Introduction
Due to the progression of neoadjuvant treatment
strategies and surgical procedures, 3- and 5-year survival rates of
patients who underwent esophagectomy with no residual tumors
improved to 71.4 and 62.8%, respectively (1). However, recurrence still occurs in
28-56% of patients who underwent radical resection of esophageal
cancer, with a median time to recurrence of 10-12 months (2,3). The
median survival time after recurrence remains poor at 8.2 months
(4). Recurrent esophageal cancer is
considered a systemic disease, for which chemotherapy with
cisplatin and 5-fluorouracil (5-FU) is used as the standard therapy
(2). In a phase II study reported
by Bleiberg et al (5),
chemotherapy with cisplatin and 5-FU yielded a response rate of 35%
and a median survival time of 7.6 months. In another phase II study
reported by Iizuka et al (6), chemotherapy with cisplatin and 5-FU
yielded a response rate of 35.9% and a median survival time of 9.5
months for patients who responded to the treatment.
Oligometastasis is a disease concept defined as a
limited number of systemic metastatic tumors, usually characterized
by fewer than five metastases, where curative strategies may be
effective (7,8). Recent reports have demonstrated that
local therapy, such as resection, radiofrequency ablation (RFA),
and radiotherapy, for oligometastasis from esophageal cancer can
have a promising long-term prognosis (8-11).
Regarding RFA, Baba et al (11) reported that the percutaneous
computed tomography (CT)-guided RFA for pulmonary metastases from
esophageal squamous cell carcinoma (ESCC) yielded 83% of local
control of ablated tumor lasting for at least 1 year. The predicted
1- and 2-year overall survival rates after lung RFA were 77.8 and
62.2%, respectively. The treatment strategy for recurrent
esophageal cancer needs to be considered individually according to
the extent of recurrence, recurrent site, and recurrent pattern
(2).
Stereotactic body radiotherapy (SBRT) has been
identified as a modern radiation technique that delivers high doses
of radiation to the target, while limiting the doses to the
surrounding healthy tissue (12).
SBRT for oligometastatic cancer has been reported to demonstrate
good disease control, with acceptable rates of acute and late grade
3 or higher toxicities (13,14).
However, even among those cases, a clinical complete response of
liver oligometastasis from ESCC is extremely rare. Here we present
a case of a patient with postoperative solitary liver metastasis
from ESCC who achieved a clinical complete response to chemotherapy
with cisplatin and 5-fluorouracil followed by SBRT.
Case report
A 76-year-old man underwent esophagectomy with
gastric tube reconstruction through the retrosternal route with
cervical anastomosis for lower thoracic ESCC. The patient was
diagnosed with T1bN0M0, stage IB according to the Union for
International Cancer Control TNM classification of malignant
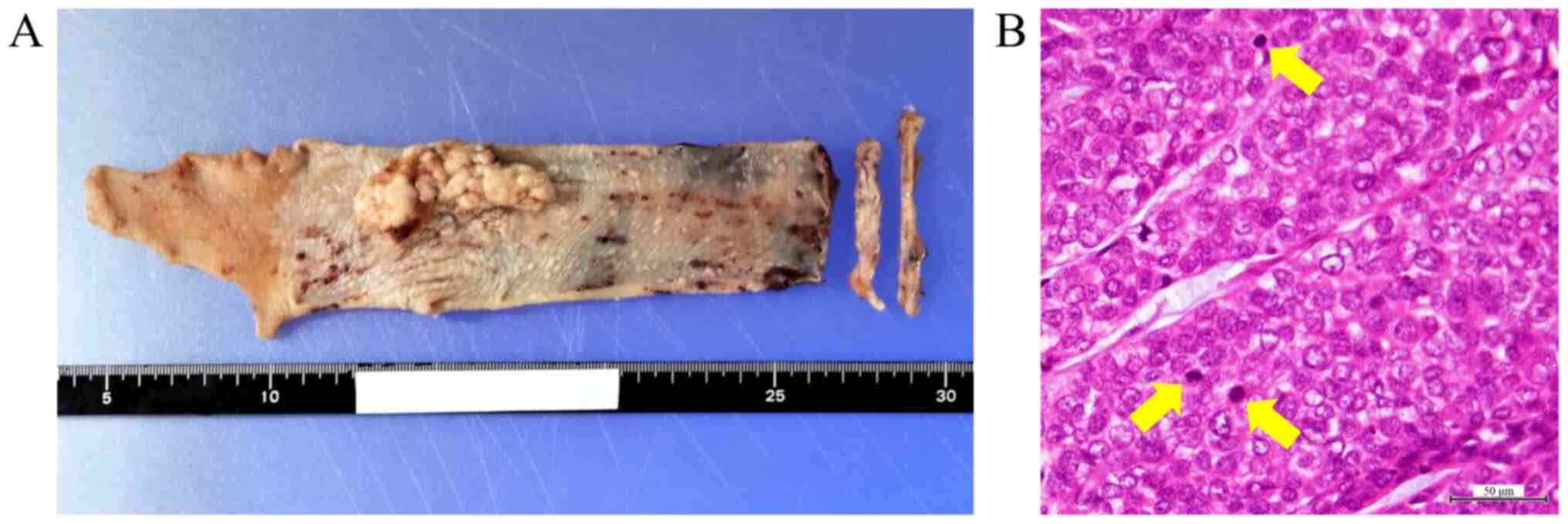
tumors, 8th edition. The histopathological findings confirmed a
poorly differentiated squamous cell carcinoma (Fig. 1). The resected margins were
determined to be free of tumor cells. No lymphatic invasion was
observed; however, slight venous invasion was detected.
Four months after surgery, abdominal
contrast-enhanced CT revealed no recurrence. Tumor markers were
within the normal ranges: Fragment of cytokeratin subunit 19 (CYFRA
21-1), 2.9 ng/ml (normal level, < 3.5 ng/ml) and squamous cell
carcinoma antigen (SCC-Ag), 1.4 ng/ml (normal level, <1.5
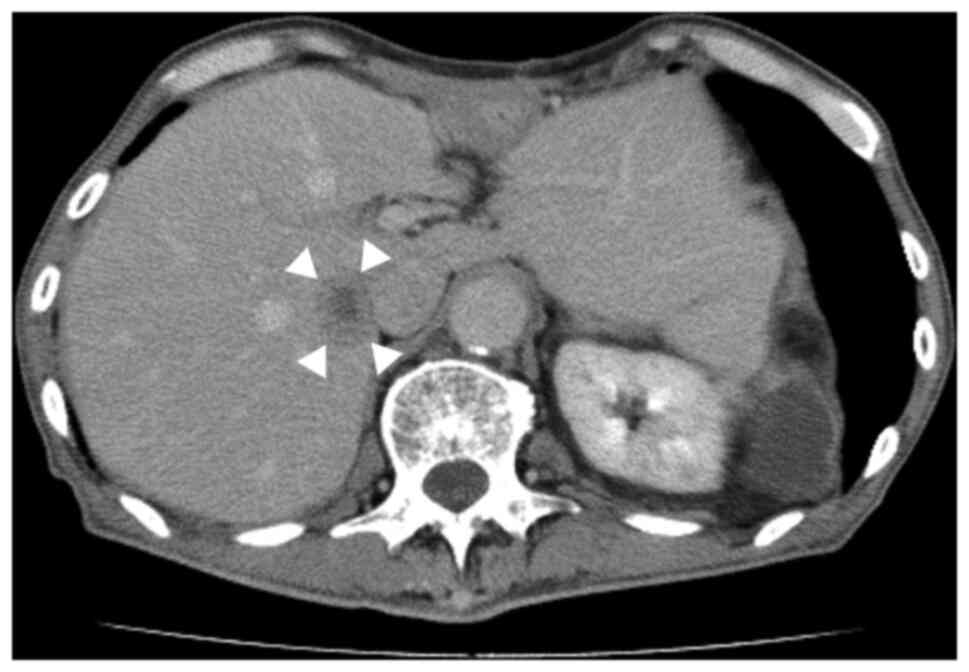
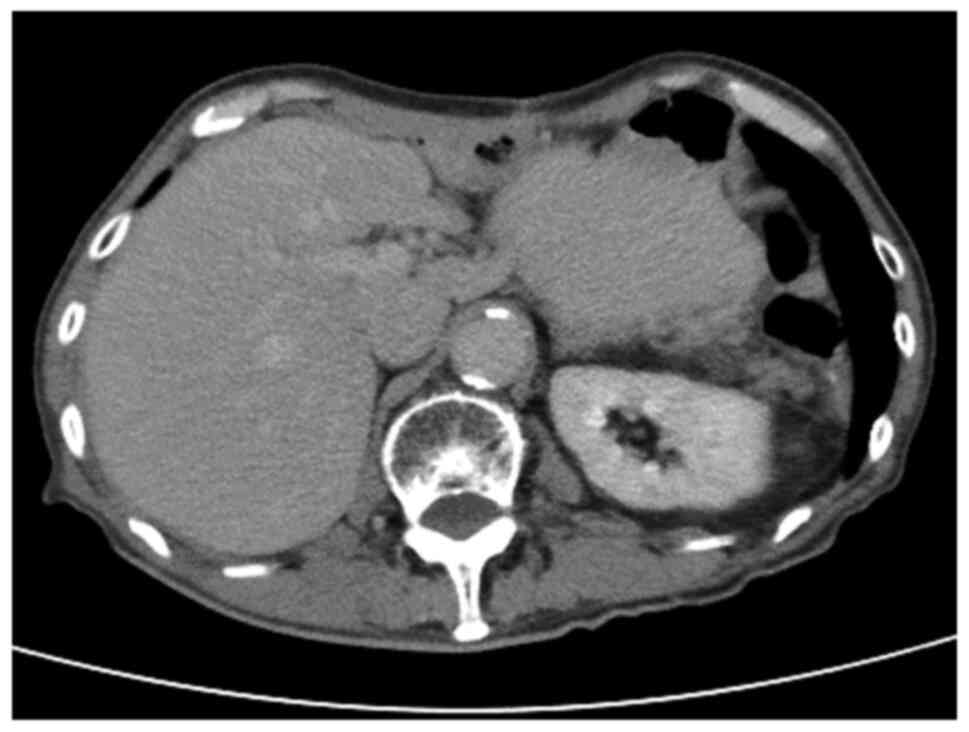
ng/ml). However, 7 months after surgery, abdominal
contrast-enhanced CT revealed a solitary hypovascular mass in
segment 7 of the liver (Fig. 2).
The mass was 28 mm in size and was located adjacent to the inferior
vena cava. No segmental enhancement of the liver parenchyma around
the liver mass at the early phase was observed, which was a
characteristic CT finding of liver abscess. Furthermore, no
enhancement of the liver mass and washout at the early and late
phases, respectively, were observed, which were characteristic CT
findings of hepatocellular carcinoma. Laboratory results showed no
inflammation. The patient was negative for both hepatitis B virus
surface antigen and hepatitis C virus antibody. Therefore, the
possibility that the liver mass was liver abscess or hepatocellular
carcinoma was ruled out. The risk of esophageal cancer recurrence
was considered high due to the pathological findings of poorly
differentiated squamous cell carcinoma and venous invasion.
Regarding tumor markers, CYFRA 21-1 was within the normal range
(2.9 ng/ml); however, SCC-Ag was slightly elevated (2.1 ng/ml).
Given these results, the patient was diagnosed with postoperative
solitary liver metastasis. Considering the early postoperative
recurrence and rapid growth within 3 months, it was determined that
systemic chemotherapy was necessary.
The patient then underwent chemotherapy consisting
of cisplatin at 75 mg/m2 administered by rapid
intravenous infusion on day 1 and 5-FU at 1,000 mg/m2
administered by continuous intravenous infusion on days 1-5,
separated by a 4-week interval. The treatment was well tolerated,
with no grade 3 or higher adverse events. After 3 courses of
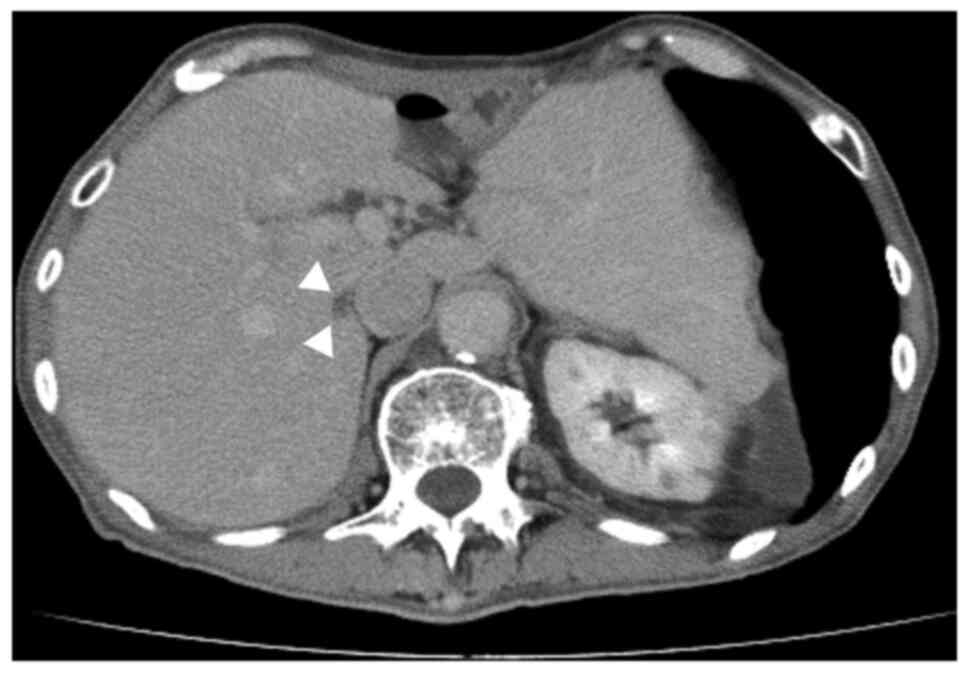
chemotherapy, abdominal contrast-enhanced CT revealed that the
liver mass has shrunk to 7 mm in size, with no evidence of further
metastatic lesions (Fig. 3).
Gd-EOB-DTPA-enhanced magnetic resonance imaging has also confirmed
the reduction in size and absence of any no other liver metastases.
After discussion at a multidisciplinary cancer conference, SBRT at
the liver mass was planned.
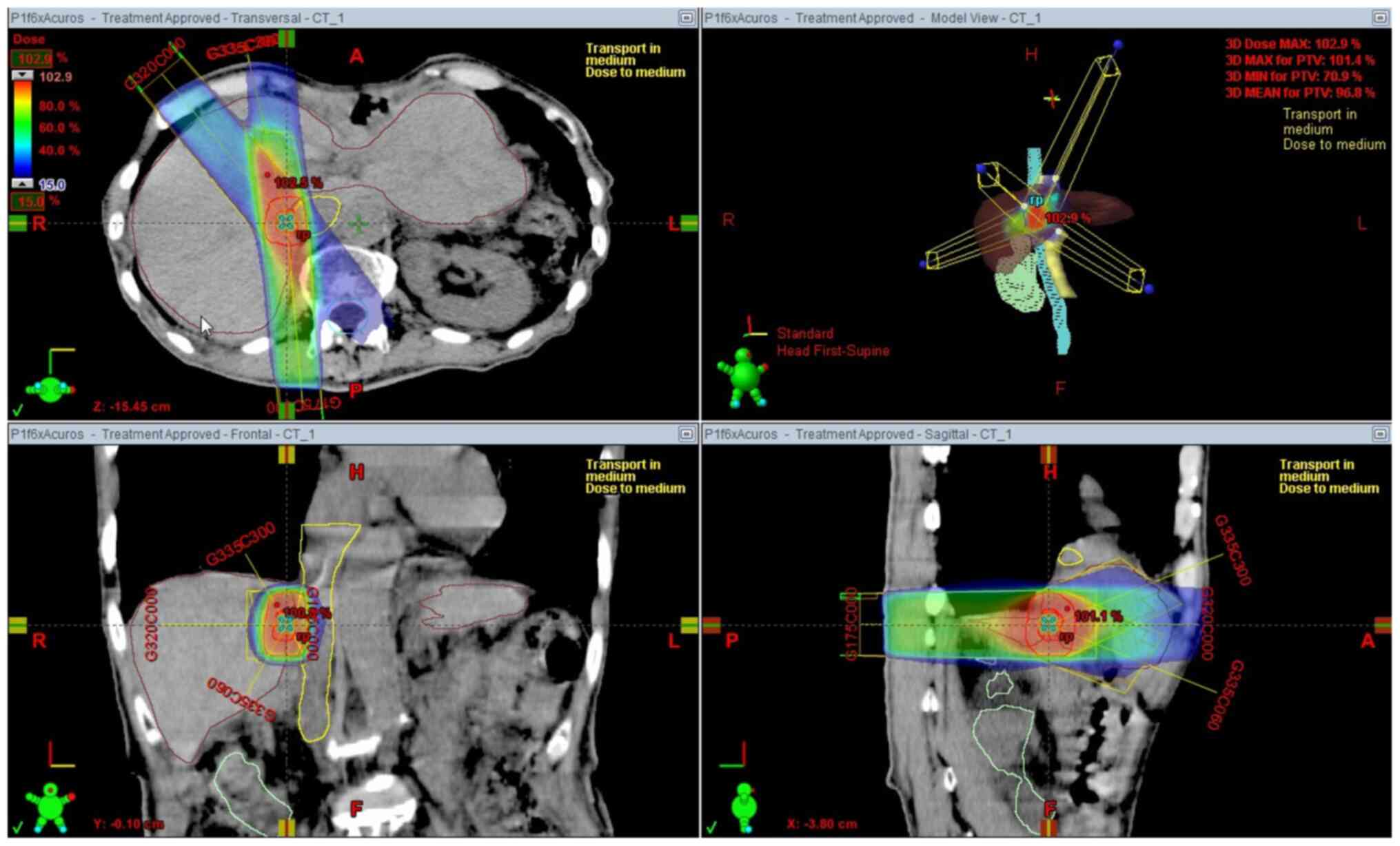
SBRT was administered with a 6 MV X-ray beam
generated by a linear accelerator (Clinac iX; Varian Medical
Systems). A total dose of 50 Gy was given in 5 fractions of 10 Gy
to the liver mass (Fig. 4). The
patient did not develop any acute or late toxicities. One month
after SBRT, abdominal contrast-enhanced CT revealed that the liver
mass has disappeared (Fig. 5).
Furthermore, positron emission tomography revealed no significant
accumulation of 18F-fluorodeoxyglucose. Thus, a clinical
complete response of the liver metastasis was achieved. The patient
received no further adjuvant chemotherapy and had no recurrence at
18 months after diagnosis of liver metastasis and 13 months after
SBRT.
Discussion
In this present case, a patient achieved a clinical
complete response to postoperative solitary liver metastasis from
ESCC with a combination of systemic therapy with chemotherapy and
local therapy with SBRT. Esophageal cancer has been determined to
be an aggressive and relatively common cancer worldwide; it is the
sixth leading cause of cancer-related mortality and the eighth most
common cancer worldwide (15). The
prognosis is generally very poor despite recent advances in
multidisciplinary treatment if distant organ metastasis occurs
after curative treatment for esophageal cancer. Thus, there has
been an urgent need to establish an effective treatment strategy
for recurrent esophageal cancer. To the best of our knowledge, this
present case is the first reported in the English literature of
liver metastasis in an ESCC patient achieving a clinical complete
response to chemotherapy followed by SBRT.
Treatment strategies for distant organ metastasis
have changed dramatically since the introduction of the concept of
oligometastasis. The perception that distant organ metastasis is a
systemic disease and not eligible for local therapy is now a thing
of the past. In general, chemotherapy works throughout the whole
body, but it appears to be marginally effective only in high
sensitivity cases (16). In
advanced/recurrent ESCC patients, the response rate of cisplatin
and 5-FU is 35-35.9%, but the evidence for prolonged survival
remains unclear, and it is usually administered with palliative
intent (2,5,6). On
the other hand, local therapy works at the targeted site and can be
curative, if treated radically. In this present case, the
recurrence was solitary, but because of the early postoperative
recurrence and rapid growth within 3 months, we were concerned
about the aggressiveness of the tumor growth and considered the
possibility of rapid multiple liver metastases and metastasis to
other sites. Therefore, we chose systemic therapy with chemotherapy
instead of local therapy. Fortunately, the tumor shrank and no
additional metastases were observed. We then chose SBRT, which has
a high local control effect, and it resulted in remission the liver
metastasis. Our treatment strategy was consistent with Morinaga
et al (17) who reported
that chemotherapy with local therapy, such as resection, RFA, and
radiotherapy, is a promising treatment modality for patients with
oligometastatic recurrence after curative resection of ESCC.
Ohkura et al (10) reported that the overall survival
rate was significantly better for patients who underwent resection
of oligometastasis from esophageal cancer than for those who did
not (3-year survival rates, 64.3 vs. 10%, respectively; 5-year
survival rates, 55.6 vs. 10%, respectively). Alternatively, SBRT is
a radiation technique that enables irradiation of a small target in
the body using a high dose. SBRT is useful in terms of quality of
life because it is deemed noninvasive and has a short treatment
time in addition to its curative potential. Rusthoven et al
(18) reported excellent results of
a multi-institutional phase I/II trial of SBRT for liver
metastases. It reported at a median follow-up of 16 months
actuarial in-field local control rates at 1 and 2 years of 95 and
92%, respectively. Among lesions < 3 cm, 2-year local control
was 100%. Furthermore, only 2% of patients experienced grade 3 or
higher toxicities. Resection of metastasis is invasive, and there
are many cases where surgery is not indicated due to lack of
surgical tolerance or patient's will. Thus, SBRT can be a good
treatment option, especially when resection of metastases is not
applicable.
Recently, a consensus recommendation by the European
Society for Radiotherapy and Oncology and European Organization for
Research and Treatment of Cancer (ESTRO-EORTC) suggested dividing
oligometastatic disease into synchronous (the interval time from
initial diagnosis to metastasis within 6 months) and metachronous
(the interval time from initial diagnosis to metastasis more than 6
months) (9,19). Furthermore, metachronous
oligometastatic disease in patients not under active systemic
therapy is classified as metachronous oligorecurrence (19). Thus, this present case falls into
this classification. Whether the treatment strategy for
metachronous oligorecurrence is a local therapy alone, systemic
therapy alone, or combination of local and systemic therapies has
not yet been established. In this present case, liver
oligometastasis from ESCC was successfully treated with systemic
therapy using chemotherapy and subsequent local therapy with SBRT.
We think it makes sense to proceed with systemic therapy before
starting local therapy, considering the possibility of microscopic
metastases to liver and other sites. In general, there is a very
poor prognosis for esophageal cancer patients with recurrence
within 12 months after radical esophagectomy (4,20).
Furthermore, liver recurrence is often associated with shortened
survival period (4). Nevertheless,
successful treatment was achieved in our case of an early
postoperative liver metastasis from ESCC.
In conclusion, this present report detailed the case
of a patient achieving a clinical complete response to
postoperative solitary liver metastasis from ESCC by chemotherapy
with cisplatin and 5-FU followed by SBRT. As this was a
single-patient case report, our findings need to be confirmed by
accumulating prospective evidence from more patients. However,
these current findings provide important information that can
contribute to the development of a treatment strategy for liver
oligometastasis from ESCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SF conceived and designed the study. SF, KOkaj, KF,
TW and YO acquired the data. SF, KOkad, TT, AG, HT, KOh, KK, KH,
HI, JIH, MY and MI analyzed and interpreted the data. SF drafted
the manuscript. SF, KOkaj and TW revised the manuscript critically
for important intellectual content. SF, KOkaj and TW are
responsible for confirming the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Kindai University Nara Hospital (approval no.
20-25; Nara, Japan).
Patient consent for publication
Written informed consent for the publication of data
and images was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Watanabe M, Tachimori Y, Oyama T, Toh Y,
Matsubara H, Ueno M, Kono K, Uno T, Ishihara R, Muro K, et al:
Comprehensive registry of esophageal cancer in Japan, 2013.
Esophagus. 18:1–24. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kitagawa Y, Uno T, Oyama T, Kato K, Kato
H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al:
Esophageal cancer practice guidelines 2017 edited by the Japan
esophageal society: Part 2. Esophagus. 16:25–43. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huddy JR, Thomas RL, Worthington TR and
Karanjia ND: Liver metastases from esophageal carcinoma: Is there a
role for surgical resection? Dis Esophagus. 28:483–487.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miyata H, Yamasaki M, Kurokawa Y,
Takiguchi S, Nakajima K, Fujiwara Y, Konishi K, Mori M and Doki Y:
Survival factors in patients with recurrence after curative
resection of esophageal squamous cell carcinomas. Ann Surg Oncol.
18:3353–3361. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bleiberg H, Conroy T, Paillot B, Lacave
AJ, Blijham G, Jacob JH, Bedenne L, Namer M, De Besi P, Gay F, et
al: Randomised phase II study of cisplatin and 5-fluorouracil
(5-FU) versus cisplatin alone in advanced squamous cell oesopahgeal
cancer. Eur J Cancer. 33:1216–1220. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iizuka T, Kakegawa T, Ide H, Ando N,
Watanabe H, Tanaka O, Takagi I, Isono K, Ishida K, Arimori M, et
al: Phase II evaluation of cisplatin and 5-fluorouracil in advanced
squamous cell carcinoma of the esophagus: A Japanese esophageal
oncology group trial. Jpn J Clin Oncol. 22:172–176. 1992.PubMed/NCBI
|
|
7
|
Hellman S and Weichselbaum RR:
Oligometastases. J Clin Oncol. 13:8–10. 1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jamel S, Tukanova K and Markar S:
Detection and management of oligometastatic disease in oesophageal
cancer and identification of prognostic factors: A systematic
review. World J Gastrointest Oncol. 11:741–749. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li J, Wen Y, Xiang Z, Du H, Geng L, Yang
X, Zhang Y, Bai J, Dai T, Feng G, et al: Radical radiotherapy for
metachronous oligometastasis after initial treatment of esophageal
cancer. Radiother Oncol. 154:201–206. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ohkura Y, Shindoh J, Ueno M, Iizuka T and
Udagawa H: Clinicopathologic characteristics of oligometastases
from esophageal cancer and long-term outcomes of resection. Ann
Surg Oncol. 27:651–659. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baba Y, Watanabe M, Kawanaka K, Iwagami S,
Ishimoto T, Iwatsuki M, Yoshida N, Yamashita Y and Baba H:
Radiofrequency ablation for pulmonary metastases from esophageal
squamous cell carcinoma. Dis Esophagus. 27:36–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dawood O, Mahadevan A and Goodman KA:
Stereotactic body radiation therapy for liver metastases. Eur J
Cancer. 45:2947–2959. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lehrer EJ, Singh R, Wang M, Chinchilli VM,
Trifiletti DM, Ost P, Siva S, Meng MB, Tchelebi L and Zaorsky NG:
Safety and survival rates associated with ablative stereotactic
radiotherapy for patients with oligometastatic cancer: A systematic
review and meta-analysis. JAMA Oncol. 7:92–106. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Palma DA, Olson R, Harrow S, Gaede S,
Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP,
et al: Stereotactic ablative radiotherapy for the comprehensive
treatment of oligometastatic cancers: Long-term results of the
SABR-COMET phase II randomized trial. J Clin Oncol. 38:2830–2838.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Palma DA, Salama JK, Lo SS, Senan S,
Treasure T, Govindan R and Weichselbaum R: The oligometastatic
state - separating truth from wishful thinking. Nat Rev Clin Oncol.
11:549–557. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Morinaga T, Iwatsuki M, Yamashita K,
Harada K, Kurashige J, Nagai Y, Iwagami S, Baba Y, Yoshida N and
Baba H: Oligometastatic recurrence as a prognostic factor after
curative resection of esophageal squamous cell carcinoma. Surg
Today. 51:798–806. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rusthoven KE, Kavanagh BD, Cardenes H,
Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin
W, Kane M, et al: Multi-institutional phase I/II trial of
stereotactic body radiation therapy for liver metastases. J Clin
Oncol. 27:1572–1578. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guckenberger M, Lievens Y, Bouma AB,
Collette L, Dekker A, deSouza NM, Dingemans AC, Fournier B,
Hurkmans C, Lecouvet FE, et al: Characterisation and classification
of oligometastatic disease: A European society for radiotherapy and
oncology and European organisation for research and treatment of
cancer consensus recommendation. Lancet Oncol. 21:e18–e28.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ghaly G, Harrison S, Kamel MK, Rahouma M,
Nasar A, Port JL, Stiles BM and Altorki NK: Predictors of survival
after treatment of oligometastases after esophagectomy. Ann Thorac
Surg. 105:357–362. 2018.PubMed/NCBI View Article : Google Scholar
|