuronen A, Leminen A, Puistola U, Vuolo-Merilä P,
Salmi T, et al: A randomised phase III study comparing high-dose
chemotherapy to conventionally dosed chemotherapy for stage III
ovarian cancer: The Finnish Ovarian (FINOVA) study. Eur J Cancer.
42:2196–2199. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Möbus V, Wandt H, Frickhofen N, Bengala C,
Champion K, Kimmig R, Ostermann H, Hinke A and Ledermann JA:
AGO-Ovar/AIO; EBMT. Phase III trial of high-dose sequential
chemotherapy with peripheral blood stem cell support compared with
standard dose chemotherapy for first-line treatment of advanced
ovarian cancer: Intergroup trial of the AGO-Ovar/AIO and EBMT. J
Clin Oncol. 25:4187–4193. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fung MF, Johnston ME, Eisenhauer EA, Elit
L, Hirte HW and Rosen B: Cancer Care Ontario Practice Guidelines
Initiative Gynecology Disease Site Group. Chemotherapy for
recurrent epithelial ovarian cancer previously treated with
platinum-a systematic review of the evidence from randomized
trials. Eur J Gynaec Oncol. 23:104–110. 2002.PubMed/NCBI
|
|
17
|
Du Bois A, Weber B, Rochon J, Meier W,
Goupil A, Olbricht S, Barats JC, Kuhn W, Orfeuvre H, Wagner U, et
al: Addition of epirubicin as a third drug to
Carboplatin-paclitaxel in first-line treatment of advanced ovarian
cancer: A prospectively randomized gynecologic cancer intergroup
trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian
Cancer Study Group and the Groupe d'Investigateurs Nationaux pour
l'Etude des Cancers Ovariens. J Clin Oncol. 24:1127–1135.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Spiliotis J, Halkia E, Lianos E, Kalantzi
N, Grivas A, Efstathiou E and Giassas S: Cytoreductive surgery and
HIPEC in recurrent epithelial ovarian cancer: A prospective
randomized phase III study. Ann Surg Oncol. 22:1570–1575.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Van Driel WJ, Koole SN, Sikorska K,
Schagen van Leeuwen JH, Schreuder HW, Hermans RH, de Hingh IH, van
der Velden J, Arts HJ, Massuger LF, et al: Hyperthermic
intraperitoneal chemotherapy in ovarian cancer. N Engl J Med.
378:230–240. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA:
Gynecologic Oncology Group. Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yi S, Zeng L, Kuan Y, Cao Z, Zheng C,
Zhang Y, Liao M and Yang L: Antiangiogenic drugs used with
chemotherapy for patients with recurrent ovarian cancer: A
meta-analysis. Onco Targets and Therapy. 10:973–984.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stone RL, Sood AK and Coleman RL:
Collateral damage: Toxic effects of targeted antiangiogenic
therapies in ovarian cancer. Lancet Oncol. 11:465–475.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Monk BJ, Han E, Joseph-Cowen CA, Pugmire G
and Burger RA: Salvage bevacizumab-(rhuMABVEGF)-based therapy after
multiple prior cytotoxic regimens in advanced refractory epithelial
ovarian cancer. Gynecol Oncol. 102:140–144. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cannistra SA: The ethics of early stopping
rules: Who is protecting whom? J Clin Oncol. 22:1542–1545.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
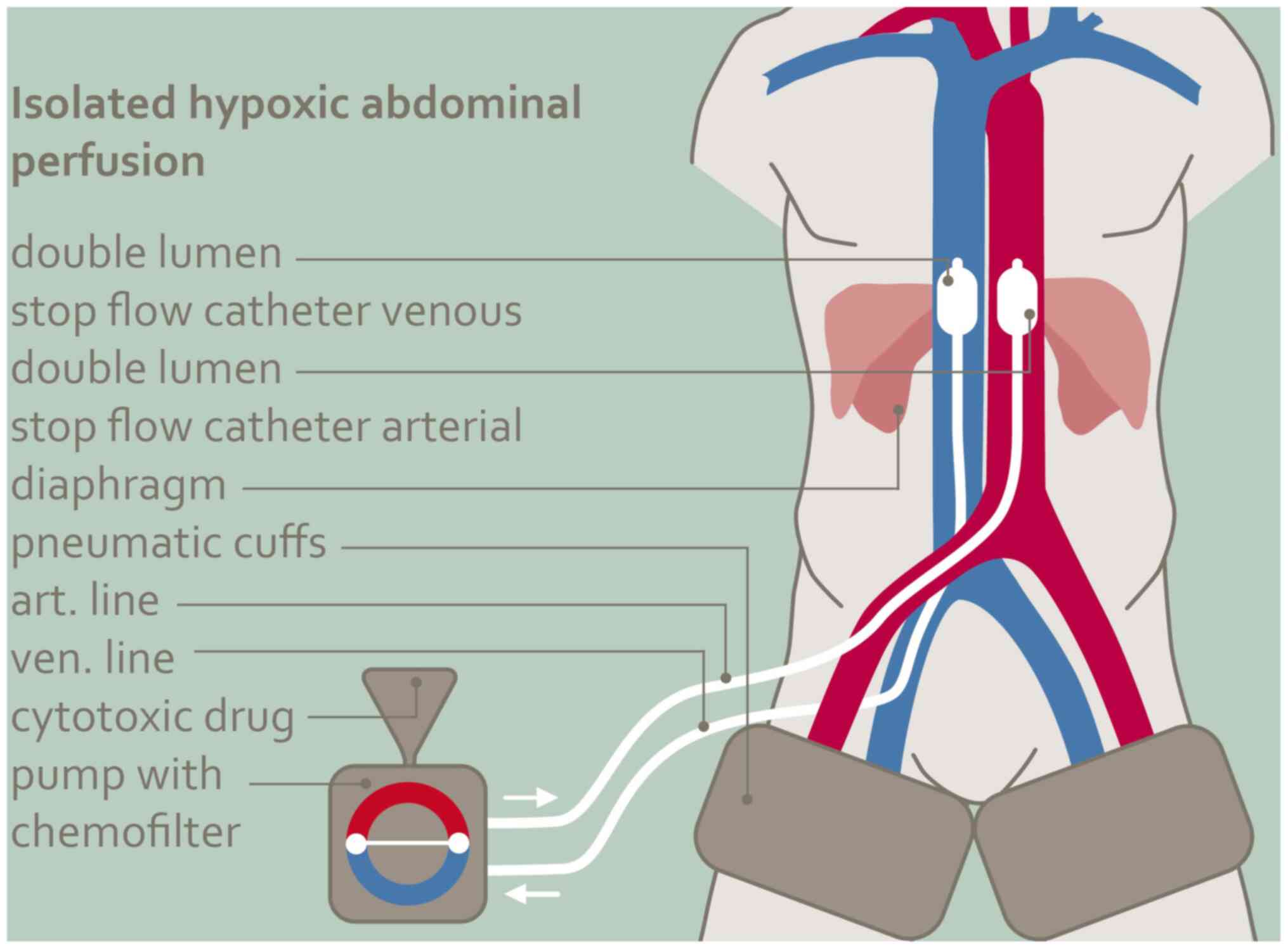
Aigner KR and Gailhofer S: High dose MMC:
Aortic stopflow infusion (ASI) with versus without chemofiltration:
A comparison of toxic side effects (abstract). Reg Cancer Treat. 6
(Suppl 1)(S3)1993.
|
|
26
|
Aigner KR, Tonn JC, Hechtel R and Seuffer
R: Die intraarterielle Zytostatikatherapie mit venöser Filtration
im halboffenen System. Onkologie. 6:74–76. 1983.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Teicher BA, Lazo JS and Sartorelli A:
Classification of antineoplastic agents by their selective
toxicities toward oxygenated and hypoxic tumor cells. Cancer Res.
41:73–81. 1981.PubMed/NCBI
|
|
28
|
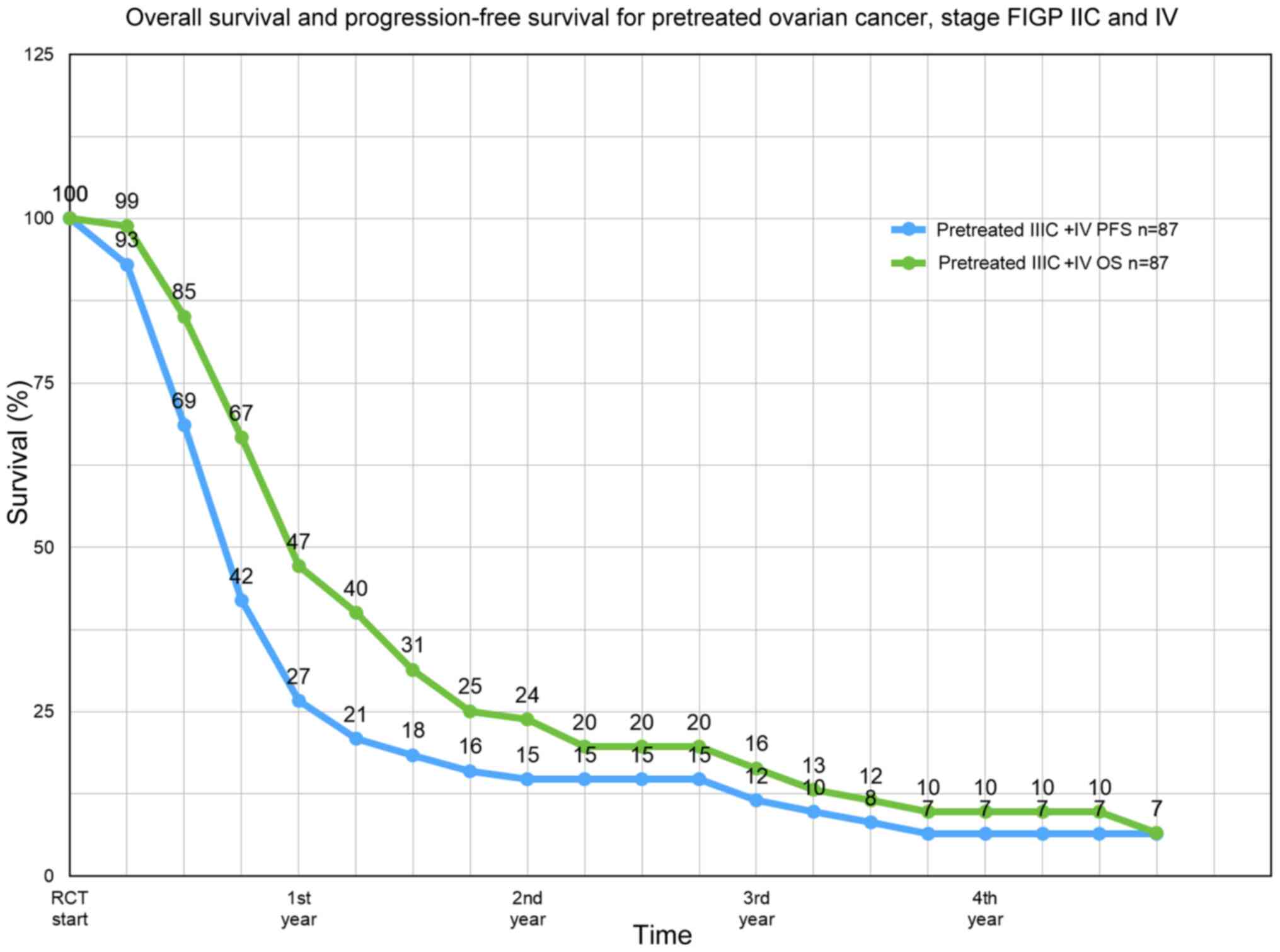
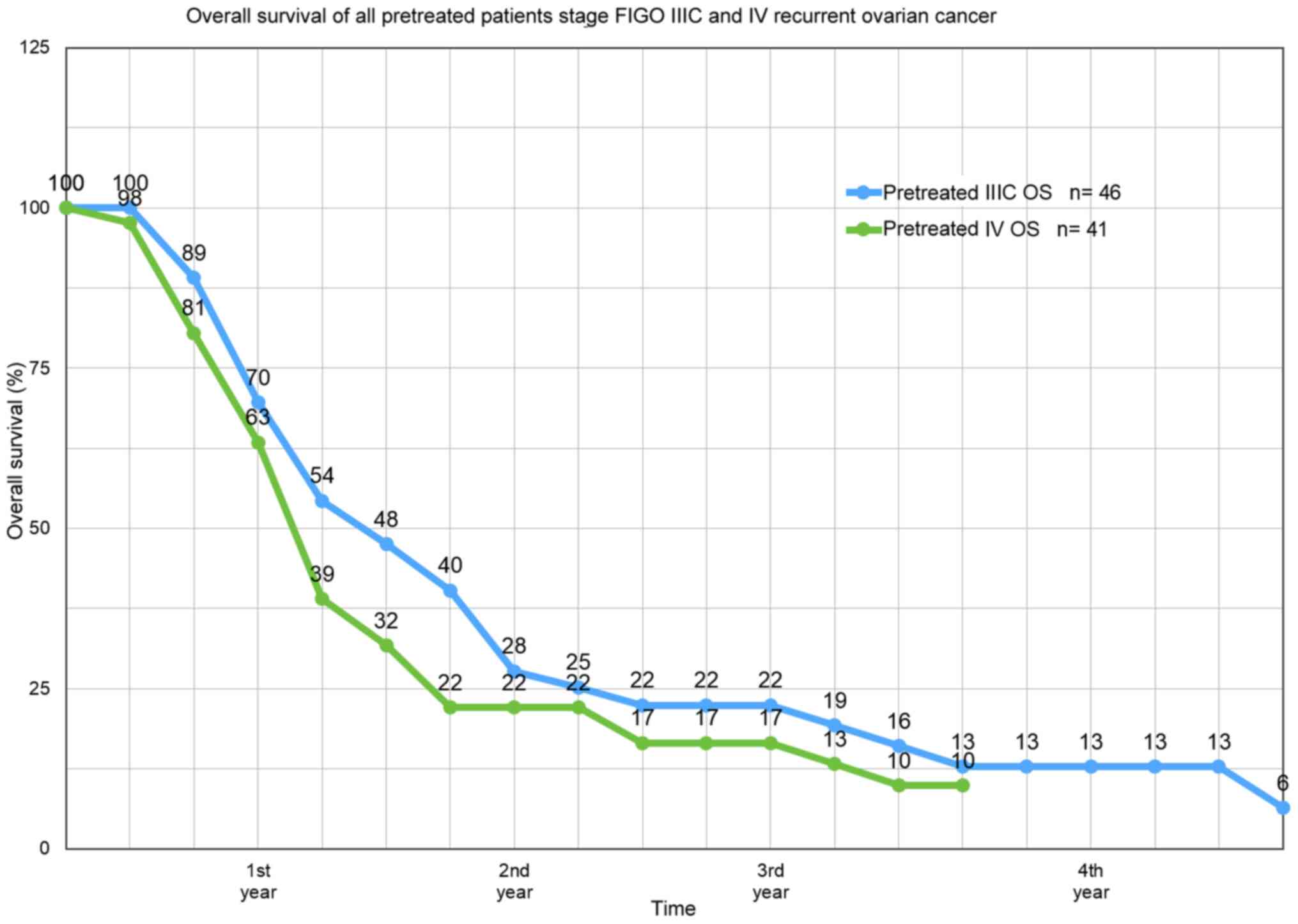
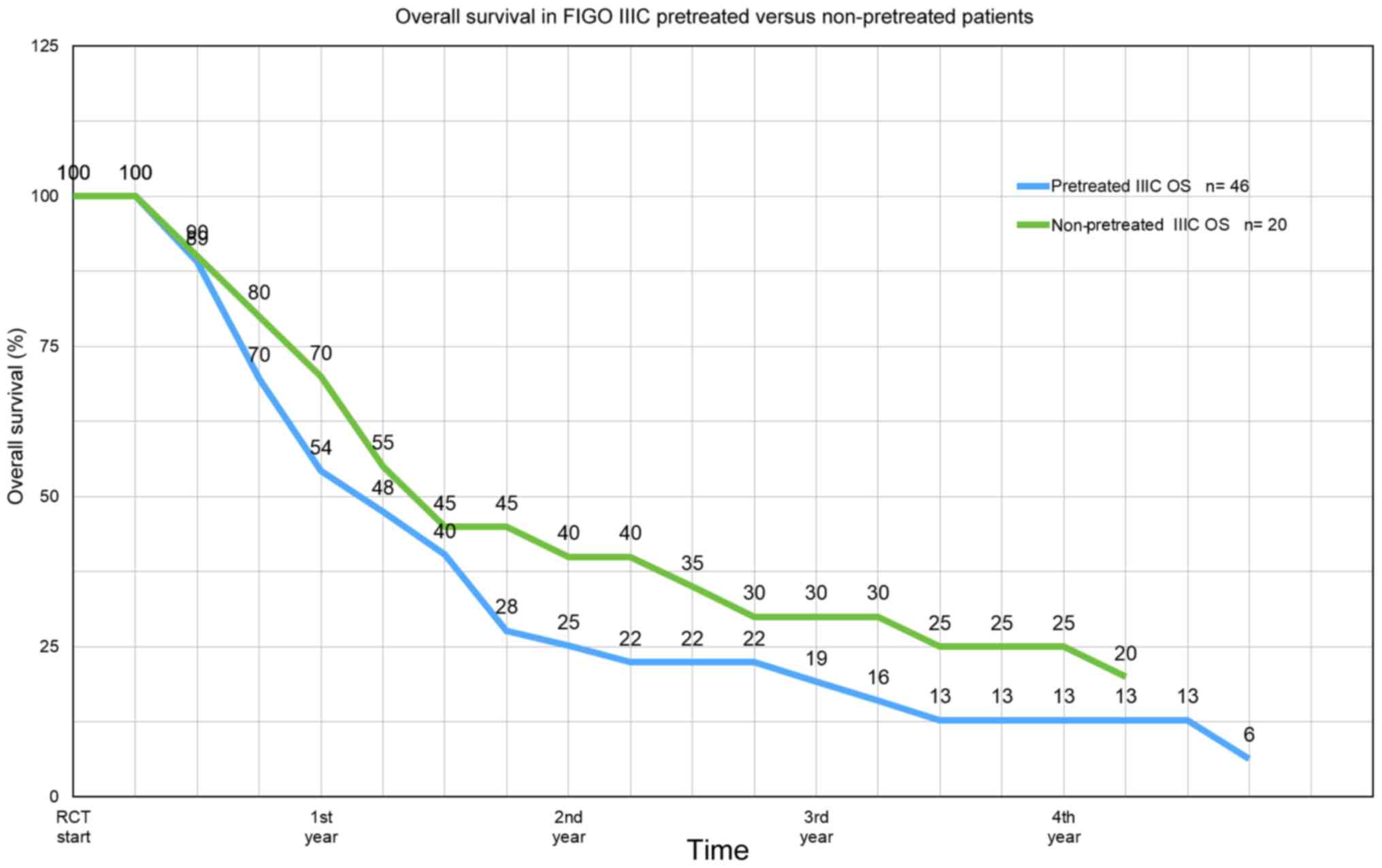
Aigner KR, Selak E, Gailhofer S and Aigner
K: Hypoxic Isolated Abdominal Perfusion (HAP) chemotherapy for
non-operable advanced staged ovarian cancer with peritoneal
carcinosis: An experience in 45 platinum-refractory ovarian cancer
patients. Indian J Surg Oncol. 10:506–514. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aigner KR and Knapp N: Toxicity Profiles
with Systemic vs. Regional Chemotherapy. In: Induction
Chemotherapy-Systemic and Locoregional. 2nd edition. KR Aigner and
FO Stephens (eds). Springer Verlag Berlin, pp497-506, 2016.
|
|
30
|
Du Bois A, Vergote I, Ferron G, Reuss A,
Meier W, Greggi S, Jensen PT, Selle F, Guyon F, Pomel C, et al:
Randomized controlled phase III study evaluating the impact of
secondary cytoreductive surgery in recurrent ovarian cancer: AGO
DESKTOP III/ENGOT ov20. J Clin Oncol. 35(5501)2017.
|
|
31
|
Shanghai Gynecologic Oncology Group.
Surgery or Chemotherapy in Recurrent Ovarian Cancer (SOC 1 Trial).
National Library of Medicine, Bethesda, MD, 2018. Available from:
www.clinicaltrials.gov/ct2/show/record/NCT01611766.
|
|
32
|
Zang R and Zhu J: Which patients benefit
from secondary cytoreductive surgery in recurrent ovarian cancer? J
Gynecol Oncol. 30(e116)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Coleman RL, Enserro D, Spirtos N, Herzog
TJ, Sabbatini P, Armstrong DK, Kim B, Fujiwara K, Walker JL, Flynn
PJ, et al: A phase III randomized controlled trial of secondary
surgical cytoreduction (SSC) followed by platinum-based combination
chemotherapy (PBC), with or without bevacizumab (B) in
platinum-sensitive, recurrent ovarian cancer (PSOC): A NRG
Oncology/Gynecologic Oncology Group (GOG) study. J Clin Oncol.
36:5501. 2018.
|
|
34
|
Coleman RL, Spirtos NM, Enserro D, Herzog
TJ, Sabbatini P, Armstrong DK, Kim JW, Park SY, Kim BG, Nam JH, et
al: Secondary surgical cytoreduction for recurrent ovarian cancer.
N Engl J Med. 381:1929–1939. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vargas HA, Burger IA, Goldman DA, Miccò M,
Sosa RE, Weber W, Chi DS, Hricak H and Sala E: Volume-based
quantitative FDG PET/CT metrics and their association with optimal
debulking and progression-free survival in patients with recurrent
ovarian cancer undergoing secondary cytoreductive surgery. Eur
Radiol. 25:3348–3353. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Van de Vaart PJ, van der Vange N,
Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink
WW, Beijnen JH, Bartelink H and Begg AC: Intraperitoneal cisplatin
with regional hyperthermia in advanced ovarian cancer:
Pharmacokinetics and cisplatin-DNA adduct formation in patients and
ovarian cancer cell lines. Eur J Cancer. 34:148–154.
1998.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vergote I, Harter P and Chiva L: Is there
a role for intraperitoneal chemotherapy, including HIPEC, in the
management of ovarian cancer? J Clin Oncol. 37:2420–2423.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gore M, du Bois A and Vergote I:
Intraperitoneal chemotherapy in ovarian cancer remains
experimental. J Clin Oncol. 24:4528–4530. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Colombo N, Sessa C, du Bois A, Ledermann
J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I,
Vergote I, et al: ESMO-ESGO Consensus Conference on Ovarian Cancer:
Pathology and molecular biology, early and advanced stages,
borderline ovarian tumours and recurrent disease. Ann Oncol.
30:672–705. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tempfer CB, Winnekendonk G, Solass W,
Horvat R, Giger-Pabst U, Zieren J, Rezniczek GA and Reymond MA:
Pressurized intraperitoneal aerosol chemotherapy in women with
recurrent ovarian cancer: A phase 2 study. Gynecol Oncol.
137:223–228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guadagni S, Clementi M, Masedu F,
Fiorentini G, Sarti D, Deraco M, Kusamura S, Papasotiriou I,
Apostolou P, Aigner KR, et al: A pilot study of the predictive
potential of chemosensitivity and gene expression assays using
circulating tumour cells from patients with recurrent ovarian
cancer. Int J Mol Sci. 21(4813)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Del Campo JM, Matulonis UA, Malander S,
Provencher D, Mahner S, Follana P, Waters J, Berek JS, Woie K, Oza
AM, et al: Niraparib maintenance therapy in patients with recurrent
ovarian cancer after a partial response to the last platinum-based
chemotherapy in the ENGOT-OV16/NOVA Trial. J Clin Oncol.
37:2968–2973. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Poveda A: Final overall survival (OS)
results from SOLO2/ENGOT-ov21: A phase III trial assessing
maintenance olaparib in patients (pts) with platinum-sensitive,
relapsed ovarian cancer and a BRCA mutation. Presented at: 2020
ASCO Virtual Scientific Program; Abstract 6002, May 12, 2020.
|
|
44
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance Olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505.
2018.PubMed/NCBI View Article : Google Scholar
|