Introduction
Clinical research has noticeably improved
progression-free survival and overall survival over the past few
decades. Nevertheless, ovarian cancer is still the leading cause of
death among all gynecological malignancies. The insidiousness of
the disease is the early peritoneal spread, the rapid development
of chemoresistance and the bypassing of the hosts immune response
(1). The recommended therapy option
is complete surgical cytoreduction and chemotherapy with a
combination carboplatin and taxanes (2-4).
Although a complete remission rate of ~80% is achieved,
platinum-resistance often occurs within two years. The shorter the
relapse-free interval, the less likely the tumor will respond again
(5-7).
A higher individual dose or dose-dense therapy could induce a new
response, but are incompatible and too toxic for the patient
(8-13).
Even modified drug combinations or high-dose chemotherapy have not
brought any real progress (14-17).
Hyperthermic intraperitoneal chemotherapy has been of recent
interest, particularly after surgical cytoreduction (18-20).
Alternatively, new drugs or targeted substances can be considered
(21-24).
Because of responsiveness increases with an increased dose or
concentration of cytostatics, we assume that isolated perfusion in
a closed circuit is able to break through chemoresistance due to
the increased exposure to cytostatic drugs.
In order to keep the systemic toxicity within
acceptable limits, and to maintain the quality of life,
chemofiltration was carried out directly after the therapy
(25,26). We herein present a cohort study of
107 patients who underwent hypoxic abdominal perfusion. All
pretreated patients had recurrent stage IIIC and IV epithelial
ovarian cancers resistant to platinum-containing drug
combinations.
Materials and methods
Patients
The study included 107 patients in the FIGO stages
IIIC and IV, who were treated in one institution between 1997 and
2017. 87 patients were previously treated with platinum-containing
combination chemotherapies, mostly taxanes and had recurrent
epithelial ovarian cancers, resistant to platinum-based drug
combination. 46 patients were stage FIGO IIIC and 41 were stage IV.
An additional 20 patients who had refused prior therapies were all
stage IIIC. 34 patients had G3 degree malignancies (Table I). All pretreated patients had
received at least two prior lines of chemotherapy. 7 had undergone
third-line and one patient fourth-line therapies. The median
platinum-free interval in 46 stage IIIC patients was 7 months and
in 41 patients in stage IV was 9 months (Table II). Performance status was mostly
stage ECOG 2 and 3. The 20 patients who had refused prior systemic
chemotherapy received the same isolated perfusion therapy as the 87
patients with recurrent disease. The possibility of debulking
surgery after diagnosis of progression was not considered feasible
in terms of patient's performance and advanced disease in all
cases.
 | Table IPatient characteristics (n=107). |
Table I
Patient characteristics (n=107).
| | Value |
|---|
| Variable | Pretreated
(n=87) | Non-pretreated
(n=20) |
|---|
| Median age,
years | 56.6 | |
| Stage, n (%) | | |
|
FIGO
IIIC | 46(53) | 20(100) |
|
FIGO IV | 41(47) | 0 (0) |
| Grading, n (%) | | |
|
G3 | 34(30) | |
 | Table IITime interval between prior treatments
and hypoxic abdominal perfusion with chemofiltration. |
Table II
Time interval between prior treatments
and hypoxic abdominal perfusion with chemofiltration.
| Stage | No. | Median platinum-free
interval before perfusion |
|---|
| FIGO IIIC
non-pretreated | 20 | No pretreatment |
| FIGO IIIC
pretreated | 46 | 7 months |
| FIGO IV
pretreated | 41 | 9 months |
Investigations were performed in compliance with the
principles of good clinical practice outlined in the Declaration of
Helsinki and federal guidelines, and had approval by the Medias
Institutional Review Committee. Informed consent was obtained from
each participant or participant's guardian. Patients were required
to have an ECOG performance status of 3 or less. Exclusion criteria
included cardiovascular disease, uncontrolled diabetes and serious
infections. The white blood count had to be no less than 2,500/µl,
and should under no circumstances be in decline before starting
therapy. The same applied to the platelet count with a limit of no
less than 100,000/µl.
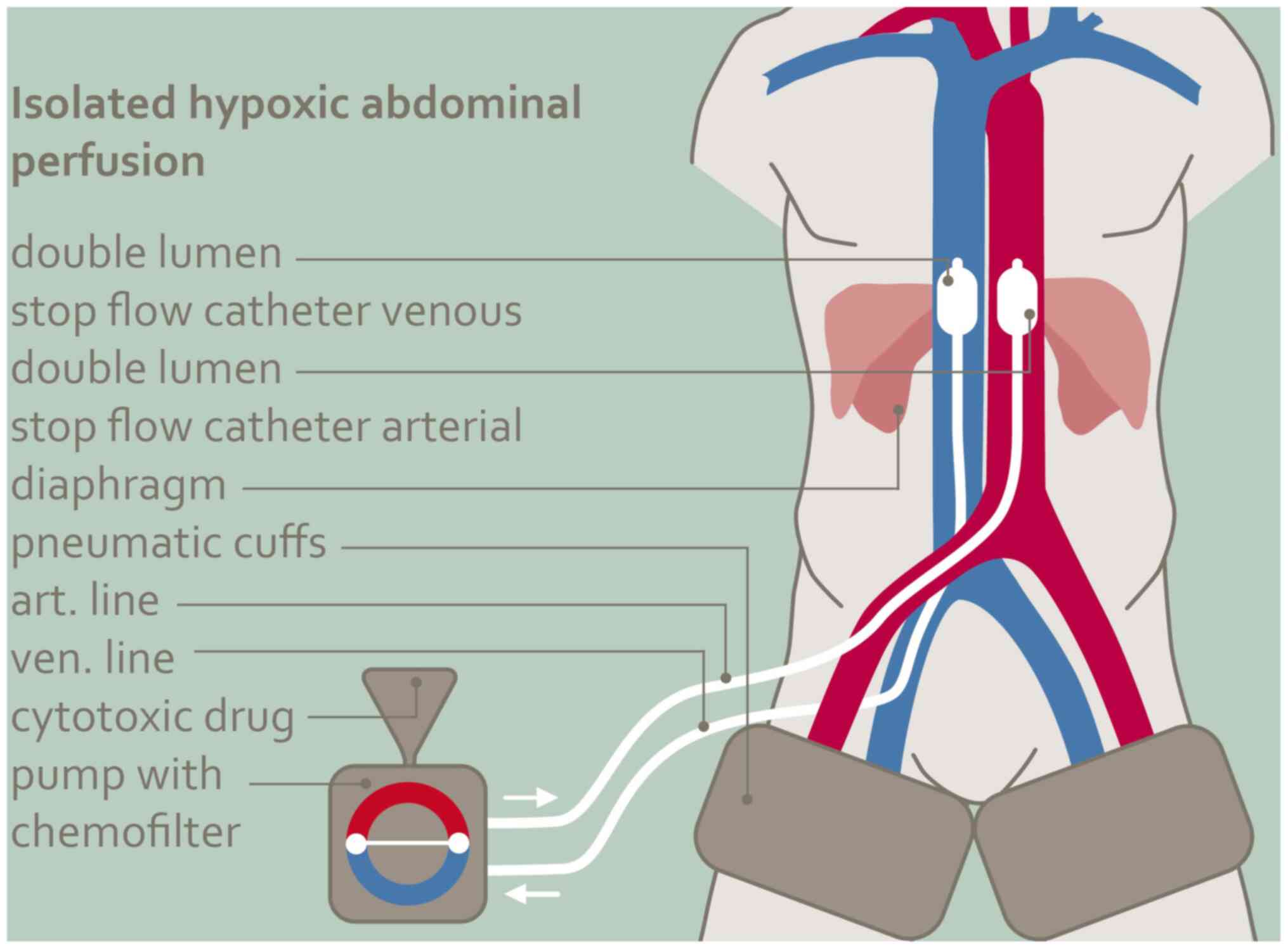
Isolated hypoxic abdominal
perfusion
The procedure (Fig.
1) is carried out with heparinization at 150 IU/kg body weight
under general anesthesia and takes a total of one and a half to two
hours. The femoral vessels, artery and vein, are exposed through a
short incision in the groin and cannulated with stop-flow balloon
catheters (Dispomedica, Hamburg). Both thighs are blocked with
inflatable pneumatic cuffs. The venous balloon is positioned in the
vena cava between the confluence of the hepatic vein and the right
atrium, the aortic balloon shortly above the diaphragm level. After
the correct position of the balloons has been documented with
contrast medium, they are unblocked again and after temporary
hyperoxygenation, the cytostatic combination is injected as a bolus
into the aorta and both balloons are blocked again immediately. To
prevent an initial acidification of the system, the balloons are
only blocked after a short prior hyperoxygenation and aortic bolus
injection of the chemotherapeutic agents. The therapy itself is
conducted for 15 min under hypoxic conditions. Mitomycin and
adriamycin unlike other cytostatics exhibit an augmented
tumoricidal effect under hypoxia. The pH value has no influence on
the efficacy of cisplatin (27). In
isolated hypoxic abdominal perfusion the average dose of cisplatin
was 60 mg, the maximum safe intra-arterial total dose of cisplatin
was 70 mg as a bolus-injection. The maximum dose of adriamycin was
50 mg and for mitomycin 20 mg totally at one shot into the aorta.
These seemingly low doses of cisplatin and adriamycin develop high
active concentrations when used intra-arterially in an isolated
circuit. Due to the intra-arterial application, the
chemotherapeutics were not dosed by bodyweight for these patients.
Plasma levels of mitomycin and adriamycin are 50 times higher in
the arterial vs. venous perfusion line for the first three minutes
and then adjust (28). Leakage
monitoring is not necessary during the 15 min isolated perfusion
period because after the balloons and thigh pressure cuffs have
been unblocked, chemofiltration via the perfusion catheters is
started immediately. Chemofiltration is continued at a maximum
blood flow rate of 500 ml per minute to a substitution volume of 4
liters. After completing chemofiltration, the catheters are removed
and the vessels repaired with running sutures.
The isolated hypoxic abdominal perfusion is
conducted in four cycles in three to four weeks intervals each. The
blood count is checked weekly, and, while approaching the lowest
Nadir, controls are carried out every second day until the blood
count starts to reemerge. The tumor marker CA12-5 is checked before
each therapy and a CT monitoring was performed two weeks after the
second and fourth perfusion each. The extent of the residual tumor
load and the tumor response were assessed according to the course
of the tumor marker, the amount of residual ascites and the CT
findings as well as the general condition of the patient. In case
of progressive peritoneal lesions or distant metastases the
treatment was discontinued.
Statistical analysis
Statistics have been calculated with 95% confidence
limits. Survival times were estimated using the Kaplan-Meier
product limit estimator and follow up for the surviving patients
was minimum 12 months. Statistical analyses were performed using
MediasStat software, version 28.5.14.
Results
Endpoints of the study
The most important endpoints of the trial were
quality of life and overall survival, followed by the response
rates. The latter was derived from the clinical response rate in
the form of the tumor marker CA 12-5, the computer tomographic
control and not least, quality of life.
Quality of life (QoL)
QoL was in particular measured in the form of the
decline or complete disappearance of ascites and especially, the
substantial improvement in pain and the often described general
discomfort. Patients who had prior systemic followed by regional
chemotherapy filled in questionnaires comparing the intensity of
the most common side effects after the respective therapies in a
scale from one to six. Patients perceived regional perfusion
therapy less stressing than conventional chemotherapy.
Response rates
A positive influence on clinical response rates was
noted among 69% of all patients in stage IIIC and IV. The rate of
complete remissions was 19,6% in stage IIIC and 14,6% in stage IV,
partial remissions 47,8 and 56,1% respectively. Complete
disappearance of ascites was observed in 43% of patients after only
two perfusions, and 19% of patients reported a 50% or more
reduction of abdominal pressure and fluid volume (Table III). A considerable improvement in
general wellbeing, with a reduction in abdominal symptoms and a
substantial decrease in pain, was reported by 74% or 3/4 of
patients with advanced ovarian carcinoma.
 | Table IIIResponse rates of pretreated
Fédération Internationale de Gynécologie et d'Obstétrique stage
IIIC and IV patients with recurrent disease. |
Table III
Response rates of pretreated
Fédération Internationale de Gynécologie et d'Obstétrique stage
IIIC and IV patients with recurrent disease.
| Response | Stage IIIC, % | Stage IV, % | Stage IIIC/IV,
% | Ascites, % |
|---|
| PD | 6.5 | 9.8 | 8.0 | 0.0 |
| SD | 26.1 | 19.5 | 23.0 | 0.0 |
| PR | 47.8 | 56.1 | 51.7 | 19.0 |
| CR | 19.6 | 14.6 | 17.3 | 43.0 |
| CR+PR | 67.4 | 70.7 | 69.0 | 62.0 |
Survival
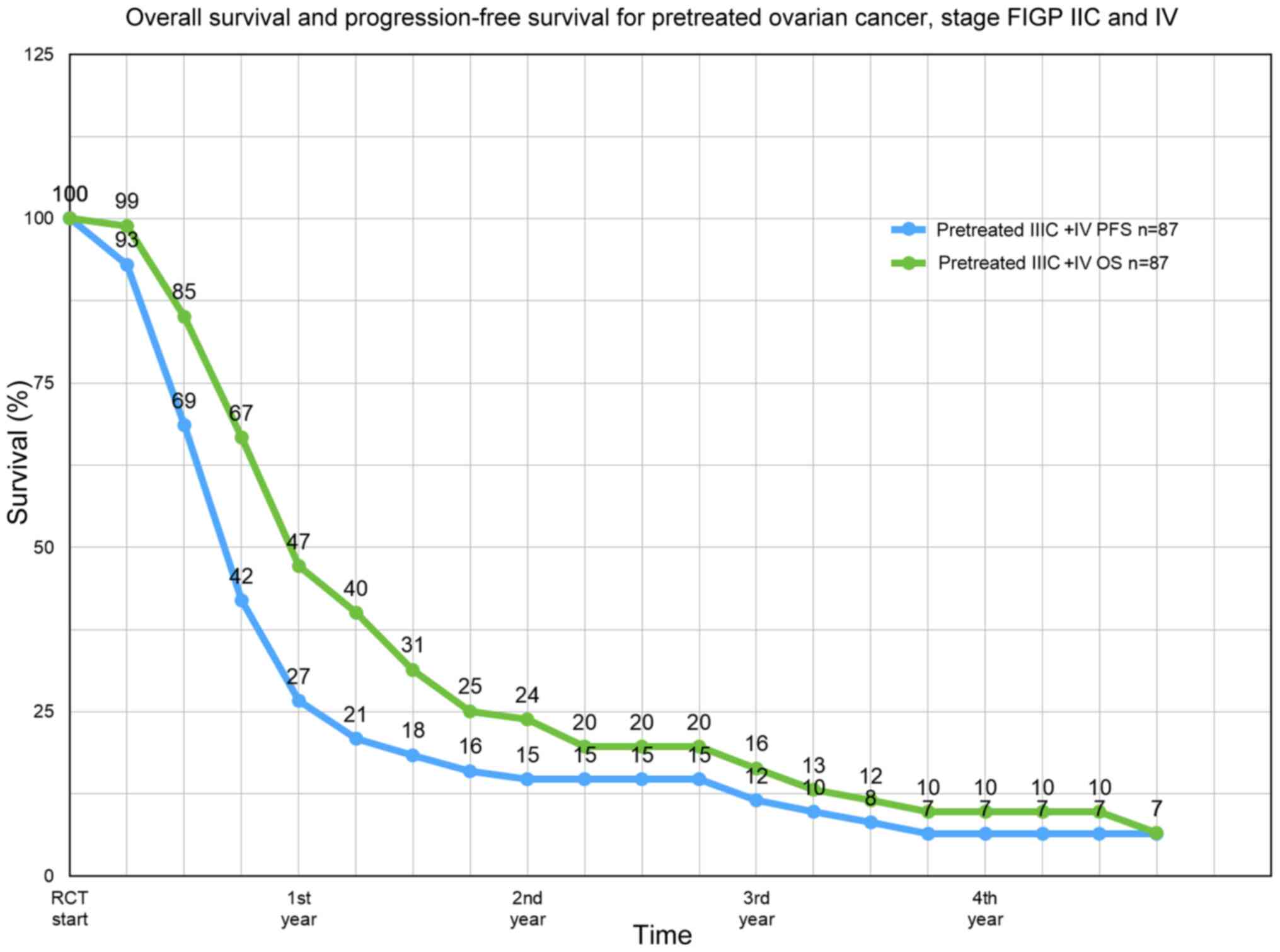
The median progression-free survival (PFS) of all 87
patients was 8 months, the median overall survival 11.9 months
(Fig. 2). The median survival rate
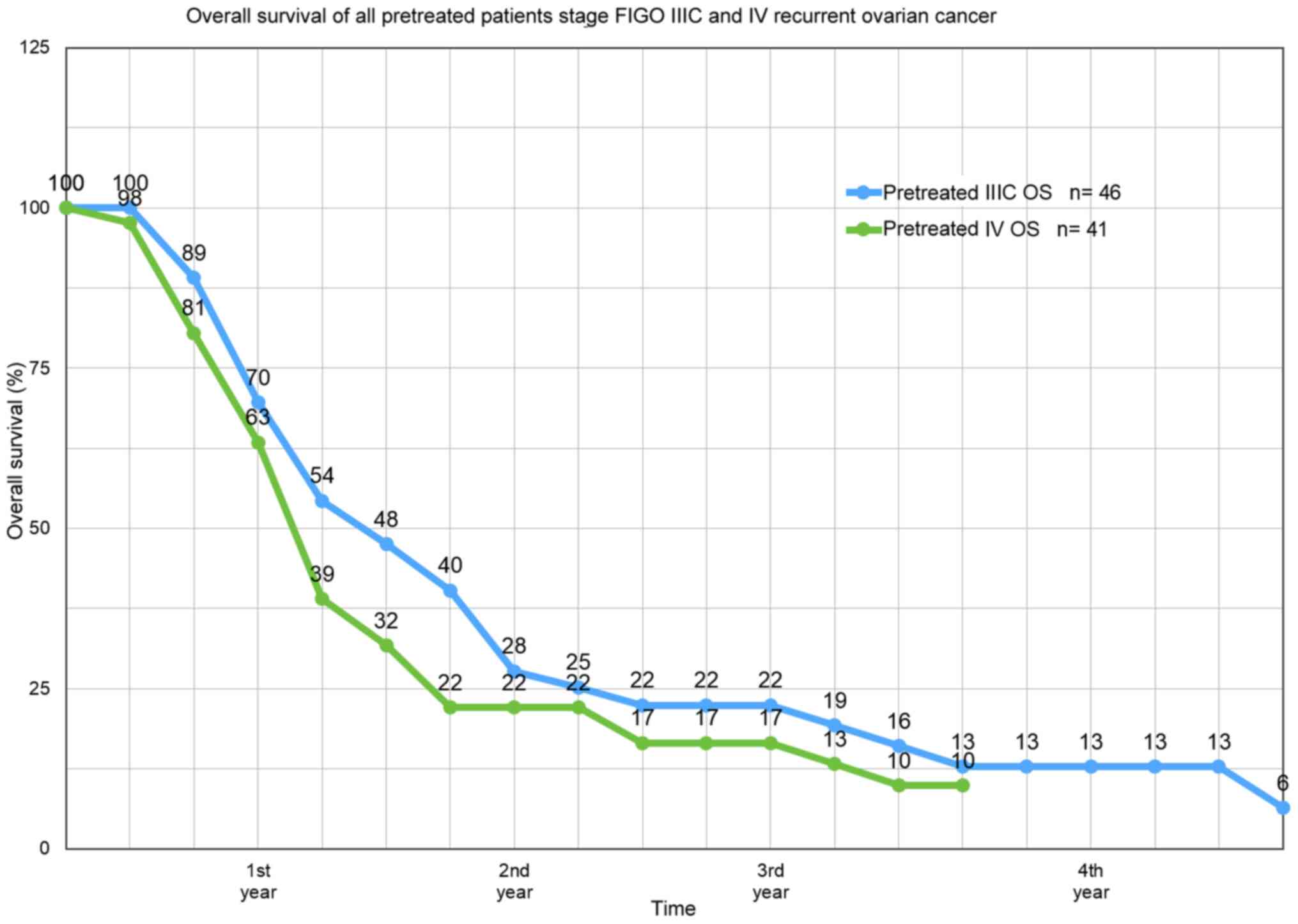
of pretreated platinum-refractory patients at FIGO IIIC stage was
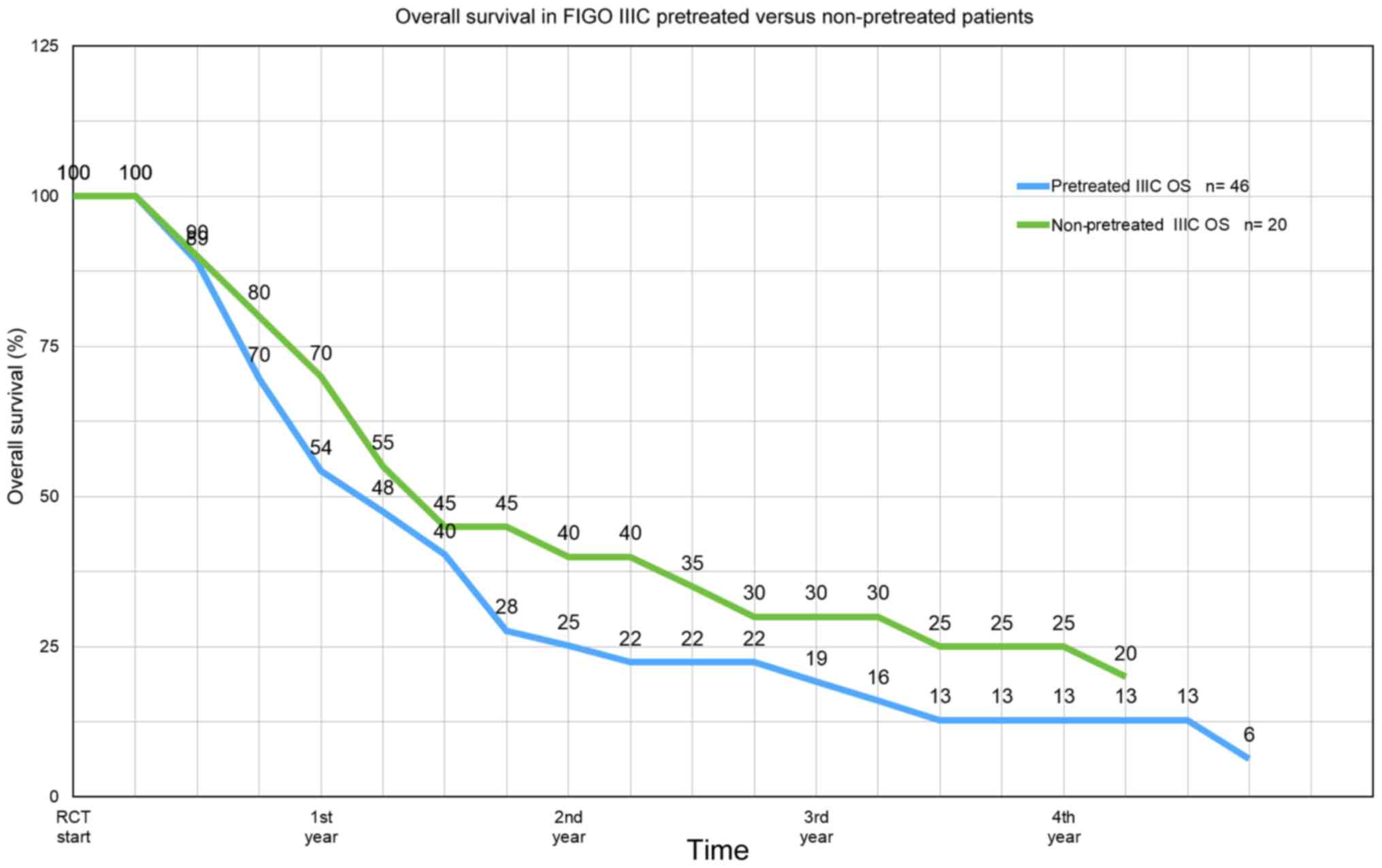
12.8 months and at stage IV, 10.9 months (Fig. 3). In stage IIIC, overall survival in
pretreated vs. non-pretreated patients at one year is 54 vs. 70%,
respectively, at two years 25 vs. 40% at three years 19 vs. 30%,
and at four years still 13 vs. 25% (Fig. 4). The response rates are summarized
in Table III. The annual survival
rates of 1-4 years for all patients together and selectively at
FIGO IIIC and IV stage are summarized in Table IV.
 | Table IVAnnual survival rates of 1-4 years of
all pretreated FIGO stage IIIC and IV patients (n=87) with
recurrent disease. |
Table IV
Annual survival rates of 1-4 years of
all pretreated FIGO stage IIIC and IV patients (n=87) with
recurrent disease.
| Stage | 1-year OS, % | 2-year OS, % | 3-year OS, % | 4-year OS, % | 13-year OS, % |
|---|
| FIGO IIIC/IV
(n=87) | 47.1 | 23.8 | 16.4 | 9.8 | - |
| FIGO IIIC
(n=46) | 54.3 | 25.2 | 19.2 | 12.8 | 3.1 |
| FIGO IV (n=41) | 39.0 | 22.0 | 13.2 | 9.5 | - |
Toxicity
Bone marrow depression ranged between WHO grade 1
and 2. Only patients with a reduced bone marrow reserve after
stressful third and fourth-line therapy had leucopenia and
thrombocytopenia grade 3 even after perfusion therapy with
chemofiltration. WHO grade 4 toxicity and neutropenic fever as well
as neuropathy in terms of hand-foot-syndrome were never observed.
In the event of rapid tumor necrosis, which can occur during the
first three post-therapeutic days, the patient report tiredness and
fatigue with a simultaneous increase in LDH and tumor marker, which
falls below the initial value within a few days. The syndrome
occurs in 10-15% of patients, accompanied by fever and lassitude
(Table V). In general, the
performance of patients improves after perfusion therapy with
chemofiltration from therapy to therapy (29).
 | Table VToxicity profile after isolated
hypoxic abdominal perfusion with cisplatin, adriamycin and
mitomycin for advanced ovarian cancer. |
Table V
Toxicity profile after isolated
hypoxic abdominal perfusion with cisplatin, adriamycin and
mitomycin for advanced ovarian cancer.
| Adverse event | Extent |
|---|
| Bone marrow
suppression | WHO grade I/II |
| Fatigue
syndrome | 15-20% |
| Transient elevation
of creatinin | 15% |
| Neutropenic
fever | 0% |
|
Hand-foot-syndrome | 0% |
Discussion
Ovarian cancer is the leading cause of death, and
cure rates between 12-14% have changed little over the past few
decades. Debulking operations in terms of complete cytoreduction
and platinum-based chemotherapy are considered the cornerstones of
current therapy.
On the remaining options, an increase in dose in
systemic chemotherapy is limited by the increasing toxicity
(8,9). Because of the angiogenic properties of
ovarian cancer, targeted therapies appeared to be a logical and
generally accepted option (21). On
the one hand, they were complicated by not inconsiderable
collateral damage such as perforations in the intestine due to
microcirculation disorders in the intestinal wall, bleedings,
proteinuria and high blood pressure. On the other hand, the
clinical results with bevacizumab were not necessarily convincing;
the median survival of 6.9 months in a group of 32 multiple
chemically pretreated patients after treatment with bevacizumab was
rated as good, but did not reach the 11.3 months of a comparable
group after isolated perfusion therapy which corresponds to an
increase of over 60% (23). Due to
the large difference in survival, this may indicate a trend towards
isolated perfusion, but is of limited importance due to the small
number of cases, not necessarily comparable patient groups and the
lack of randomization. In spite of all restrictions, in addition to
the good survival times, the minor side effects with mostly rapid
improvement in the quality of life through ‘segmental’ limited
chemotherapy with subsequent detoxification are of primary
importance. The basic prerequisite for any cancer treatment should
be the prolongation of life while maintaining and if possible
improving the quality of life. No other fundamental necessity for
any treatment to be recommended should actually apply (24). Side effects scales in patients who
were progressive after systemic therapies of different tumor
entities and then received regional chemotherapy (RCT) showed
significantly fewer side effects and better quality of life after
regional chemotherapy (29). The
reduction or removal of all tumor masses is a prerequisite for
thorough treatment of advanced ovarian cancer. Debulking surgery
with no or less than 1 cm residual tumor is also considered a
decisive factor for the long-term prognosis. Basically, the aim is
to achieve this, but the greater the tumor mass, the more difficult
it is to achieve.
Primary surgery for complete cytoreduction is not
possible in advanced stage IV ovarian cancers; these patients can
only be treated with chemotherapy.
The DESKTOP III study, comparing chemotherapy and
tumor debulking surgery vs. chemotherapy alone was the first
prospectively randomized trial showing an overall survival benefit
of debulking surgery in recurrent ovarian cancer (30). In other studies with secondary
cytoreduction combined with platinum-based chemotherapy in relapsed
ovarian cancer, no differences were found in progression-free
survival and overall survival, which may be due to different
clinical expertise in the radicality of cytoreduction, but also in
biological criteria and medication, including the role of
bevacizumab in combination therapy is still unclear (31-34).
The preoperative tumor load also influences survival, just as
solitary recurrent disease is associated with a much better life
expectancy (35).
HIPEC pursues the purpose of increased exposure to
all peritoneal surfaces after debulking surgery and the best
results are expected after debulking to zero or at least less than
1 cm residual disease because the depth of penetration of
cytostatics under hyperthermic peritoneal irrigation is at most 2
mm (36). Although survival
benefits have been shown in randomized studies (4,18,19),
the HIPEC procedure is not regarded as the standard of care for
first-line therapy of ovarian cancer, not least because of its high
toxicity (37-39).
On the other hand, novel methodologies are under
investigation, such as PIPAC, pressurized intraperitoneal aerosol
chemotherapy (40) and precision
chemotherapy using liquid biopsies for chemosensitivity and tumors
gene expression assays (41).
The goal of all efforts in the treatment of ovarian
cancer, and especially advanced ovarian cancer, is to increase the
efficiency of the therapy used without affecting the quality of
life due to unacceptable toxicity. It is a well-known rule that a
higher local active drug concentration causes a better response.
The limiting factor in higher exposure to cytostatics that may be
effective is toxicity. With high-dose therapy limited to one region
of the body only (28), the tumor
can be permanently damaged with good long-term results. A further
prolongation of progression-free and overall survival after initial
isolated perfusion according to recent data (42-44)
might be maintenance therapy with PARP-inhibitors.
Hypoxic abdominal perfusion with chemofiltration on
the other hand, is relatively uncomplicated with some vascular
surgery experience (28), without
the need for follow-up treatment in an intensive care unit.
Patients report little or very rarely about relevant side effects,
while a decrease in tumor markers and stressful symptoms such as
ascites or general discomfort is observed very often. The most
striking feature of the isolated perfusion therapy is the high
effectiveness with quick onset of tumor response with hardly any
side effects and preserved, often improved quality of life.
Acknowledgements
The authors wish to acknowledge Mr. Giuseppe
Zavattieri (Department for Surgical Oncology, Medias Klinikum
Burghausen, Burghausen, Germany) for his help and assistance in
preparing the manuscript and statistics used in this report.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KRA, SG and KA conceived the study. KRA and SG
developed the methodology and assessed the authenticity of the
data. KRA, SG and KA validated the data. KRA and KA performed
formal analysis. KRA and SG performed the investigation. KRA and KA
provided resources. KRA, SG and KA curated the data. KRA wrote the
original draft. KRA and KA reviewed and edited the manuscript. KRA
was involved in visualization. KRA supervised the study. KRA was
responsible for project administration. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Investigations were performed in compliance with the
principles of good clinical practice outlined in the Declaration of
Helsinki and federal guidelines, and had approval by the Medias
Institutional Review Committee (permit number MIRB20200515;
Burghausen, Germany). Written informed consent was obtained from
each participant or participant's guardian.
Patient consent for publication
Consent for publication was obtained from any
individual person whose data are included in this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gourley C and Bookman MA: Evolving
concepts in the management of newly diagnosed epithelial ovarian
cancer. J Clin Oncol. 37:2386–2398. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Meyer LA, Cronin AM, Sun CC, Bixel K,
Bookman MA, Cristea MC, Griggs JJ, Levenback CF, Burger RA,
Mantia-Smaldone G, et al: Use and effectiveness of neoadjuvant
chemotherapy for treatment of ovarian cancer. J Clin Oncol.
34:3854–3863. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ,
Panici PB, et al: Neoadjuvant chemotherapy or primary surgery in
Stage IIIC or IV ovarian cancer. N Engl J Med. 363:943–953.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vergote I, Van Nieuwenhuysen E and
Vanderstichele A: How to select neoadjuvant chemotherapy or primary
debulking surgery in patients with Stage IIIC or IV ovarian
carcinoma. J Clin Oncol. 34:3827–3828. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gore ME, Fryatt I and Wiltshaw E:
Treatment of relapsed carcinoma of the ovary with cisplatin or
carboplatin following initial treatment with these compounds.
Gynecol Oncol. 36:207–211. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393.
1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ozols RF: Treatment of recurrent ovarian
cancer: Increasing options-‘recurrent’ results. J Clin Oncol.
15:2177–2180. 1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gore M, Mainwaring P, A'Hern R, MacFarlane
V, Slevin M, Harper P, Osborne R, Mansi J, Blake P, Wiltshaw E and
Shepherd J: Randomized trial of dose-intensity with single-agent
carboplatin in patients with epithelial ovarian cancer. London
Gynaecological Oncology Group. J Clin Oncol. 16:2426–2434.
1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jodrell DI, Egorin MJ, Canetta RM,
Langenberg P, Goldbloom EP, Burroughs JN, Goodlow JL, Tan S and
Wiltshaw E: Relationships between carboplatin exposure and tumor
response and toxicity in patients with ovarian cancer. J Clin
Oncol. 10:520–528. 1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McGuire WP, Hoskins WJ, Brady MF, Homesley
HD, Creasman WT, Berman ML, Ball H, Berek JS and Woodward J:
Assessment of dose-intensive therapy in suboptimally debulked
ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol.
13:1589–1599. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Markman M, Liu PY, Moon J, Monk BJ,
Copeland L, Wilczynski S and Alberts D: Impact on survival of 12
versus 3 monthly cycles of paclitaxel (175 mg/m²) administered to
patients with advanced ovarian cancer who attained a complete
response to primary platinum-paclitaxel: Follow-up of a Southwest
Oncology Group and Gynecologic Oncology Group phase 3 trial.
Gynecol Oncol. 114:195–198. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Omura GA, Brady MF, Look KY, Averette HE,
Delmore JE, Long HJ, Wadler S, Spiegel G and Arbuck SG: Phase III
trial of paclitaxel at two dose levels, the higher dose accompanied
by filgrastim at two dose levels in platinum-pretreated epithelial
ovarian cancer: An intergroup study. J Clin Oncol. 21:2843–2848.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thigpen JT: Dose-intensity in ovarian
carcinoma: Hold, enough? J Clin Oncol. 15:1291–1293.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Grénman S, Wiklund T, Jalkanen J, Kuoppala
T, Mäenpää J, Kuronen A, Leminen A, Puistola U, Vuolo-Merilä P,
Salmi T, et al: A randomised phase III study comparing high-dose
chemotherapy to conventionally dosed chemotherapy for stage III
ovarian cancer: The Finnish Ovarian (FINOVA) study. Eur J Cancer.
42:2196–2199. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Möbus V, Wandt H, Frickhofen N, Bengala C,
Champion K, Kimmig R, Ostermann H, Hinke A and Ledermann JA:
AGO-Ovar/AIO; EBMT. Phase III trial of high-dose sequential
chemotherapy with peripheral blood stem cell support compared with
standard dose chemotherapy for first-line treatment of advanced
ovarian cancer: Intergroup trial of the AGO-Ovar/AIO and EBMT. J
Clin Oncol. 25:4187–4193. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fung MF, Johnston ME, Eisenhauer EA, Elit
L, Hirte HW and Rosen B: Cancer Care Ontario Practice Guidelines
Initiative Gynecology Disease Site Group. Chemotherapy for
recurrent epithelial ovarian cancer previously treated with
platinum-a systematic review of the evidence from randomized
trials. Eur J Gynaec Oncol. 23:104–110. 2002.PubMed/NCBI
|
|
17
|
Du Bois A, Weber B, Rochon J, Meier W,
Goupil A, Olbricht S, Barats JC, Kuhn W, Orfeuvre H, Wagner U, et
al: Addition of epirubicin as a third drug to
Carboplatin-paclitaxel in first-line treatment of advanced ovarian
cancer: A prospectively randomized gynecologic cancer intergroup
trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian
Cancer Study Group and the Groupe d'Investigateurs Nationaux pour
l'Etude des Cancers Ovariens. J Clin Oncol. 24:1127–1135.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Spiliotis J, Halkia E, Lianos E, Kalantzi
N, Grivas A, Efstathiou E and Giassas S: Cytoreductive surgery and
HIPEC in recurrent epithelial ovarian cancer: A prospective
randomized phase III study. Ann Surg Oncol. 22:1570–1575.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Van Driel WJ, Koole SN, Sikorska K,
Schagen van Leeuwen JH, Schreuder HW, Hermans RH, de Hingh IH, van
der Velden J, Arts HJ, Massuger LF, et al: Hyperthermic
intraperitoneal chemotherapy in ovarian cancer. N Engl J Med.
378:230–240. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA:
Gynecologic Oncology Group. Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yi S, Zeng L, Kuan Y, Cao Z, Zheng C,
Zhang Y, Liao M and Yang L: Antiangiogenic drugs used with
chemotherapy for patients with recurrent ovarian cancer: A
meta-analysis. Onco Targets and Therapy. 10:973–984.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stone RL, Sood AK and Coleman RL:
Collateral damage: Toxic effects of targeted antiangiogenic
therapies in ovarian cancer. Lancet Oncol. 11:465–475.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Monk BJ, Han E, Joseph-Cowen CA, Pugmire G
and Burger RA: Salvage bevacizumab-(rhuMABVEGF)-based therapy after
multiple prior cytotoxic regimens in advanced refractory epithelial
ovarian cancer. Gynecol Oncol. 102:140–144. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cannistra SA: The ethics of early stopping
rules: Who is protecting whom? J Clin Oncol. 22:1542–1545.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aigner KR and Gailhofer S: High dose MMC:
Aortic stopflow infusion (ASI) with versus without chemofiltration:
A comparison of toxic side effects (abstract). Reg Cancer Treat. 6
(Suppl 1)(S3)1993.
|
|
26
|
Aigner KR, Tonn JC, Hechtel R and Seuffer
R: Die intraarterielle Zytostatikatherapie mit venöser Filtration
im halboffenen System. Onkologie. 6:74–76. 1983.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Teicher BA, Lazo JS and Sartorelli A:
Classification of antineoplastic agents by their selective
toxicities toward oxygenated and hypoxic tumor cells. Cancer Res.
41:73–81. 1981.PubMed/NCBI
|
|
28
|
Aigner KR, Selak E, Gailhofer S and Aigner
K: Hypoxic Isolated Abdominal Perfusion (HAP) chemotherapy for
non-operable advanced staged ovarian cancer with peritoneal
carcinosis: An experience in 45 platinum-refractory ovarian cancer
patients. Indian J Surg Oncol. 10:506–514. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aigner KR and Knapp N: Toxicity Profiles
with Systemic vs. Regional Chemotherapy. In: Induction
Chemotherapy-Systemic and Locoregional. 2nd edition. KR Aigner and
FO Stephens (eds). Springer Verlag Berlin, pp497-506, 2016.
|
|
30
|
Du Bois A, Vergote I, Ferron G, Reuss A,
Meier W, Greggi S, Jensen PT, Selle F, Guyon F, Pomel C, et al:
Randomized controlled phase III study evaluating the impact of
secondary cytoreductive surgery in recurrent ovarian cancer: AGO
DESKTOP III/ENGOT ov20. J Clin Oncol. 35(5501)2017.
|
|
31
|
Shanghai Gynecologic Oncology Group.
Surgery or Chemotherapy in Recurrent Ovarian Cancer (SOC 1 Trial).
National Library of Medicine, Bethesda, MD, 2018. Available from:
www.clinicaltrials.gov/ct2/show/record/NCT01611766.
|
|
32
|
Zang R and Zhu J: Which patients benefit
from secondary cytoreductive surgery in recurrent ovarian cancer? J
Gynecol Oncol. 30(e116)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Coleman RL, Enserro D, Spirtos N, Herzog
TJ, Sabbatini P, Armstrong DK, Kim B, Fujiwara K, Walker JL, Flynn
PJ, et al: A phase III randomized controlled trial of secondary
surgical cytoreduction (SSC) followed by platinum-based combination
chemotherapy (PBC), with or without bevacizumab (B) in
platinum-sensitive, recurrent ovarian cancer (PSOC): A NRG
Oncology/Gynecologic Oncology Group (GOG) study. J Clin Oncol.
36:5501. 2018.
|
|
34
|
Coleman RL, Spirtos NM, Enserro D, Herzog
TJ, Sabbatini P, Armstrong DK, Kim JW, Park SY, Kim BG, Nam JH, et
al: Secondary surgical cytoreduction for recurrent ovarian cancer.
N Engl J Med. 381:1929–1939. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vargas HA, Burger IA, Goldman DA, Miccò M,
Sosa RE, Weber W, Chi DS, Hricak H and Sala E: Volume-based
quantitative FDG PET/CT metrics and their association with optimal
debulking and progression-free survival in patients with recurrent
ovarian cancer undergoing secondary cytoreductive surgery. Eur
Radiol. 25:3348–3353. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Van de Vaart PJ, van der Vange N,
Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink
WW, Beijnen JH, Bartelink H and Begg AC: Intraperitoneal cisplatin
with regional hyperthermia in advanced ovarian cancer:
Pharmacokinetics and cisplatin-DNA adduct formation in patients and
ovarian cancer cell lines. Eur J Cancer. 34:148–154.
1998.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vergote I, Harter P and Chiva L: Is there
a role for intraperitoneal chemotherapy, including HIPEC, in the
management of ovarian cancer? J Clin Oncol. 37:2420–2423.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gore M, du Bois A and Vergote I:
Intraperitoneal chemotherapy in ovarian cancer remains
experimental. J Clin Oncol. 24:4528–4530. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Colombo N, Sessa C, du Bois A, Ledermann
J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I,
Vergote I, et al: ESMO-ESGO Consensus Conference on Ovarian Cancer:
Pathology and molecular biology, early and advanced stages,
borderline ovarian tumours and recurrent disease. Ann Oncol.
30:672–705. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tempfer CB, Winnekendonk G, Solass W,
Horvat R, Giger-Pabst U, Zieren J, Rezniczek GA and Reymond MA:
Pressurized intraperitoneal aerosol chemotherapy in women with
recurrent ovarian cancer: A phase 2 study. Gynecol Oncol.
137:223–228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guadagni S, Clementi M, Masedu F,
Fiorentini G, Sarti D, Deraco M, Kusamura S, Papasotiriou I,
Apostolou P, Aigner KR, et al: A pilot study of the predictive
potential of chemosensitivity and gene expression assays using
circulating tumour cells from patients with recurrent ovarian
cancer. Int J Mol Sci. 21(4813)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Del Campo JM, Matulonis UA, Malander S,
Provencher D, Mahner S, Follana P, Waters J, Berek JS, Woie K, Oza
AM, et al: Niraparib maintenance therapy in patients with recurrent
ovarian cancer after a partial response to the last platinum-based
chemotherapy in the ENGOT-OV16/NOVA Trial. J Clin Oncol.
37:2968–2973. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Poveda A: Final overall survival (OS)
results from SOLO2/ENGOT-ov21: A phase III trial assessing
maintenance olaparib in patients (pts) with platinum-sensitive,
relapsed ovarian cancer and a BRCA mutation. Presented at: 2020
ASCO Virtual Scientific Program; Abstract 6002, May 12, 2020.
|
|
44
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance Olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505.
2018.PubMed/NCBI View Article : Google Scholar
|